The increasing number of hip fractures (HF) in older people is a major health care concern( Reference Konnopka, Jerusel and König 1 ), having drastic consequences on functional and self-care abilities of those affected( Reference Roth, Kammerlander and Gosch 2 – Reference Vochteloo, Moerman and Tuinebreijer 4 ). Although some patients are able to achieve almost complete functional restoration, the majority suffers from permanent disability and decline of autonomy and quality of life( Reference Roth, Kammerlander and Gosch 2 – Reference Vochteloo, Moerman and Tuinebreijer 4 ). Considering that the average HF patient is increasingly dependent and in need of care even before HF( Reference Baker, Salar and Ollivere 5 ), huge hospital and long-term costs of care are to be expected( Reference Konnopka, Jerusel and König 1 ). Successful interventions to support the restoration of functional capacity after HF are required, and they should address nutrition as one of the important modifiable factors influencing the regeneration process( Reference Roth, Kammerlander and Gosch 2 , Reference Inzitari, Doets and Bartali 6 , Reference Fiatarone Singh 7 ).
Malnutrition is highly prevalent in the older hospitalized HF population( Reference Murphy, Brooks and New 8 – Reference Corish and Kennedy 11 ), has amplifying effects on age-related decline in muscle mass and strength (sarcopenia)( Reference Inzitari, Doets and Bartali 6 , Reference Corish and Kennedy 11 , 12 ) and is associated with an increased risk of complications, rehospitalisation and mortality in these patients( Reference Inzitari, Doets and Bartali 6 , Reference Li, Cheng and Liang 9 – Reference Corish and Kennedy 11 , Reference Sorensen, Kondrup and Prokopowicz 13 ).
Thus, we previously examined functional and clinical trajectories according to pre-fracture nutritional status in a sample of geriatric HF patients comprising all cognitive and functional levels( Reference Goisser, Schrader and Singler 14 ). In these patients, a worse pre-fracture nutritional status was constantly associated with a worse functional status from before fracture to 6 months after hospital discharge, but unexpectedly not with significantly worse trajectory of functional recovery or worse clinical course.
It has been repeatedly shown that the nutritional status of older patients often deteriorates during hospitalization for various reasons( Reference Corish and Kennedy 11 , Reference Volkert, Saeglitz and Gueldenzoph 15 – Reference Bell, Bauer and Capra 20 ), with insufficient dietary intake (DI) being one of the main contributing factors( Reference Corish and Kennedy 11 , Reference Thibault, Chikhi and Clerc 21 – Reference Hiesmayr, Schindler and Pernicka 23 ).
Low DI has been reported in geriatric HF patients postoperatively( Reference Anbar, Beloosesky and Cohen 24 – Reference Delmi, Rapin and Bengoa 29 ), throughout hospital stay( Reference Murphy, Brooks and New 8 , Reference Nematy, Hickson and Brynes 19 , Reference Bell, Bauer and Capra 20 , Reference Botella-Carretero, Iglesias and Balsa 30 – Reference Bastow, Rawlings and Allison 32 ) and during rehabilitation( Reference Paillaud, Bories and Le Parco 18 , Reference Myint, Wu and Wong 33 ). In these patients, low voluntary DI has been linked with deterioration of nutritional status( Reference Nematy, Hickson and Brynes 19 , Reference Bell, Bauer and Capra 20 , Reference Hoekstra, Goosen and de Wolf 25 ) and worse clinical outcome( Reference Anbar, Beloosesky and Cohen 24 , Reference Eneroth, Olsson and Thorngren 27 – Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Myint, Wu and Wong 33 ) compared with patients receiving interventions to increase DI. Li et al. ( Reference Li, Cheng and Liang 9 ) reported worse functional trajectories over 12 months after hospitalisation in geriatric HF patients being associated with worse nutritional status as assessed at the end of hospital stay.
Most studies assessing the association of DI with functional development after HF could not show an effect of higher DI on functional recovery in older HF patients( Reference Sullivan, Nelson and Klimberg 26 , Reference Gunnarsson, Lönn and Gunningberg 28 , Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Myint, Wu and Wong 33 ), whereas some did find such effects( Reference Paillaud, Bories and Le Parco 18 , Reference Bastow, Rawlings and Allison 32 ). However, these studies were constricted to hospital stay( Reference Sullivan, Nelson and Klimberg 26 , Reference Gunnarsson, Lönn and Gunningberg 28 , Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Bastow, Rawlings and Allison 32 ) or started only in rehabilitation after acute hospital stay( Reference Paillaud, Bories and Le Parco 18 , Reference Myint, Wu and Wong 33 ). Some of them also excluded relevant patient subgroups like functionally or cognitively impaired patients( Reference Paillaud, Bories and Le Parco 18 , Reference Bastow, Rawlings and Allison 32 , Reference Myint, Wu and Wong 33 ) or malnourished persons( Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Myint, Wu and Wong 33 ), although these patients constitute a considerable portion of those sustaining HF( Reference Murphy, Brooks and New 8 , Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Sullivan, Nelson and Klimberg 26 , Reference Bastow, Rawlings and Allison 32 , Reference Seitz, Adunuri and Gill 34 , Reference Foss, Jensen and Kehlet 35 ). To the best of our knowledge, there is no previous study examining the association of voluntary postoperative DI with functional and clinical course of older HF patients over a period of 6 months after hospitalisation and including all relevant patient subgroups.
