Geriatric syndromes are a loosely defined group of conditions highly prevalent in older adults but not considered as discrete diseases; these include frailty, among others(Reference Inouye, Studenski and Tinetti1). Frailty is a state of decreased physical functioning and a significant complication of ageing that increases the risk for incident falls, fractures, disability, co-morbidity, health care expenditure and premature mortality(Reference Shardell, Hicks and Miller2–Reference Woods, LaCroix and Gray4). In 2010, the proportion of US adults aged 65 years and older was 13 %; however, by 2030 it is projected that about 20 % of the population will be older than 65 years of age, a 54 % increase. This shift in age demographics will have a substantial impact on the prevalence of frailty and highlights the need for identifying clinical- and population-based strategies to decrease the prevalence and consequences of frailty(Reference Crespo, Smit and Andersen5, Reference Vincent and Velkoff6).
Several studies have shown a potential association between nutrition and frailty. Specifically, low energy and protein intake and low serum nutrients have shown to be positively associated with frailty(Reference Beasley, LaCroix and Neuhouser7, Reference Bartali, Frongillo and Bandinelli8). Studies have either been relatively small or with women only. Food insufficiency occurs when persons sometimes or often do not have enough food to eat. Food-insufficient older adults have been shown to have poorer dietary intake, nutritional status and health status than food-sufficient older adults(Reference Lee and Frongillo9). Although little is known about food insufficiency as it relates to frailty, conceivably if food insufficiency is associated with poorer nutritional status, it may also be associated with physical functioning(Reference Lee and Frongillo10) and frailty. Given the potential importance of nutrition on frailty and the amenability to intervention, we examine the association among nutritional status, food insufficiency and prevalent frailty in a representative sample of the US older adult population.
Methods
Study participants
The study population consists of adults aged 60 years and older, who took part in the Third National Health and Nutrition Examination Survey (NHANES III). Briefly, the NHANES III is a nationally representative sample of the population that used a stratified random sample of the civilian non-institutionalised population, drawn from fifty states in the USA and the District of Columbia during 1988–94(11). The analytic sample for the present study consists of older adults (60+ years) with complete data on each frailty criteria (n 4731). This particular survey period was chosen for the availability of frailty measures, which are not available in more recent survey years. The NHANES III was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the National Center for Health Statistics ethics committee. Written informed consent was obtained from all survey participants. The secondary data analysis for the present study was deemed exempt by the Oregon State University Institutional Review Board.
Frailty
A clinical definition of the frailty phenotype has been developed by Fried et al. (Reference Fried, Tangen and Walston3) for the general ageing population and is widely used and validated by others(Reference Woods, LaCroix and Gray4, Reference Graham, Snih and Berges12–Reference Puts, Visser and Twisk14). The definition is based on meeting three of the following five criteria: unintentional weight loss, slowness, muscle weakness, exhaustion and low physical activity. Given that our goal was to examine the association between energy intake and frailty, and because information on unintentional weight loss is not available in NHANES III, we excluded weight loss in our definition of frailty. This approach is similar to and modelled after the definition used by Bartali et al. (Reference Bartali, Frongillo and Bandinelli8) in the Invecchiare in Chianti (InCHIANTI) study. InCHIANTI (Ageing in the Chianti area) is a community-based study of risk factors for disability performed in 1299 participants aged 65 years or older in Italy. The definition of frailty in the InCHIANTI study follows four of the five domains of frailty by Fried et al. (Reference Fried, Tangen and Walston3). Similarly, we defined frailty based on the four domains by Fried et al. (Reference Fried, Tangen and Walston3). These are: (1) slow walking, (2) muscular weakness, (3) exhaustion and (4) low physical activity. Participants were classified as frail if they met two or more of these four criteria. Participants who met one of the four criteria were classified as pre-frail and participants meeting none of the criteria were classified as not frail.
The specific measurement tools for each of the four frailty criteria were selected based on similar measurements or similar questions to those used by Fried et al. (Reference Fried, Tangen and Walston3) and the measurement tools that have been operationalised by other studies on frailty in NHANES III(Reference Wilhelm-Leen, Hall and Deboer15–Reference Wilhelm-Leen, Hall and K Tamura17). A timed 8-foot walk test was performed twice and the best time (s) for the 8-foot walk was used for each participant. A participant was classified as a slow walker if their best time for the 8-foot walk was within the slowest quintile adjusted for sex. Participants were asked whether they had no difficulty, some difficulty, much difficulty or were unable to lift or carry something as heavy as 10 pounds (like a sack of potatoes or rice) when they were by themselves and without the use of aids. Participants who responded to this question as having some or much difficulty or unable to lift or carry that amount were classified as having muscular weakness. Participants were also asked whether they had no difficulty, some difficulty, much difficulty or were unable to walk from one room to another on the same level. Participants who responded to this question as having some or much difficulty or unable to walk from room to room were classified as having exhaustion. Participants who considered themselves as less active when compared with most men/women of the same age were classified as having low physical activity.
