Soya foods may protect against chronic diseases(Reference Barnes, Boersma, Patel, Kirk, Darley-Usmar, Kim and Xu1, Reference Adlercreutz2). Isoflavones in soyabeans, i.e. daidzein, genistein and glycitein, are active substances that have chemical structures similar to mammalian oestrogens(Reference Barnes, Peterson, Grubbs and Setchell3). In food, they are primarily found as β-glucosides with and without additional malonates and acetate esters(Reference Franke, Hankin, Yu, Maskarinec, Low and Custer4). Once ingested, these conjugated isoflavones undergo hydrolysis by β-glucosidases mainly from intestinal bacteria, releasing the principal bioactive aglucone (glucose-free) form that is absorbed, whereas the highly water-soluble glucosides are not absorbed(Reference Xu, Harris, Wang, Murphy and Hendrich5–Reference Franke, Custer and Hundahl8). Therefore, intestinal bacteria are crucial for the absorption and bioavailability of isoflavones(Reference Xu, Harris, Wang, Murphy and Hendrich5, Reference Franke, Custer and Hundahl8, Reference Atkinson, Frankenfeld and Lampe9). It is the aglycones (sugar-free forms) that show an affinity for oestrogen receptors and have other non-hormonal effects on the cell machinery(Reference Setchell6). The isoflavone daidzein is metabolized to equol and O-desmethylangolensin by gut bacteria and excreted predominantly through the urine(Reference Axelson, Sjovall, Gustafsson and Setchell10). The ability to produce equol is limited to 30–50 % of the population but whether this metabolic feature results in more beneficial health effects from soya consumption remains uncertain(Reference Atkinson, Frankenfeld and Lampe9, Reference Setchell, Brown and Lydeking-Olsen11). The extent of isoflavone metabolism varies among individuals and may be influenced by additional dietary factors(Reference Lampe, Gustafson, Hutchins, Martini, Li, Wahala, Grandits, Potter and Slavin12, Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey13).
Dietary isoflavonoids are specific to soya foods and urinary isoflavonoid excretion (UIE) serves as an excellent marker for the bioavailability of isoflavones(Reference Seow, Shi, Franke, Hankin, Lee and Yu14, Reference Maskarinec, Singh, Meng and Franke15). As a result of their rapid metabolism, urinary appearance of isoflavonoids reflects circulating levels when the timing of specimen collection is accurately considered(Reference Franke, Custer and Hundahl8, Reference Grace, Taylor and Low16, Reference Ritchie, Morton, Deighton, Blake and Cummings17). Due to micro-organism-induced hydrolysis in fermented soya products, e.g. tempeh and miso, the predominant form of isoflavones in these foods are aglucones. Consuming fermented soya foods was reported to lead to higher levels of absorption and urinary isoflavone recovery in some(Reference Hutchins, Slavin and Lampe18–Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20) but not in other studies(Reference Tsangalis, Wilcox, Shah and Stojanovska21–Reference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord23). We hypothesized that women will excrete more isoflavonoids and produce more equol after consuming equivalent isoflavone amounts in one serving of miso soup than in one serving of soya milk because the aglucone form present in fermented soya products is more bioavailable than the conjugated isoflavones in the non-fermented soya milk.
Methods
Participants
We recruited a convenience sample through employees, families and friends of the Cancer Research Center of Hawaii. Eligible participants were at least 18 years of age, at least 50 % Japanese ancestry, current residents of Hawaii and free of any known soya allergies. Women with Japanese ancestry were chosen because they were expected to have had previous soya food exposure and a more comparable gut flora than subjects with different ancestries(Reference Song, Atkinson, Frankenfeld, Jokela, Wahala, Thomas and Lampe24). During the intervention, none of the participants were taking antibiotics, food supplements or probiotics that could alter the intestinal flora. The study protocol was approved by the Committee on Human Subjects at the University of Hawaii and all subjects gave written informed consent.