We therefore assessed this relationship in an HF patient sample including all cognitive and functional levels under the hypothesis that low postoperative DI would be associated with worse postoperative functional and clinical course.
Methods
Study design and recruitment
For this observational study with follow-up after 6 months, all patients aged ≥ 75 years with surgically repaired proximal femoral (subtrochanteric, pertrochanteric and femoral neck) fracture (in this text further referred to as hip fracture (HF)) consecutively admitted to the Department of Trauma and Orthopaedic Surgery of a large urban, maximum care hospital (Klinikum Nürnberg, Germany) from October 2011 to June 2012 were asked to participate. Exclusion criteria were a presumed terminal state, transfer from other hospitals or hospital departments, German-language skills insufficient for answering questions, known cancer-related pathological fractures, cancer with acute radiation or chemotherapy, postoperative transfer to another department or intermediate care unit for more than 72 h (i.e. unavailable for assessment). For this analysis, only patients with complete follow-up assessment and DI data were included.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the ethics committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany. Written informed consent was obtained from every participant or participants' legal custodian. Standardized data acquisition was carried out by three trained persons at four points of time: addressing the situation before fracture (T1, as retrospective interview), within 48 h postoperatively (T2), at discharge from hospital (T3) and in a follow-up telephone interview 6 months after discharge (T4). In addition, patients' medical charts were reviewed and proxies or caregivers were contacted for additional information when necessary, for example, in patients with dementia or delirium.
Baseline characteristics
Participants' demographic characteristics (age, sex, housing situation) were obtained from patients or their proxies in personal interviews, and clinical characteristics (number and type of co-morbidities and medication) from medical documentation. Co-morbidities were grouped according to the German version of the ICD-10 system (International Classification of Diseases, 10th revision)( 36 ). The severity of co-morbid conditions was determined with the Charlson Comorbidity Index (0–37 points)( Reference Charlson, Pompei and Ales 37 ). Polypharmacy was defined as taking more than five different drugs per day.
Nutritional status before HF (T1) was determined retrospectively at T2 by interviewing participants or proxies using the Mini Nutritional Assessment-Long Form (0–30 points)( Reference Murphy, Brooks and New 8 , Reference Guigoz, Vellas and Garry 38 ). Depending on total Mini Nutritional Assessment score, patients were classified as well nourished (>23·5 points), at risk of malnutrition (17–23·5 points) or malnourished ( < 17 points). Information on previous weight loss and previous reduction of DI was taken from Mini Nutritional Assessment questions A and B, respectively, and dichotomized as weight loss ≥ 1 kg in the previous 3 months (yes/no) and any reduction of DI in the previous 3 months (yes/no).
Participants' postoperative cognitive status was evaluated by Mini Mental State Examination (0–30 points)( Reference Folstein, Folstein and McHugh 39 ) at T2. Patients were considered as having no cognitive impairment if achieving >26 points, mildly to moderately impaired with 10–26 points and severely impaired with < 10 points( Reference Loveman, Green and Kirby 40 ) or if the test could not be completed for cognitive reasons. Depressive symptoms at T2 were assessed with the fifteen-item Geriatric Depression Scale (0–15 points)( Reference Yesavage, Brink and Rose 41 ), with a result ≥ 6 points indicating presence of symptoms.
Dietary intake
Postoperative DI was determined with the plate diagram method( Reference Bjornsdottir, Oskarsdottir and Thordardottir 42 , Reference Goisser, Schrader and Ender 43 ). Starting with breakfast on the first day after surgery, each participants' DI was estimated over 4 d by trained study staff after each main meal and recorded as having eaten all (100 %), three-quarters (75 %), half (50 %), one-quarter (25 %) or nothing (0 %) of the whole meal served (Fig. 1), irrespective of which single components had been eaten or left over. Snacks and oral nutritional supplements were not considered. For all patients with at least eleven of twelve possible plate diagrams completed, the overall DI was calculated as the estimated intake percentage of all meals recorded and then stratified as DI >50 %, >25–50 % or ≤ 25 %.

Fig. 1 Plate diagram record sheet for 1 d. ●, All; ![]() , 3/4;
, 3/4; ![]() , 1/2;
, 1/2; ![]() , 1/4; ○, nothing eaten.
