Pseudomonas aeruginosa is a Gram-negative bacterium and an opportunistic pathogen that exhibits resistance to most available antibiotics( Reference Oliver, Canton and Campo 1 ). P. aeruginosa is involved in nosocomial infections and is the predominant pathogen in the lungs of patients with cystic fibrosis( Reference Davies 2 ). P. aeruginosa infection promotes an abnormal, up-regulated pro-inflammatory cytokine profile and inhibits host immune responses that contribute to its pathogenicity( Reference Cantin 3 , Reference Lyczak, Cannon and Pier 4 ). The failure to clear infection causes massive and persistent neutrophil influx, which can directly damage tissues and cause additional increases in pro-inflammatory cytokines, leading to bronchiectasis and fibrosis, followed by respiratory failure and death( Reference Davis, Drumm and Konstan 5 ). In this context, breaking the cycle of repetitive and eventually persistent infections, inflammation and influx of neutrophils is critical to treat cystic fibrosis successfully.
A growing number of studies have demonstrated an important role of specific dietary lipids in health and disease. n-3 Long-chain (LC) PUFA and alkylglycerols are two different lipids that abundantly accumulate in the liver of certain species of marine fish, such as rays and chimeras, respectively. n-3 LC-PUFA mainly exhibit anti-inflammatory properties by promoting the synthesis of anti-inflammatory factors and inhibiting n-6 LC-PUFA-induced inflammation (for a review, see Miles & Calder( Reference Miles and Calder 6 )). Dietary n-3 LC-PUFA, mainly EPA and to a lesser extent DHA in a 2:1 ratio, play a key role in improving the outcomes of lung infections resulting from P. aeruginosa ( Reference Pierre, Husson and Le Berre 7 , Reference Tiesset, Pierre and Desseyn 8 ). Although EPA and DHA are grouped together as the n-3 PUFA, there is substantial evidence suggesting that the individual fatty acids may have selective and potentially independent effects (for a review, see Anderson & Ma( Reference Anderson and Ma 9 )). In most dietary sources of n-3 LC-PUFA used in previous experiments, EPA was more abundant than DHA in a 2:1 ratio, suggesting that most of the anti-inflammatory effects observed were mainly due to EPA. However, it remains unclear whether DHA alone or in a DHA/EPA mixture with a 2:1 ratio is able to improve pulmonary inflammation induced by P. aeruginosa infection and more importantly to increase the survival rate after the infection.
Alkylglycerols are bioactive ether lipids particularly abundant in oil extracted from the liver of sharks and chimeras where they constitute up to 50 % of the lipid fraction. Alkylglycerols have been shown to play a critical role in the modulation of immunity by enhancing macrophage activation and increasing plasma levels of Ig in rodents( Reference Yamamoto, St Claire and Homma 10 – Reference Mitre, Etienne and Martinais 13 ). Besides their roles at the host level, in vitro studies have indicated alkylglycerols to be antimicrobial agents( Reference Ved, Gustow and Mahadevan 14 , Reference Brissette, Cabacungan and Pieringer 15 ).
In this context, the aim of the present study was to test the impact of (1) an n-3 PUFA mixture enriched in DHA/EPA with a 2:1 ratio and (2) alkylglycerols on the survival of mice subjected to an acute P. aeruginosa lung infection. To test this hypothesis, oils extracted from rays and chimeras, naturally enriched in DHA/EPA (2:1) and alkylglycerols, respectively, were incorporated in diets and given to mice for 5 weeks before the induction of P. aeruginosa infection. The impact of the diets on the survival rate was evaluated, and we particularly focused on the inflammatory status and pulmonary functions of the infected mice to assess the influence of those lipids on their immune system.
Materials and methods
Mice and diets
Male C57BL/6 mice (Janvier), aged 5 weeks old, were provided food and water ad libitum and housed in a light-controlled (12 h light–12 h dark, lights on at 07.00 hours) and temperature-controlled (20°C) environment.