Data collection
Demographic, familial and medical information was collected by questionnaire. Regular soya intake was assessed with a twelve-item questionnaire which elicited the frequency and average serving size of soya food consumed during the last 12 months(Reference Williams, Maskarinec, Hebshi, Oshiro, Murphy and Franke25). To obtain a summary score, we multiplied the frequencies of intake for each of the twelve soya foods by the estimated isoflavone content from the food composition database maintained by the Nutrition Support Shared Resource at the Cancer Research Center of Hawaii. Isoflavone concentrations for soya foods in this database were primarily derived from an analysis of representative local foods(Reference Franke, Hankin, Yu, Maskarinec, Low and Custer4). An additional lifetime questionnaire estimated soya exposure since birth using the following stages: infancy (1 year), childhood (1–9 years), adolescence (10–19 years), early adulthood (20–29 years) and late adulthood (30+ years)(Reference Maskarinec, Takata, Franke, Williams and Murphy26). Participants indicated the annual frequency of four categories of soya foods (tofu; soyabeans and sprouts; soyabean drink or milk; and other soya products) for each period. To obtain a summary score, we multiplied the frequency of intake for each of the four foods by the estimated isoflavone amount and added the total amounts of the four foods in each stage of life to determine an annual intake for each stage.
Intervention and urine collection
The 6 d study protocol included three spot urine collections after a soya-free day and three overnight urine collections after consumption of a standardized portion of miso soup or soya milk. According to the HPLC analyses, one serving of soya milk (250 ml Edensoy® Original Organic soya milk) contained 48·3 mg isoflavones as aglycone equivalents which was matched with 31 g Haccho miso (again determined by HPLC) to be dissolved in one cup of water; 98 % of the isoflavones in miso were aglycones but only 2 % in the soya milk. During and 1 d before starting the intervention, participants were instructed to abstain from all soya foods other than those provided to them for the study. Half of the participants started with soya milk (group A) as follows. On day 1, at around 18.00 hours, the women collected a spot urine sample, consumed one container of soya milk, and collected all urine thereafter until they got up the next morning (overnight urine). On day 2, participants proceeded with their normal diets. On day 3, subjects followed the same protocol as day 1, but drank one serving of miso soup. On day 4, participants again returned to their normal diets. On day 5, subjects followed the same protocol as day 1 exactly, collected a spot urine sample at around 18.00 hours, consumed one container of soya milk, and collected all overnight urine. The other half of the participants (group B) followed the same protocol except starting with miso soup (day 1) followed by soya milk (day 3) and finally miso soup again (day 5). The urine collection containers contained ascorbic and boric acid to prevent bacterial contamination and degradation of analytes. The samples were stored in refrigerators and transported in chilled coolers. The urine samples were aliquoted into 2 ml vials and stored at − 80°C until analysed. Based on the collection times recorded by each subject, we calculated the total number of overnight collection hours.
Urine analysis
Daidzein, genistein and equol were analysed from urine by LC–MS using a triple quadruple TSQ Ultra system (ThermoFisher, San Jose, CA, USA) with electrospray ionization in negative mode and multiple reaction monitoring(Reference Franke, Custer, Wilkens, LeMarchand, Goodman and Kolonel27, Reference Blair, Appt, Franke and Clarkson28). In brief, triply 13C-labelled internal standards of daidzein, genistein and equol (University of St Andrews, UK) were added to 100 μl urine and hydrolysed for 1 h at 37°C with glucuronidase and sulphatase (Roche Applied Sciences, Indianapolis, IN, USA) followed by phase separation with diethyl ether(Reference Franke, Custer, Wang and Shi29). The diethyl ether fractions were dried under nitrogen and re-dissolved in a 1:1 mixture of methanol–sodium acetate buffer (0·2 m, pH 5). The extract (25 μl) was analysed by LC–MS/MS after separation on a BetaBasic C8-column (100 mm × 2·1 mm internal diameter, 3 μm) coupled to a BetaBasic C8-precolumn (10 mm × 2·1 mm internal diameter, 3 μm; both from ThermoFisher)(Reference Franke, Custer, Wilkens, LeMarchand, Goodman and Kolonel27, Reference Blair, Appt, Franke and Clarkson28, Reference Dai, Franke, Yu, Shu, Jin, Hebert, Custer, Gao and Zheng30). The elution was performed with methanol–acetonitrile–water (10:10:80 to 33:33:34) in 6 min, holding there for 1 min before changing in 0·1 min to the starting mixture for equilibration. Ammonium hydroxide (aqueous 2·5 % at 10 μl/min) was infused to enhance the signal. Daidzein was monitored using the transitions (m/z) 253·020 to 222·988, 207·980 and 131·949; for genistein the transitions were 269·090 to 159·050, 133·035 and 132·032, and for equol they were 241·130 to 134·950, 121·000 and 118·960. Limits of quantitation for all analytes were 1·5 nmol/l for daidzein and genistein and 3·0 nmol/l for equol for post-intervention samples and half of those values for pre-intervention samples due to differences in concentration steps prior to analysis. Mean intra- and inter-day CV of LC–MS/MS quantitation for daidzein (422 nmol/l), genistein (35·9 nmol/l) and equol (9·9 nmol/l) were 8·3, 13·6 and 2·9 %, and 7·3, 17·9 and 3·5 %, respectively. Recoveries were 72–88 %. Urinary creatinine concentrations were measured with a Roche-Cobas MiraPlus chemistry analyser using a kit from Randox Laboratory (Crumlin, UK) that is based on a kinetic modification of the Jaffe reaction. Limits of quantitation were < 15 μmol/l and the mean inter-assay CV was 0·8 % at 187 μmol/l. The sum of dadzein, genistein and equol was expressed in nmol isoflavonoid/mg creatinine. We also calculated the isoflavonoid excretion as nmol/h based on urine weight, hours of urine collection and isoflavonoid concentration in urine.