, 1/4; ○, nothing eaten.
Functional status and functional course
The ability to perform basic activities of daily living (ADL) was assessed for T1, T2, T3 and T4 with the ten-item Barthel index score (0–100 points)( Reference Mahoney and Barthel 44 ). Postoperative ADL loss in Barthel index points (T2 − T1), residual ADL loss at T3 (T3 − T1) and total residual ADL loss at T4 (T4 − T1) were calculated. Participants' mobility levels (bedridden or chair-bound, mobile only with helper (with or without assistive devices for locomotion) or mobile alone (with or without assistive devices for locomotion)) at T1, T2, T3 and T4 were asked and recorded.
Clinical course
In-hospital mortality, number and type of complications during hospital stay (including all clinically diagnosed conditions occurring in hospital that needed treatment or affected the patients' management) and length of stay were extracted from medical documentation. At follow-up (T4), information about mortality and rehospitalisation of participants was gathered from patients or proxies.
Data analysis and statistics
Continuous data were examined for normal distribution by exact Kolmogorov–Smirnov test and are presented as median and interquartile range (25th–75th percentiles) since most parameters were distributed non-normally. Categorical data are presented as absolute numbers and percentages. Data were stratified into three groups according to DI level (>50 %, >25–50 % or ≤ 25 %). Differences in continuous variables between the groups were tested for significance with Kruskal–Wallis test, followed by pairwise exact Mann–Whitney U test. Prevalence rates of categorical variables were compared between DI groups and tested for statistically significant differences with χ2 or modified Fisher's exact test, as appropriate. A P-value < 0·05 (two-sided) was considered statistically significant after correction for multiple comparisons with the Benjamini–Hochberg procedure( Reference Benjamini and Hochberg 45 ).
ANOVA with repeated measures was performed to assess whether there was a significant difference between DI groups in change of ADL scores over time. Polynomial contrasts were used to account for the unequal time intervals between measurements. When a significant Mauchly's test indicated that the assumption of sphericity had been violated, df were corrected using Huyhn–Feldt estimates of sphericity. The explained variance (%) is based on partial η2. For each significant effect, post hoc analysis between DI groups was performed using the simple Bonferroni correction to control for the effect of multiple comparisons.
In addition, the ANOVA model was extended to an ANCOVA with repeated measures to adjust for the influence of potentially confounding factors. The confounders taken into consideration were included in the model as covariates if continuous (age, number of co-morbidities, Mini Nutritional Assessment points), otherwise as between-subjects factor (sex, postoperative cognitive impairment according to Mini Mental State Examination score (dichotomized to: no or mild cognitive impairment (20–30 points)/moderate to severe cognitive impairment ( < 20 points))). All potential confounders showing a significant interaction effect with ADL levels over time and an impact>10 % on the time × DI group interaction effect were included in the final model.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0.0.1 (IBM Corporation).
Results
Study sample
During the 9-month recruitment period, 236 persons with proximal femoral fractures were admitted to the Department of Trauma and Orthopaedic Surgery; of which, 132 were eligible. Of the 132 persons, 117 (89 % of eligible patients) agreed to participate (Fig. 2). At follow-up (T4), seventeen persons had died (mortality rate 14·5 %) and three refused further participation, resulting in ninety-seven patients (83 % of study participants) with complete follow-up assessment. After excluding nine participants with incomplete DI data (for various reasons, e.g. plate cleared up before DI estimation or meal shared with relatives), eighty-eight patients (75 % of initial study participants) remained for the present analysis. No significant differences regarding age, sex, type of fracture or other baseline characteristics were found between the final study sample (n 88) and those who refused participation at baseline (n 15). Those lost for analysis (n 29) were also not significantly different in baseline characteristics to the final study sample, with the exception that these persons more often were male (lost for analysis 38 % v. study sample 18 %; P= 0·042), more often had malignant tumours (14 v. 0 %; P= 0·003) and had a higher Charlson Comorbidity Index score (median 3 (25th–75th percentile 2–4) v. 2 (25th–75th percentile 1–3) points; P= 0·003). Mortality until T4 did not differ between the DI levels (n 13 of those that had died had complete DI data; DI >50 %: 14 % (n 4); DI >25–50 %: 10 % (n 4); DI ≤ 25 %: 17 % (n 5); P= 0·654).

Fig. 2 Flow chart of study participation. T1, before fracture; T2, postoperatively; T3, at hospital discharge; T4, 6 months after T3.
Baseline characteristics and dietary intake
Eighty-one per cent of the study participants were female, mean age was 84 (sd 5) years (maximum 97 years). Before fracture, 67 % were living in a private household, 9 % in assisted housing and 24 % were admitted from a nursing home. All but one participant had multiple co-morbidities (median 8 (25th–75th percentile 5–11) medical conditions in addition to HF), the main co-morbidities being cardiovascular (86 %), urogenital (80 %), metabolic (67 %) and musculoskeletal diseases (55 %). At T1, 35 % of the patients were at risk of malnutrition and 17 % malnourished according to the Mini Nutritional Assessment score.