Dietary intake and food insufficiency
A 24 h recall was collected during the visit to the mobile examination centre. Dietary intake may differ by weekday, especially on weekend days. To capture intake on all days of the week, the 24 h recalls were collected on every day of the week. The dietary interviewers used the Dietary Data Collection system, which is an automated standardised interactive dietary interview and coding system. The system was specifically developed for NHANES III by the University of Minnesota Nutrition Coordinating Center(Reference Feskanich, Sielaff and Chong18). Participants with 24 h recalls who were noted as incomplete or as unreliable during the interview were excluded (n 178). All the energy intake values from participants with reliable 24 h recalls were included in the final analysis, after preliminary analysis showed similar results with and without excluding outlying intakes (data not shown).
Food insufficiency was determined by asking participants if the food eaten by them and/or their families was enough food to eat, sometimes not enough food to eat or often not enough to eat. Respondents were considered to be food insufficient if they reported sometimes not enough food to eat or often not enough to eat. This has been found to be a reliable measure of food insufficiency(Reference Briefel and Woteki19) and has been used by others(Reference Dixon, Winkleby and Radimer20, Reference Alaimo, Briefel and Frongillo21).
Biochemical variables
Blood concentrations were determined on a specimen obtained by venepuncture during the visit to the mobile examination centre. Details of the laboratory procedures can be found in the Laboratory Procedures Used in NHANES III(11). Albumin was measured with the Boehringer Manneheim Diagnostics albumin system and the bromocresol purple binding agent. Folate was measured by using the Bio-Rad Laboratories ‘Quantaphase Folate’ radioassay kit. Serum levels of vitamins (vitamins A, B12, C and E, carotenoids) were measured by isocratic HPLC, with detection at three different wavelengths. Serum Se was measured using atomic absorption spectrophotometry.
Covariates
We selected covariates based on known factors associated with frailty and/or nutritional status, including age, BMI, race–ethnicity, sex, smoking, education, income and presence of chronic diseases. Self-reported race and ethnicity were used to classify participants as non-Hispanic white, non-Hispanic black or Mexican-American (i.e. persons of Mexican origin living in the USA). Age was defined as the age in years at the time of the household interview. Education was based on the number of years the participant attended and completed school, and coded as less than high school, high school and more than high school. During the medical examination, height was measured using a stadiometer and weight was measured on a balance beam scale. Height and weight data were then used to calculate BMI (weight (kg)/height2 (m)). BMI was categorised into underweight (BMI < 18·5 kg/m2), normal weight (BMI 18·5–24·9 kg/m2), overweight (BMI 25–29·9 kg/m2) and obese (BMI ≥ 30 kg/m2) groups. Smoking history was assessed during the interview and classified into current, former or never smokers. For the present study, presence of current or history of chronic diseases related to physical function was based on affirmative responses to the following physician-diagnosed self-reported chronic conditions: lupus, osteoarthritis, rheumatoid arthritis, bronchitis, stroke, asthma, congestive heart failure, emphysema, heart attack, cancer and chronic low back pain.
Analysis
Total and adjusted means, variances and prevalences were calculated using multivariate linear and multinomial logistic regression models. Sample weights, provided by the National Center for Health Statistics, were used to correct for differential selection probabilities and to adjust for non-coverage and non-response. Logistic regression models with frailty as the outcome were used to obtain adjusted OR. Covariates that were significantly (P< 0·05) associated with frailty in the univariate models were included in the multivariate models, namely age, BMI, race–ethnicity, sex, smoking, education, income and presence of chronic diseases. Additional models related to the macronutrients also adjusted for energy intake where appropriate. All analyses were completed using STATA (version 10.0, StataCorp LP).