The control, DHA/EPA (2:1) and alkylglycerol diets were adapted from the AIN-93G (American Institute of Nutrition-93 Growth) rodent diet (American Institute of Nutrition( Reference Reeves, Rossow and Lindlauf 16 )) and presented the same content of energy, proteins, minerals, micronutrients and vitamins (Table 1). Briefly, lipids from soyabean oil were substituted with an isoenergetic amount of vegetable oil mixture for the control diet and with an isoenergetic amount of vegetable oil mixture plus oils extracted from the liver of rays and chimeras, which naturally demonstrate a high rate of DHA/EPA in a 2:1 ratio or alkylglycerols, respectively, for the diets enriched in DHA/EPA (2:1) and alkylglycerols (Table 2). Fish oils were extracted from rays and chimeras, and provided by Copalis and used by Nutricia Research to prepare the diets. Our aim was to supply the mice in each group with the same amounts of α-linolenic acid (18 : 3n-3) and linoleic acid (18 : 2n-6), the precursors of n-3 and n-6 PUFA, respectively, and to substitute the lipids of the AIN-93G diet with either ray oil, naturally enriched in DHA/EPA (2:1), or chimera oil, naturally enriched in alkylglycerols (10 g/kg diet); the control diet had none of these compounds (Table 3). Mice were randomised into three groups and fed with the control diet or the isoenergetic, isolipidic diets containing DHA/EPA (2:1) or alkylglycerols for 5 weeks before the induction of P. aeruginosa infection and until the end of the protocols.
Table 1 Composition of the experimental diets*
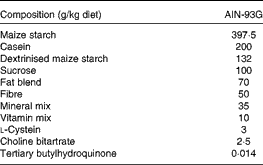
AIN-93G, American Institute of Nutrition-93 Growth.
* Control, DHA/EPA (2:1) and alkylglycerol diets were adapted from the AIN-93G rodent diet. Each of the diets presents a similar distribution of the AIN-93G compounds except for the lipid content that was adapted in order to supply DHA/EPA in a 2:1 ratio and alkylglycerols for two of the diets without affecting the energy and lipid contents.
Table 2 Composition of fat blends*
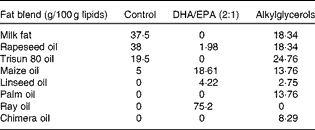
* Lipids (from soyabean oil) were substituted by three different isoenergetic fat blends: vegetable oil mixture for the control diet; vegetable oil mixture plus ray oil for the DHA/EPA (2:1) diet; vegetable oil mixture plus chimera oil for the alkylglycerol diet.
Table 3 Fatty acid composition of the experimental diets*
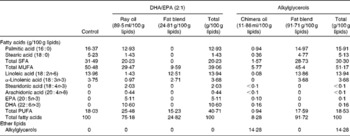
* The lipid mixtures obtained from vegetable oils or vegetable oils plus ray or chimera oils were isolipidic and isoenergetic. Our aim was to supply the mice in each group with the same amounts of α-linolenic acid (18 : 3n-3) and linoleic acid (18 : 2n-6), the precursors of n-3 and n-6 PUFA, respectively.
Acute Pseudomonas aeruginosa pulmonary infection and survival
The P. aeruginosa strain O1 (PAO1) was grown overnight (14 h) in Luria broth medium in a shaking and temperature-controlled (37°C) environment. The bacteria were then pelleted, washed twice and grown in fresh Luria broth for 3 h at 37°C. Once the exponential phase was reached, the bacteria were pelleted, washed twice and resuspended in 0·9 % sterile saline buffer to obtain 1 × 109 colony-forming units/ml. Male C57BL/6 mice, aged 10 weeks old, were anaesthetised with sevoflurane inhalation (Sevorane; Abbott) and placed in dorsal recumbency for endotracheal instillation of 50 μl of the PAO1 suspension (5 × 107 colony-forming units/mouse) using a 24 G animal feeding needle. Mice were then checked and weighed daily; survival was assessed over a period of 10 d after the onset of the infection.
Pulmonary bacterial clearance
Mice were killed at 8, 24 or 36 h after the instillation of P. aeruginosa for the evaluation of pulmonary bacterial load. Briefly, the lungs were excised aseptically, weighed and homogenised in 0·9 % sterile saline buffer. To determine the number of P. aeruginosa colony-forming units, a 100 μl aliquot of the homogenates was inoculated quantitatively by 10-fold serial dilutions on bromocresol purple agar plates (Biomerieux Laboratories) and incubated for 24 h at 37°C.