Postoperatively, 22 % of the participants had no cognitive impairment, 51 % mild to moderate and 18 % severe cognitive impairment. Six per cent refused to be tested and 3 % were too deaf. Depressive symptoms were detected in 15 %, in 18 % severe cognitive impairment impeded testing and again 6 % refused to be tested.
Median DI was 43 % (25th–75th percentile 25–52) of the meals offered (range 6–79 %). Twenty-five (28 %) participants ate on average >50 %, thirty-eight (43 %) ate >25–50 % and twenty-five (28 %) patients ate ≤ 25 %. Only one person ate >75 %. In Table 1, the subjects' baseline characteristics are presented according to their postoperative DI level. The groups were comparable regarding sex, BMI, pre-fracture housing situation, number and prevalence of co-morbidities, Charlson Comorbidity Index score and prevalence of polypharmacy at T1, but patients with DI ≤ 25 % were slightly (though not significantly) older. These patients also had a significantly worse pre-fracture nutritional status according to the Mini Nutritional Assessment score and more often reported loss of weight and/or reduced DI in the 3 months before fracture than patients with higher DI. Participants with lower DI also suffered more often from cognitive impairments, and accordingly more of these patients were unable to perform the Geriatric Depression Scale (Table 1).
Table 1 Baseline characteristics of geriatric hip fracture patients according to postoperative dietary intake (DI) (Number of patients and percentages; median values with their 25th–75th percentiles)

P, points; MNA, Mini Nutritional Assessment-Long Form; WN, well-nourished; RMN, at risk of malnutrition; MN, malnourished.
* Results for the ≤25 % DI group were significantly different from those of the >50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
† Results for the ≤25 % DI group were significantly different from those of the >25–50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
‡ P for differences between all groups by Kruskal–Wallis, χ2 or Fisher's exact test.
§ n 87 (no weight: n 1 in DI ≤ 25 % group).
∥ n 80 (refused test or too deaf: n 4 each in DI>50 % and DI >25–50 % groups).
¶ n 67 (refused test or cognitively impaired: n 2 in DI>50 %, n 10 in DI >25–50 %, n 10 in DI ≤ 25 % group).
Functional status and functional course
Median Barthel index score for all participants was 90 (25th–75th percentile 75–95) points at T1, decreased to 25 (25th–75th percentile 15–39) points at T2 and then gradually increased to 40 (25th–75th percentile 25–64) points at T3 and to 75 (25th–75th percentile 30–90) points at T4 (Fig. 3), when 68 % of patients had not regained their pre-fracture level of independence in ADL (T4 − T1 < 0 points). Thirty-four per cent had a total residual loss >20 points.

Fig. 3 Functional course of geriatric hip fracture patients as boxplots of Barthel index scores (basic activities of daily living; ADL) at four points in time according to postoperative dietary intake (DI). Data are medians, with interquartile ranges represented by vertical bars. * Median value was significantly different from that of the >50 % DI group (P< 0·05; Mann–Whitney U test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure). † Median value was significantly different from that of the >25–50 % DI group (P< 0·05; Mann–Whitney U test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure). ![]() , >50 % (n 25);
, >50 % (n 25); ![]() , >25–50 % (n 38);
, >25–50 % (n 38); ![]() , ≤ 25 % (n 25). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
, ≤ 25 % (n 25). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
There was no significant difference in ADL scores between DI groups at T1 (Fig. 3). However, patients with postoperative DI ≤ 25 % had significantly higher postoperative ADL loss, residual ADL loss at T3 and remaining total residual ADL loss at T4 compared with patients with higher DI (Table 2). Accordingly, patients with lowest DI had significantly lower ADL scores at all times after surgery (Fig. 3) and significantly more of these patients suffered from remaining ADL losses >20 points at T3 and T4 (Table 2).
Table 2 Changes in Barthel index scores (basic activities of daily living; ADL) of geriatric hip fracture patients from before fracture to 6 months after hospital discharge according to postoperative dietary intake (DI) (Number of patients and percentages; median values with their 25th–75th percentiles)

T2, postoperatively; T1, before fracture; P, points; T3, at hospital discharge; T4, 6 months after T3.
* Results for the ≤25 % DI group were significantly different from those of the >50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
† Results for the ≤25 % DI group were significantly different from those of the >25–50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
‡ P for differences among all three groups by Kruskal–Wallis, χ2 or Fisher's exact test.