Statistical analysis
All data management and statistical analyses were performed using SAS release 9.1 (SAS Institute, Cary, NC, USA). To define equol producer status, we used 0·05 nmol/mg creatinine as a cut-off value. Two-sample t tests were used to compare study characteristics by group at baseline. Due to their non-normal distribution, we log transformed the UIE. We examined overall mean differences of UIE after soya milk and miso soup intake in one model that included all six UIE values for each woman. This examination was carried out using maximum likelihood estimation of a mixed general linear model that takes into account the covariance structure of the repeated measures within subjects(Reference Littell, Milliken, Stroup and Wolfinger31). The order (group A or B), day, age, weight and equol producer status were included into the model as covariates. In addition, we computed adjusted least square means of UIE for the three food categories (no soya, soya milk, miso soup).
Results
We enrolled twenty-one participants with a mean age of 49·4 (range 20–83) years (Table 1). The mean body weight was 58·0 (sd 11·7) kg. All women reported at least 50 % Japanese ancestry. Self-reported isoflavone intake during the previous 12 months varied among the participants with a mean of 20·1 (range 0·1–85·6) mg/d. While self-reported early life soya intake was only 0·98 (range 0–7·1) servings/d, adult soya intake was 1·7 (range 0·2–10·2) servings/d. Although group B reported higher soya and isoflavone intake than group A during their previous life, none of the differences was statistically significant. The time during which overnight urine was collected covered a mean of 10·8 (sd 1·5) h and did not differ by group (11·0 v. 10·5 h; P = 0·67).
Table 1 Characteristics of the study population

* Self-reported intake during the previous 12 months according to a FFQ.
† Two-sample t tests using log-transformed values for difference between groups A and B.
The unadjusted mean UIE was 135 nmol/mg creatinine after first soya milk as compared to 148 nmol/mg creatinine after first miso soup intake (Fig. 1). When we expressed UIE as an hourly rate(Reference Franke, Halm, Custer, Tatsumura and Hebshi32), the results remained unchanged; the respective medians for soya milk and miso soup were 6·39 and 6·57 μmol/h. The correlation between the two UIE values expressed in different units was 0·95. Based on all 6 d, the mean UIE on days after soya food intake were significantly higher than on the days without soya intake (P < 0·001; Fig. 2). In a mixed model that included the baseline UIE values, the difference in UIE after soya milk v. miso soup consumption was not significant (P = 0·87). The respective adjusted mean UIE for no soya, soya milk and miso soup were 5·7, 116·9 and 111·7 nmol/mg creatinine, respectively. Order (group A or B) was not significant (P = 0·98). Inclusion of body weight and age did not change the effect estimate for UIE (P = 0·19 and P = 0·38, respectively).
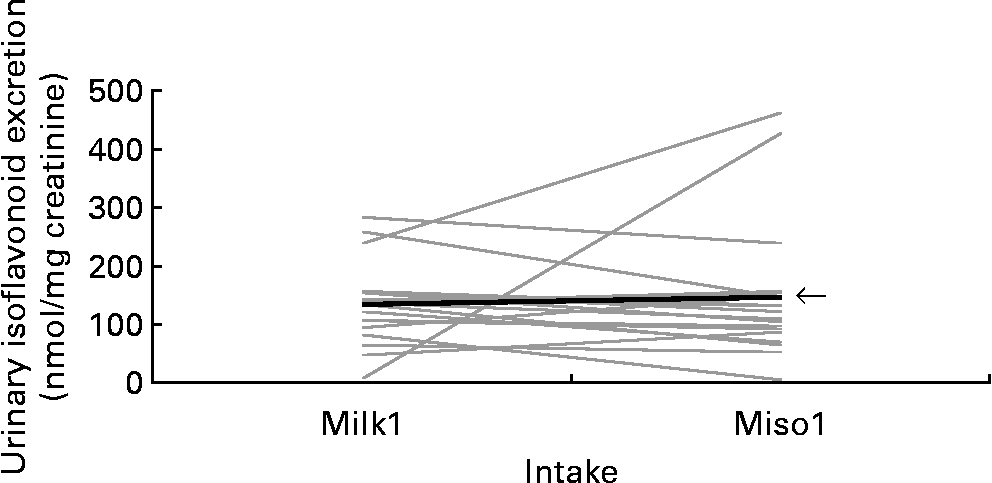
Fig. 1 Urinary isoflavonoid excretion on days 2 and 4 comparing first soya milk (Milk1) and miso soup (Miso1) intake. Each line represents one of the twenty-one Japanese-American women. ![]() , Mean.