Results
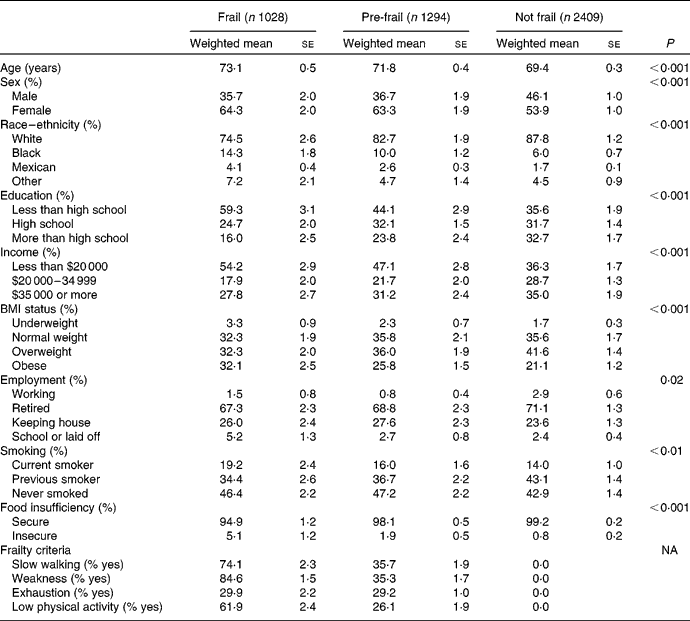
The prevalence of frailty in the US adults aged 60 years and older was 21·7 %, and the prevalence of pre-frailty was 27·4 %. Characteristics of frail, pre-frail and not frail older adults are shown in Table 1. Among frail people, weakness was the most common criteria met, followed by slow walking, low physical activity and exhaustion (85, 74, 62 and 30 %, respectively). Frail people were older, less educated, at lower income levels, more likely to be female and current smokers and less likely to be white than adults who were not frail. Frail people were more likely to be underweight or obese than people who were not frail. In addition, frail people were more likely to report being food insufficient. In fact, people who were frail were 4·69 (95 % CI 1·73, 12·67) times more likely to report food insufficiency than people who were not frail, after adjusting for age, sex, race–ethnicity, smoking status, education, BMI and co-morbidity (data not shown). Pre-frail people were 2·14 (95 % CI 0·79, 5·76) times more likely to report food insufficiency than people who were not frail. In general, pre-frail older adults had values for demographics, BMI and food insufficiency in between the values for frail and not frail adults. For example, the prevalence of obesity was 32 % among frail people, 26 % among pre-frail people and 21 % among not frail people.
Table 1 Characteristics of the Third National Health and Nutrition Examination Survey of adults aged 60 years and older by frailty (Weighted mean values or percentages with their standard errors)

NA, not applicable.
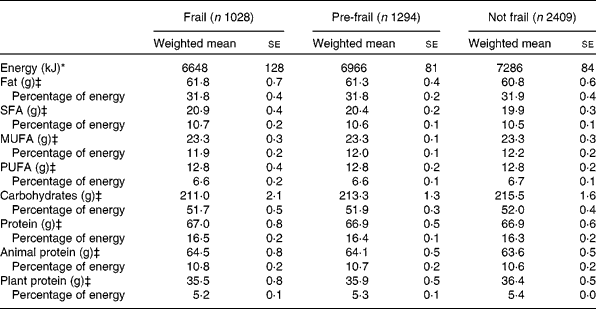
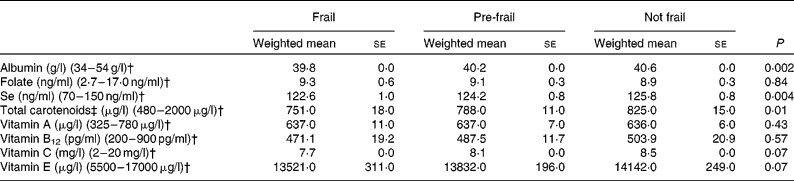
Energy intake was lowest in people who were frail, followed by pre-frail and highest in people who were not frail, independent of BMI (see Table 2). Crude dietary intake of grams of total fat, monounsaturated fat, carbohydrates and protein were lower in people who were frail (57·9 (se 1·7), 21·7 (se 0·6), 201 (se 4·1) and 64·0 (se 1·2), respectively) than people who were not frail (63·6 (se 1·1), 24·4 (se 0·4), 223 (se 3·0) and 68·9 (se 0·7), respectively) (data not shown). However, after adjusting for differences in energy intake, intake of all macronutrients was similar among frail and not frail people (see Table 2). For example, protein intake, either as a percentage of total energy intake or as grams adjusted for energy intake, was similar for frail, pre-frail and not frail people. Plant and animal protein intakes were also similar. Biomarkers of nutritional status are shown in Table 3. Serum albumin, carotenoids and Se levels were significantly lower in people who were frail than people who were not frail.