ANOVA with repeated measures resulted in a significant time × DI group interaction (F(5·2) = 2·26, P= 0·047, explained variance (partial η2) = 5·1 %), indicating a dependence of ADL development over time from DI level. Post hoc analysis of pairwise group comparisons revealed that the difference was significant only between the groups with the lowest and the highest DI (P= 0·004).
Of the potential confounders, number of co-morbidities (F(2·6) = 4·44, P= 0·007, partial η2= 4·9 %), points in Mini Nutritional Assessment (F(2·6) = 2·57, P= 0·020, partial η2= 4·0 %) and postoperative cognitive impairment according to the Mini Mental State Examination score (F(2·6) = 5·31, P= 0·002, partial η2= 5·8 %) also showed a significant interaction effect with ADL level over time. Of these, only the inclusion of Mini Nutritional Assessment points had an impact >10 % on the time × DI group interaction, increasing the effect of DI level on temporal ADL course (DI group × time: F(5·2) = 2·73, P= 0·019, partial η2= 6·1 %; Mini Nutritional Assessment points × time: F(2·6) = 4·47, P= 0·007, partial η2= 5·1 %).
Regarding mobility, at T1, all but one participant were mobile on their own; one patient needed a helper for locomotion (Table 3). At T2, all patients were bedridden or chair-bound and had to try to start walking again with helpers and assistive devices. At T3, 85 % of patients had not regained their pre-fracture mobility level: 21 % were bedridden or chair-bound, 66 % were mobile only with helpers and 14 % were mobile on their own with assistive devices, with significantly worse mobility in patients with lower postoperative DI (Table 3), because more of them had not regained their pre-fracture mobility level at T3 (DI >50 %: 64 %; DI >25–50 %: 90 %; DI ≤ 25 %: 100 %; P= 0·001). At T4, 2 % of all patients were still bedridden or chair-bound and 16 % were mobile only with a helper. Seventeen per cent had not regained their pre-fracture mobility level, and this was still seen more often in patients with lowest postoperative DI (DI >50 %: 12 %; DI >25–50 %: 11 %; DI ≤ 25 %: 32 %; P= 0·087).
Table 3 Mobility level of geriatric hip fracture patients before fracture, at hospital discharge and 6 months later, according to postoperative dietary intake (DI) (Number of patients and percentages)
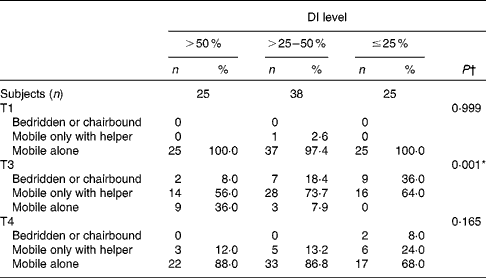
T1, before fracture; T3, at hospital discharge; T4, 6 months after T3.
* Results for the ≤25 % and >25–50 % DI groups were significantly different from those of the >50 % DI group (P< 0·05; χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
† P for differences between all three groups by χ2 or Fisher's exact test.
Clinical course
Mean length of hospital stay was 13 (sd 5) d (median 11 (25th–75th percentile 9; 15) d, range 5–42 d), with no difference between DI groups. Ninety-three per cent of patients suffered from at least one postoperative complication, the main problems being electrolyte imbalance (67 %), infections (49 %) and delirium (44 %). Patients with lowest DI suffered from a higher number of complications and more often had (urinary tract) infections and dehydration (Table 4). During follow-up, 46 % had been readmitted to a hospital at least once, with no difference between DI groups (Table 4).
Table 4 Clinical course of geriatric hip fracture patients according to postoperative dietary intake (DI) (Number of patients and percentages; median values with their 25th–75th percentiles)

T3, at hospital discharge; T4, 6 months after T3.
* Results for the ≤25 % DI group were significantly different from those of the >50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
† Results for the ≤25 % DI group were significantly different from those of the >25–50 % DI group (P< 0·05; Mann–Whitney U, χ2 or Fisher's exact test after correction for multiple tests in pairwise comparisons: Benjamini–Hochberg procedure).
‡ P for differences between all groups by Kruskal–Wallis, χ2 or Fisher's exact test.
Discussion
In this observational study with 6 months follow-up, the majority of geriatric HF patients had an alarmingly low voluntary postoperative DI, with 72 % eating only < 50 % of meals served over the first four postoperative days. Patients with lowest DI had significantly lower ADL scores at all times after surgery than patients with higher DI and more of them suffered from persistent mobility losses up to 6 months after HF, irrespective of functional status before HF.