, Mean.

Fig. 2 Urinary isoflavonoid excretion before (days 1, 3 and 5; □) and after soya food intake (days 2, 4 and 6; ![]() , soya milk; ■, miso soup) in twenty-one Japanese-American women in two groups (A and B). Values are medians.
, soya milk; ■, miso soup) in twenty-one Japanese-American women in two groups (A and B). Values are medians.
To assess the repeatability of UIE after consuming the same food, we compared the first and second soya milk intake for group A (Fig. 3) and the first and second miso soup intake for group B (Fig. 4). There was no significant difference in UIE between first and second intake for group A or for group B (P = 0·65 and P = 0·81, respectively).
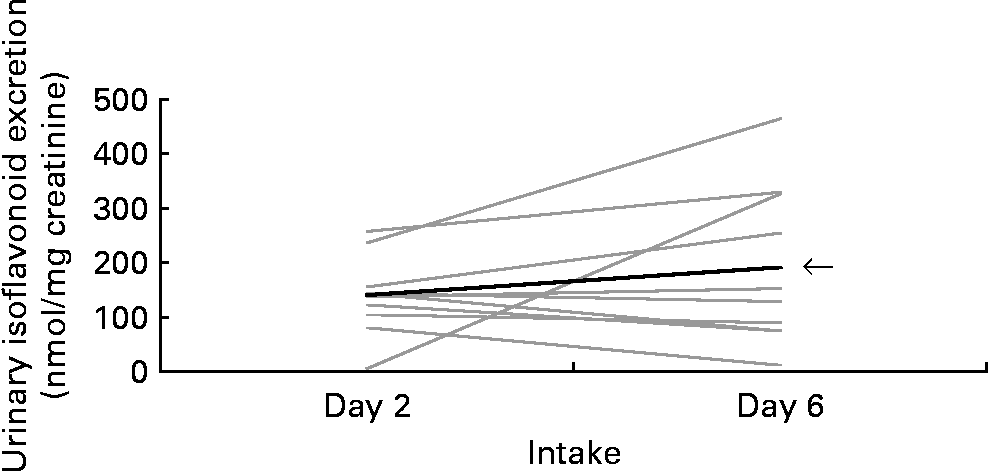
Fig. 3 Urinary isoflavonoid excretion after soya milk intake on days 2 and 6. Each line represents one of the ten group A Japanese-American woman. ![]() , Mean.
, Mean.
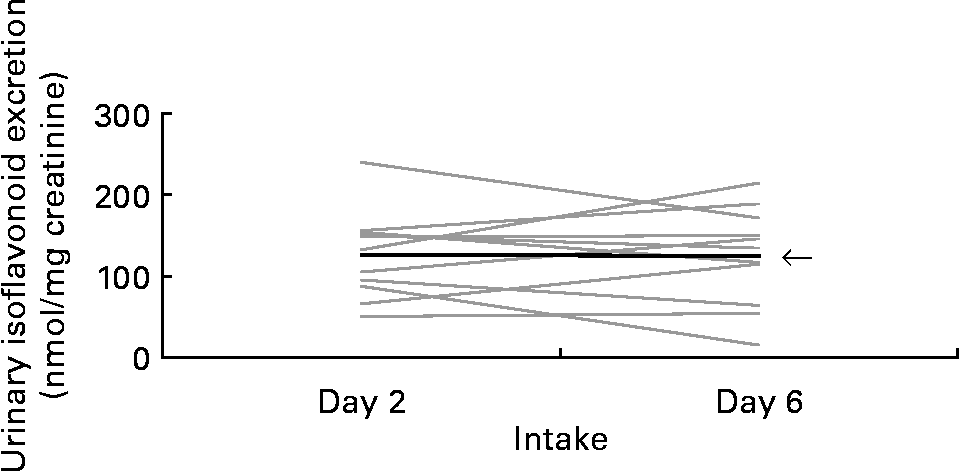
Fig. 4 Urinary isoflavonoid excretion after miso soup intake on days 2 and 6. Each line represents one of the eleven group B Japanese-American woman. ![]() , Mean.