Table 2 Dietary intake of macronutrients in the Third National Health and Nutrition Examination Survey of adults aged 60 years and older by frailty† (Weighted mean values with their standard errors)

* P< 0·001.
† Adjusted for age, sex, race–ethnicity, smoking, education, income, BMI and co-morbidity.
‡ Also adjusted for energy intake.
Table 3 Serum biomarkers of nutritional status in the Third National Health and Nutrition Examination Survey of adults aged 60 years and older by frailty* (Weighted mean values with their standard errors)

* Adjusted for age, sex, race–ethnicity, smoking, education, income, BMI and co-morbidity.
† Normal reference range.
‡ Sum of β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene.
To examine if one particular criterion for frailty accounts for most of the lower energy intake, we evaluated average energy intake between people who met or did not meet each of the four frailty criteria (see Table 4). Energy intake was lower in people who met each of the criteria than people who did not meet each of the respective criteria. For example, energy intake was lower in people who reported exhaustion than in people who reported no exhaustion. Similarly, underweight, obesity and food insufficiency were consistently higher in people who met each individual criterion than people who did not meet that particular criterion.
Table 4 Energy intake, underweight and obesity, and food insufficiency for each of the frailty criteria in the Third National Health and Nutrition Examination Survey of adults aged 60 years and older* (Weighted mean values with their standard errors and percentages)

* All P values for differences by frailty criteria were significant at P< 0·01. P values were obtained using linear regression for continuous variables and χ2 tests for categorical variables.
† Adjusted for age, sex, race–ethnicity, smoking status, education, income, BMI and co-morbidity.
We examined frailty by BMI categories and found that the prevalence of frailty was highest among people who were obese (20·8 %), followed by overweight (18·4 %), normal weight (16·1 %) and lowest among people who were underweight (13·8 %) (P< 0·01). We also compared energy intake levels for people who are frail and not frail by BMI categories. For each BMI category, energy intake was consistently lower in people who were frail than in people who were not frail (P< 0·01). The difference in energy intake between people who were frail and not frail was highest among people who were obese (1367 kJ), followed by overweight (1294 kJ), normal weight (1220 kJ) and lowest among people who were underweight (1148 kJ). Energy intakes by BMI category among pre-frail older adults were in between the values for frail and not frail adults (data not shown).
Discussion
We examined dietary intake and markers of nutritional status in relation to frailty in a population of US adults aged 60 years and older. Energy intake was lowest in frail, intermediate in pre-frail and highest in people who were not frail, independent of BMI. The present findings are in agreement with Bartali et al. (Reference Bartali, Frongillo and Bandinelli8), who, using a similar definition of frailty as the present study, examined frailty in 802 persons aged 65 years and older in the InCHIANTI study. They reported energy intake to be lower in frail adults independent of BMI.
In contrast to other studies, we found that protein intake, either as energy-adjusted grams of protein or as a percentage of energy intake from protein, did not differ between people who were frail or not frail. The Women's Health Initiative observed that protein intake as a percentage of energy intake, but not absolute protein intake, was positively associated with the incidence of frailty in 24 417 women aged 65–79 years(Reference Beasley, LaCroix and Neuhouser7). The InCHIANTI study reported that low protein intake, defined as the lowest quintile of intake in g/d, was positively associated with frailty(Reference Bartali, Frongillo and Bandinelli8). Differences in the present findings may, in part, be due to differences in study design, study geographical location and analysis, where the Women's Health Initiative study examined the association of protein intake with the incidence of frailty in US women only and the InCHIANTI study reported prevalence of low protein (lowest quintile) intake by frailty status in Italy. NHANES III includes a single 24 h recall, which provides reasonable group means, but less reliably classifies people into low intakes of nutrients. Differences may also, in part, be due to differences in the frailty definition: the Women's Health Initiative study used the five domains of frailty including unintentional weight loss, while the present study and the InCHIANTI study used four domains excluding weight loss. We analysed the present results by including a fifth domain to reflect low BMI (BMI < 18·5 kg/m2) in our definition of frailty, as done by others(Reference Wilhelm-Leen, Hall and Deboer15, Reference Smit, Crespo and Michael16), and results did not change substantially (data not shown).