Indeed, the majority of our participants had a relatively high pre-fracture functional level compared to other studies in geriatric HF patients that also did not exclude functionally and/or cognitively impaired persons or nursing home residents( Reference Baker, Salar and Ollivere 5 , Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Foss, Jensen and Kehlet 35 , Reference Gumieiro, Rafacho and Gonçalves 46 ). Otherwise, the present study sample was comparable to these studies regarding distribution of sex( Reference Baker, Salar and Ollivere 5 , Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Bell, Bauer and Capra 20 , Reference Seitz, Adunuri and Gill 34 , Reference Foss, Jensen and Kehlet 35 , Reference Gumieiro, Rafacho and Gonçalves 46 ), fracture type( Reference Baker, Salar and Ollivere 5 , Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Foss, Jensen and Kehlet 35 , Reference Gumieiro, Rafacho and Gonçalves 46 ), BMI( Reference Koren-Hakim, Weiss and Hershkovitz 10 ), number and severity of co-morbidities( Reference Koren-Hakim, Weiss and Hershkovitz 10 ), prevalence of malnutrition and risk of malnutrition( Reference Koren-Hakim, Weiss and Hershkovitz 10 ), proportion of cognitively impaired patients( Reference Bell, Bauer and Capra 20 , Reference Seitz, Adunuri and Gill 34 ) and nursing home residents( Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Bell, Bauer and Capra 20 , Reference Foss, Jensen and Kehlet 35 ), but were slightly older( Reference Koren-Hakim, Weiss and Hershkovitz 10 , Reference Bell, Bauer and Capra 20 , Reference Seitz, Adunuri and Gill 34 , Reference Gumieiro, Rafacho and Gonçalves 46 ).
In the present study, DI was determined with plate diagrams. With this method, a patient's intake is estimated visually as a portion of the whole meal offered irrespective of exactly how much of each single component has actually been eaten. This method yields only semi-quantitative results; however, in a validation study that we have previously published as an abstract( Reference Goisser, Schrader and Ender 43 ), it showed high agreement with the gold standard weighing record when applied for 4 d in a row, and was able to correctly identify patients with low intake of energy and/or protein. The plate diagrams were filled out by specially trained and experienced study personnel with a direct view of the plate, as opposed to assessment by staff during daily routine, and thus can be deemed to be very reliable. Literature suggests that regular ward staff, which has to cope with many other tasks at the same time, has a tendency to overestimate DI of older patients( Reference Castellanos and Andrews 47 ).
A limitation of the present study is that for this analysis only main meals could be taken into account, leading to a potential underestimation of absolute DI. Snacks provided by relatives and oral nutritional supplements were recorded when noticed but we decided not to include these data into DI estimation, as systematic monitoring was not guaranteed. However, according to the records taken, snacks or supplements were generally consumed very rarely, leading to the conclusion that underestimation should be only marginal. Moreover, this information bias can be assumed to be largely the same for all three groups and thus should not lead to systematic error, although this cannot be known for sure. In another study including patients from all ages with all kinds of diseases, it was estimated that in hospitals the intake from snacks and supplements provides about 25–30 %/d of extra energy and protein( Reference Dupertuis, Kossovsky and Kyle 22 ). In view of the fact that the three main meals provided daily by hospital catering contained on average 8400 kJ (2000 kcal) and 80 g protein/d, mean daily energy and protein intake per patient from main meals in the present study can very roughly be estimated as < 2100 kJ and < 20 g protein/d when DI ≤ 25 %, and as < 4200 kJ and < 40 g protein/d when DI >25–50 %. Even if an additional daily intake from snacks and supplements of about 30 % is assumed, this would only result in approximately < 2700 kJ and < 26 g protein/d, and about < 5400 kJ and < 52 g protein/d, respectively, which would still be far below most patients' requirements( Reference Miller, Daniels and Bannerman 48 ).
Such a very low postoperative DI in geriatric HF patients has previously been found in other studies, where an average intake between 2780 kJ/d over the first three postoperative days( Reference Eneroth, Olsson and Thorngren 27 ) and 3965 kJ/d over the first postoperative week( Reference Sullivan, Nelson and Klimberg 26 ) was reported (calculated from estimated portion ingested of all food items and snacks served). Anbar et al. ( Reference Anbar, Beloosesky and Cohen 24 ) observed a mean energy intake from food of 3250 kJ/d plus 400 kJ/d from supplements and a mean protein intake of 37 g/d (assessed with food records) over the whole hospital stay (13 (sd 6) d). Considering that other studies( Reference Nematy, Hickson and Brynes 19 , Reference Myint, Wu and Wong 33 ) reported mean energy intakes from 4220 to 4340 kJ/d at admission to rehabilitation 2–4 weeks after surgery, it can be assumed that also in many of our patients DI remained very low after the fourth postoperative day. Further, taking into account that most patients of the present study already went through a prolonged period of mandatory fasting before surgery (median 33 (25th–75th percentile 20–47) h, which is comparable to reports from other HF studies( Reference Anbar, Beloosesky and Cohen 24 , Reference Sullivan, Nelson and Klimberg 26 )), without much doubt in most of our participants DI was far from sufficient to cover the basic nutritional needs for at least 1 week and probably even longer.