, Mean.
Equol excretion was observed in seven of our twenty-one (33 %) participants. Three of the seven women showed equol excretion on all feeding days, three only on the initial feeding day, and one on feeding days 2 and 4. We observed no significant difference in UIE between soya milk and miso soup for the seven equol producers (P = 0·88). Based on the mixed model, there was no difference in overall UIE between equol producers and non-producers (P = 0·87).
Discussion
In this small intervention study among twenty-one women with Japanese ancestry, we observed no significant difference in overnight UIE after intake of one serving of soya milk or miso soup with equivalent isoflavone content. Expressing UIE as nmol/mg creatinine or as nmol/h gave similar P values(Reference Franke, Halm, Custer, Tatsumura and Hebshi32) and adjustment for potential confounders did not change the result. For the majority of subjects, the UIE did not vary by food (Fig. 1). The results are contrary to our expectation of a higher UIE for miso soup that contains readily available aglucones, whereas the isoflavones in soya milk require hydrolysis prior to uptake. Repeated intake of the same food on different days showed high reproducibility within subjects. There was also no difference in UIE among the subgroup of seven equol producers or between them and the non-equol producers. The fact that one-third of the participants excreted equol agreed with previous reports for Caucasians(Reference Setchell, Brown and Lydeking-Olsen11) but is low for persons of Asian ancestry(Reference Akaza, Miyanaga and Takashima33).
The present results agree with some previous reports(Reference Tsangalis, Wilcox, Shah and Stojanovska21–Reference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord23, Reference Xu, Wang, Murphy and Hendrich34, Reference Tsunoda, Pomeroy and Nestel35), but they are in conflict with others(Reference Hutchins, Slavin and Lampe18–Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20, Reference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi36, Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37). Urinary isoflavonoid recovery did not differ significantly after a meal of cooked soyabeans, tofu, texturized vegetable protein or tempeh(Reference Xu, Wang, Murphy and Hendrich34) between subjects who ingested soyabean isoflavone glycosides or red clover isoflavone aglycones(Reference Tsunoda, Pomeroy and Nestel35), and in a cross-over trial that provided regular soya milk containing mostly glucosides and soya milk treated by probiotic bifidobacteria to produce aglucones(Reference Tsangalis, Wilcox, Shah and Stojanovska21). Similarly, equivalent bioavailability was observed when isoflavones were given as glucosides as naturally present in soya drinks and after enzymatically hydrolysing these drinks to produce aglucones(Reference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord23), and bioavailability of isoflavones was similar among American women after consumption of aglucone or glucoside tablets(Reference Zubik and Meydani22). A comparison of isolated aglucones and conjugated isoflavones also showed identical uptakes for both(Reference Franke, Custer and Hundahl8). On the other hand, a randomized cross-over trial described higher urinary isoflavonoid recovery after eating tempeh than soyabean pieces(Reference Hutchins, Slavin and Lampe18), plasma concentrations over 24 h were higher after the administration of aglucone tablets than an equivalent glucoside preparation(Reference Izumi, Piskula, Osawa, Obata, Tobe, Saito, Kataoka, Kubota and Kikuchi19), isoflavone aglycones were absorbed in greater amounts than their glycosides in a comparison of regular soya milk, fermented soya milk and β-glucosidase-treated soya milk(Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20), and tempeh resulted in larger areas under the curve than textured vegetable protein(Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37). Still another report demonstrated higher bioavailability of glucosides v. aglucones when the area under the plasma curve after oral dosage of 50 mg β-glucosides (daidzin, genistin) was compared to that after intake of the equivalent amount of aglycones (daidzein, genistein)(Reference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi36).