Along with lower energy intake, frail people were more likely to be food insufficient than not frail people. Food insufficiency reflects an inadequate amount of food due to lack of resources and, to our knowledge, has not been examined in relation to frailty by others. The lower energy intake and higher food insufficiency among people with frailty suggests that food sufficiency and energy intake may be important in the assessment and treatment of frailty. Further, the present findings of a higher prevalence of both underweight and obesity among people who are frail are also in agreement with other studies(Reference Hubbard, Lang and Llewellyn22–Reference Bylow, Hemmerich and Mohile24). Hubbard et al. (Reference Hubbard, Lang and Llewellyn22) in the English Longitudinal Study on Ageing showed a U-shaped association between BMI and frailty. Similarly, Blaum et al. (Reference Blaum, Xue and Michelon23) in the Women's Health and Aging Study showed a positive association between obesity and frailty. Thus, obesity does not preclude frailty, and the lower energy intake and higher food insufficiency in frail people occur across all categories of BMI.
The present findings of lower serum Se and carotenoids, and borderline lower levels of serum vitamins C and E are also in support of others(Reference Semba, Bartali and Zhou25–Reference Schalk, Deeg and Penninx27). Semba et al. (Reference Semba, Bartali and Zhou25) using data from the Women's Health and Aging Study, found that women who were frail had lower serum Se and vitamin E. Similarly, lower serum albumin has been shown to be associated with greater loss of muscle mass(Reference Visser, Kritchevsky and Newman26). The lower serum albumin among frail people in the present study may be an early indicator of impending muscle strength decline, as suggested by the results by Schalk et al. (Reference Schalk, Deeg and Penninx27). Thus, the lower energy intake, lower serum albumin and lower serum nutrients indicate a lower nutritional status in people who are frail compared with people who are not frail. Energy balance may be present in persons who have both lower energy intake and lower physical activity. Low physical activity is one of the criteria of frailty, and energy intake was lower among people who were frail, suggesting potential energy balance in people who are frail. Regardless of the possible energy balance, frail people experienced lower serum nutrients and higher food insufficiency.
Strengths of the present study include dietary intake, serum nutrients and frailty measures on a representative sample of the civilian, non-institutionalised older US adult population and detailed data on important covariates. Limitations include the use of cross-sectional data, which limits our ability to assess cause-and-effect relationships. Nevertheless, these data are very useful in estimating frailty and describing nutritional status in the US population. Frailty measures were available for NHANES III (1988–94) and were not available for more recent survey years. Given the ageing population and the importance of physical function and nutrition in older adults, what we learn from NHANES III can be applicable today. The 24 h recall does not reflect usual or individual intake, yet it does provide reasonable group estimates of dietary intake(Reference Karvetti and Knuts28) for comparison of intake among frail, pre-frail and not frail people. The present estimates of food insufficiency are based on a single question and compared with more comprehensive measures may underestimate the prevalence of food insufficiency(Reference Frongillo, Rauschenbach and Olson29). Pilot testing of the NHANES food insufficiency measure has shown it to be reliable(Reference Briefel and Woteki19), and others have published results using these data(Reference Dixon, Winkleby and Radimer20, Reference Alaimo, Briefel and Frongillo21).
The definition of frailty was based on a modification of the Fried et al. (Reference Fried, Tangen and Walston3) criteria to fit the available NHANES data, excluding unintentional weight loss. The criterion ‘weakness’ was based on interview data, as measured grip strength was not available in NHANES. Other criteria were based on interview questions in both Fried et al. (Reference Fried, Tangen and Walston3) and the present study, with questions varying slightly. The definition of frailty used in the present study is consistent with the definition of frailty used by others(Reference Wilhelm-Leen, Hall and Deboer15, Reference Smit, Crespo and Michael16) and has strong face validity. Moreover, the present study focused on energy intake and serum nutrients as a measure of nutritional status rather than weight loss. This approach allowed us to take a close look at the association between BMI and frailty.
Conclusions
In summary, we found that the prevalence of frailty increases with increasing BMI and that energy intake is consistently lower among frail than not frail older adults. Further, frail older adults are more likely to be food insufficient and have lower serum Se, carotenoids and albumin levels than older adults who are not frail. More research is needed on the energy balance of frailty, and on interventions that allow simultaneously improving nutritional status and food insufficiency among frail older adults, while not necessarily increasing BMI. In the meantime, the present results suggest that targeted interventions should focus on promoting availability and access to nutritious foods among older adults with frailty.
Acknowledgements
The work was partially supported by grants from the General Research Fund Award, Oregon State University (E. S.) and the National Institutes of Health (1 R25 GM086349-01: C. J. C.). E. S., K. M. W.-S. and C. J. C. designed the study; E. S. and P. D. L. analysed the data; K. M. W.-S., P. D. L. and A. M. T. interpreted the results and contributed to the writing of the paper; E. S. and C. J. C. wrote the paper; E. S. had responsibility for final content; all authors have approved the final manuscript. None of the authors claims a conflict of interest.