There are other studies that report a slightly higher mean DI of 4600–5400 kJ/d and up to 1 g protein/kg body weight/d over the first seven to fourteen postoperative days( Reference Hoekstra, Goosen and de Wolf 25 , Reference Delmi, Rapin and Bengoa 29 , Reference Botella-Carretero, Iglesias and Balsa 30 ). However, in these studies, none of the participants was malnourished before HF or was cognitively impaired, whereas the results of the present study show that significantly more patients with low postoperative DI were already malnourished before HF, had lost weight and/or had reduced DI, and all of them were at least mildly cognitively impaired after surgery. Excluding such patients thus might lead to records of higher DI, as seen in the aforementioned reports.
The high prevalence of malnutrition and cognitive impairment in patients with low DI (or vice versa) reflects a general state of frailty and reveals the highly worrying fact that precisely the most vulnerable patients seem to be predisposed to low postoperative DI (which also might be a sign for a generally low DI). Comparable findings have been reported by other groups( Reference Murphy, Brooks and New 8 , Reference Nematy, Hickson and Brynes 19 , Reference Bell, Bauer and Capra 20 , Reference Hoekstra, Goosen and de Wolf 25 , Reference Lumbers, New and Gibson 31 , Reference Bastow, Rawlings and Allison 32 ), indicating that nutritional problems that occurred before HF (not surprisingly) continued or exacerbated in hospital, and that these problems were not properly addressed by hospital care. The cumulating energy and nutrient deficit caused by inadequate DI (before and) throughout hospital stay can very likely be assumed to contribute substantially to these patients' reduced capacity for rehabilitation( Reference Corish and Kennedy 11 ).
In accord with this hypothesis, in the present study, long-term impairments in ADL and mobility were seen significantly more often and more pronounced in patients with lowest postoperative DI. Such a relation has been reported as early as 1983 by Bastow et al. ( Reference Bastow, Rawlings and Allison 32 ), who found that patients with lower DI (mean 4200 kJ/d from fifth postoperative day to discharge after about 40 d, calculated from estimated portion ingested of all food items and snacks served) needed more time to restore independent mobility than patients with a higher DI (that was either achieved voluntarily or by supplementary nightly enteral tube feeding). In 2000, Paillaud et al. ( Reference Paillaud, Bories and Le Parco 18 ) observed the same relation in patients of a rehabilitation clinic, where those patients with lowest voluntary DI about 3 weeks after surgery needed considerably more time to self-sufficient mobility than those with highest DI. In contrast, other studies found no significant differences in functional development according to DI( Reference Sullivan, Nelson and Klimberg 26 , Reference Gunnarsson, Lönn and Gunningberg 28 , Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Myint, Wu and Wong 33 ). However, all previous studies only had short follow-up periods and were all primarily designed to evaluate the effect of increasing DI through supplementation, and therefore did not differentiate their control groups in patients with higher or lower voluntary DI. To our knowledge, this is the first time that differences in voluntary postoperative DI were analysed for their influence on functional development after HF over four points in time until 6 months after hospitalisation and including patients from all functional and cognitive levels. In most previous studies, the exclusion of relevant patient subgroups like functionally or cognitively impaired patients( Reference Paillaud, Bories and Le Parco 18 , Reference Bastow, Rawlings and Allison 32 , Reference Myint, Wu and Wong 33 ) or malnourished persons( Reference Botella-Carretero, Iglesias and Balsa 30 , Reference Myint, Wu and Wong 33 ) most likely led to the exclusion of those with lowest DI and probably worst functional recovery.
The patients with lowest DI also suffered from significantly more postoperative in-hospital complications, thus adding to the growing body of evidence that insufficient DI is associated with a worse postoperative clinical course, especially with the development of postoperative infections( Reference Anbar, Beloosesky and Cohen 24 , Reference Eneroth, Olsson and Thorngren 27 – Reference Delmi, Rapin and Bengoa 29 ). However, all postoperative nutritional intervention studies to prevent such complications up to now yielded only conflicting results( Reference Corish and Kennedy 11 , Reference Avenell and Handoll 49 ), and thus it has to be taken into account that association does not necessarily implicate causality. Foss et al. ( Reference Foss, Jensen and Kehlet 35 ) correctly stated that it should also be considered that suffering from complications may lead to insufficient DI. Moreover, other factors such as the deleterious impact of HF and surgery on the general health of these patients might influence the prevalence of both, and the same effect has of course to be taken into consideration for postoperative functional development too. Certainly, more well-designed high-quality trials addressing and elucidating this relationship are needed, preferably including clinical and biochemical data from the short-term recovery period, in order to shed more light on this aspect.