Interventions that measured isoflavones in blood agree that the peak plasma level is achieved faster when aglucones as opposed to glucosides are consumed(Reference Izumi, Piskula, Osawa, Obata, Tobe, Saito, Kataoka, Kubota and Kikuchi19, Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20, Reference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi36, Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37). In the present study, it was not possible to assess the speed of isoflavone absorption during the first hours of consumption as described in studies that collected repeated blood samples(Reference Franke, Custer and Hundahl8, Reference Izumi, Piskula, Osawa, Obata, Tobe, Saito, Kataoka, Kubota and Kikuchi19, Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20, Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37). However, the parallel pattern of plasma isoflavone levels and UIE when plotted as a function of time has been shown(Reference Franke, Custer and Hundahl8). Published reports are consistent in that isoflavones in liquids rather than solids are absorbed faster, but cumulative uptake may be higher for solids(Reference Franke, Custer and Hundahl8, Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37, Reference de Pascual-Teresa, Hallund, Talbot, Schroot, Williams, Bugel and Cassidy38). Therefore, fermentation of soya foods leads to faster absorption and higher peak levels, but it appears likely that similar amounts of isoflavonoids from non-fermented products will become available over time.
The results of the present study have to be considered in light of several limitations, foremost the small sample size. Given the strong variation across subjects, the minimum difference by food that could have been detected with the present study, given a power of 0·80, would have been 49 nmol/mg creatinine. Because all subjects were free-living, we were not able to verify the food intake and the exact times of soya consumption. In fact, it appears that at least one subject may not have consumed the soya milk at all (Fig. 1). Although our choice of two liquid soya foods eliminated bias due to the differential uptake according to the texture of the soya foods(Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37, Reference de Pascual-Teresa, Hallund, Talbot, Schroot, Williams, Bugel and Cassidy38), the women may have consumed the study foods as part of a regular meal whose composition may have affected the uptake of the isoflavones(Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell37). On the other hand, previous studies do not indicate a major effect of regular diet on the excretion of isoflavones(Reference Xu, Wang, Murphy and Hendrich34, Reference Tsunoda, Pomeroy and Nestel35). Another issue was the lack of 24 h urine collections. Overnight urine samples represent the UIE that occurred over 10–12 h. Therefore, we have no information on isoflavonoid excretion during the next day. However, the time frame in the present study covered approximately 70 % of the total UIE(Reference Franke, Custer and Hundahl8). Total excretion levels could have also been determined in 24 h urine samples but extensive periods of urine collection tend to result in low compliance.
The homogeneous ethnic background of the women with regular soya intake in the past was a strength because it reduced possible variation in UIE due to the development of specialized intestinal flora adapted to the breakdown of soya foods(Reference Song, Atkinson, Frankenfeld, Jokela, Wahala, Thomas and Lampe24). Individual differences in this cross-over study were minimized since the subjects served as their own controls and baseline UIE values were included in the statistical models. Results could be different in other ethnic groups who were not exposed to soya foods since childhood(Reference Song, Atkinson, Frankenfeld, Jokela, Wahala, Thomas and Lampe24). The wide age range in the present study, as well as unmeasured genetic and lifestyle factors, e.g. alcohol intake and exercise, may have also affected isoflavone uptake. Although UIE does not directly assess isoflavone absorption, the use of urine seemed adequate to estimate isoflavone bioavailability due to the high correlation of isoflavone appearance patterns in plasma and urine(Reference Franke, Custer and Hundahl8), the general high correlation between plasma and urine values when samples are collected correctly(Reference Franke, Halm, Custer, Tatsumura and Hebshi32, Reference Fanti, Asmis, Stephenson, Sawaya and Franke39), and the strong correlation between plasma and urine values as determined in different experimental settings(Reference Grace, Taylor and Low16, Reference Ritchie, Morton, Deighton, Blake and Cummings17).
The interest in isoflavone uptake from fermented soya foods is based on the idea that the aglucones may be more readily bioavailable and more beneficial to human health(Reference Kano, Takayanagi, Harada, Sawada and Ishikawa20). Due to the continued ambiguity, the present study examined this question using an ethnically homogeneous population, a cross-over design, two liquid soya foods and a timed urine collection. The present results do not support the idea that the consumption of fermented soya foods results in higher isoflavonoid exposure than the intake of unfermented soya foods. Future studies with repeated blood and/or urine collections over more than 24 h among a larger study population are necessary to assess the speed of isoflavonoid uptake. At this time, the overall evidence does not justify nutritional recommendations that favour fermented soya foods as long as the intestinal microflora is capable of hydrolysing the isoflavone glucosides from unfermented soya foods.
Acknowledgements
We are grateful to the Meiji-Yasuda Foundation for supporting this intervention, the study participants, and Laurie Custer for isoflavonoid analysis of the urine specimens. Thanks to Beth Hopping for her help with the manuscript. Support by NCI (CA71789) is appreciated. None of the authors had a conflict of interest related to this project.