Length of hospital stay and rehospitalisation did not differ significantly between DI groups. However, in the German disease-related group reimbursement system currently established in the health care system, both parameters are hugely influenced by a variety of factors (e.g. minimal and maximal reimbursed length of stay per diagnosis) and do not always primarily reflect the individual patients' condition. Due to differences in health care system structures, such results are also hardly comparable between countries, sometimes not even between hospitals( Reference Liem, Kammerlander and Suhm 50 ).
Of course, it is not only nutritional deficits that cause unfavourable functional outcomes, but in the present study, the consideration of potential confounders through ANCOVA did not change the result that postoperative DI has a significant influence on functional development after HF. This underlines that nutrition is one important contributing factor in the rehabilitation process, and that nutritional deficits have to be addressed as part of a complex care concept considering all relevant factors that affect rehabilitation success( Reference Inzitari, Doets and Bartali 6 , Reference Fiatarone Singh 7 ).
Limitations
One major limitation of the present study is that the statistical power for detecting differences between DI groups is limited by the rather small overall sample size. Another problem is that we could not systematically assess and analyse the patients' individual reasons for low DI, which would be key information for the development of suitable, patient-tailored nutritional interventions to positively influence DI. Study staff impression is that among the reasons heard most often from patients, relatives and ward staff, ‘refuses to eat’ (especially in those cognitively impaired), ‘no appetite’, ‘don't like the food’, ‘cannot chew the food’, ‘nausea’, ‘not feeling well’ and ‘too tired’ were prominent, but also ‘didn't get the help needed’. These and many more reasons for low DI in hospitalised geriatric HF patients have also been detected and discussed rather comprehensively by other groups( Reference Corish and Kennedy 11 , Reference Bell, Bauer and Capra 20 , Reference Dupertuis, Kossovsky and Kyle 22 , Reference Nieuwenhuizen, Weenen and Rigby 51 , Reference Patel and Martin 52 ). Sadly, as seen again in the present study, even today, nutritional deficits frequently remain undetected in clinical routine( Reference Corish and Kennedy 11 , Reference Volkert, Saeglitz and Gueldenzoph 15 ). As there is hardly any situation more convenient to initiate nutritional therapies than the hospital setting, suitable interventions ideally should start there and continue in rehabilitation. To cope with the challenges presented to health care systems by demographic change, it is necessary to be more cost-effective by ‘making the most’ of the hospitalisation period with its high inevitable fixed costs by providing optimal treatment that minimises follow-up costs( Reference Wyers, Reijven and Evers 53 ).
Conclusions
In the present study, significantly more patients with lower postoperative DI turned out to have already been in a state of frailty before HF, as they were malnourished and cognitively impaired, indicating that the most vulnerable patients seem to be highly predisposed to low postoperative DI.
Obviously, this subgroup of patients would need to be in the focus of care and get specific nutritional support to achieve adequate DI. Ideally, any therapy should not only help to maintain their functional capacity by covering present nutritional needs, but should also compensate for previously accumulated nutritional deficits. Therefore, future research will need to focus on suitable, patient-tailored nutritional intervention strategies that help to achieve adequate DI in the vulnerable group of malnourished older HF patients to support their functional and clinical recovery, thereby elucidating which kind of therapeutic concepts are most beneficial for these patients regarding functional and clinical course after HF.
Acknowledgements
The authors are grateful to all study participants and their proxies as well as the staff at the orthogeriatric and other Departments of Trauma and Orthopaedic Surgery and the hospital kitchen at Klinikum Nürnberg for their cooperation and support. We also wish to thank Christin Ender (Nutritional Scientist) and Katja Purucker (MD) for their very valuable help in conducting the present study.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. S. G. and D. V. were supported by the Theo and Friedl Schöller-Foundation, Nürnberg, Germany, and S. G. was scholarship holder of a Bavarian Equal Opportunities Sponsorship (Förderung von Frauen in Forschung und Lehre – Promoting Equal Opportunities for Women in Research and Teaching) of the Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany. These funding sources had no role in the design or analysis of the study or the writing of this article.
The authors declare that there are no known conflicts of interest associated with this publication. The primary author is a current PhD candidate. The present work was performed in partial fulfilment of the requirements for obtaining the degree ‘Doktor der Humanbiologie (Dr rer. biol. hum.)’ at the Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany.
The authors' contributions are as follows: S. G. was primarily responsible for patient recruitment, data acquisition, analysis and interpretation, and for drafting of the manuscript. E. S. contributed to study conception and data interpretation. K. S. contributed to study conception, data acquisition and interpretation. O. G. contributed to data analysis and manuscript revision. T. B., R. B., H.-J. B. and C. C. S. assisted with study conception and patient recruitment. D. V. was mainly responsible for study design and conception and contributed to data interpretation and analysis as well as to manuscript drafting and revision. The final version of this manuscript has been approved by all named authors, and there are no other persons who fulfilled the criteria for authorship but are not listed.