Bronchoalveolar lavage
Bronchoalveolar lavages were performed by inserting a blunt 20 G needle in the trachea and injecting/reaspirating 0·5 ml followed by 1 ml of 0·9 % sterile saline in the lungs. Samples (1·2–1·5 ml) of the bronchoalveolar lavage fluid (BALF) were collected from the infected lungs and centrifuged for 10 min at 1000 g . The supernatant or BALF was processed for cytokine assays while the pellet was resuspended in 1 ml of 0·9 % sterile saline to determine the proportion of neutrophils and macrophages. Briefly, the cells collected from the lungs of the infected mice were counted and organised in monolayers with a cytocentrifuge (Thermo Electron). The cells were stained with Wright–Giemsa solutions (CML) for the determination of different morphotypes. Proportions of neutrophils and macrophages were obtained from the samples of 200 cells per mouse.
Cytokine assays
Lung homogenates and the BALF were used to quantify the concentrations of IL-10 and TNF-α, respectively, in mice infected with P. aeruginosa using ELISA kits (BD System). Absorbance at 450 nm was determined using a microplate reader, which was used to quantify the concentrations of IL-10 and TNF-α in the lungs homogenised in 0·9 % sterile saline or the BALF collected from mice in each group.
Alveolar–capillary barrier permeability
Mice from the control, DHA/EPA (2:1) and alkylglycerol groups were injected intraperitoneally with 0·2 mg fluorescein isothiocyanate (FITC)-albumin diluted in 0·9 % sterile saline at 6, 14 and 34 h after the onset of the infection. At 2 h later, mice were killed, blood was collected by cardiac puncture and bronchoalveolar lavages were performed as described above. Alveolar–capillary barrier permeability was determined by calculating the ratio between the fluorescence measured in the BALF and in the serum.
Time points of the study
Based on previous experiments( Reference Tiesset, Pierre and Desseyn 8 ) and the survival rate of mice in the present study, bacterial load and expression of the anti-inflammatory molecule IL-10 were assessed in the lungs of mice at 8, 24 and 36 h after the onset of the infection. We evaluated the expression of the pro-inflammatory molecule TNF-α, the recruitment of neutrophils and macrophages, and pulmonary injuries in the BALF of mice at 8, 16 and 36 h after the onset of the infection.
Statistical analysis
Results are presented as means with their standard errors. Multiple comparisons were analysed using a two-way ANOVA with two independent factors (diet and time of infection). Multiple comparisons between the treatment groups and the control group were corrected with Dunnett's test. Multiple comparisons within groups between the different time points of infection were corrected using Tukey's test. Cumulative survival rates were compared using the log-rank statistical analysis. The threshold for significance was set at P< 0·05.
Ethics statement
The protocols used herein were approved by the INSERM guidelines for the care and use of laboratory animals and by the Animal Care Ethics Committee of the region Nord-Pas-de-Calais (protocol CEEA 142011). The animals and experiments used in the present study were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding mammalian research.
Results
DHA/EPA (2:1) improve the survival rate of mice infected with Pseudomonas aeruginosa
In the control mice, P. aeruginosa infection induced 68 % mortality within 6 d and most of these mice (51 %) died within 48 h after the onset of P. aeruginosa lung infection (Fig. 1(a)). The survival rate of mice fed the alkylglycerol diet was similar to that of the control mice (Fig. 1(a)). Interestingly, the DHA/EPA (2:1)-enriched diet significantly increased the resistance to P. aeruginosa infection starting at day 2 after the instillation of the bacteria (P< 0·05 v. control diet; log-rank test; Fig. 1(a)).
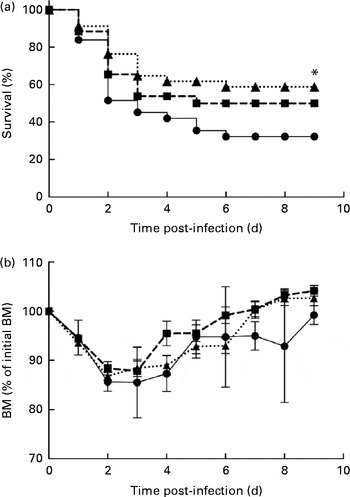
Fig. 1 Impact of diets enriched in DHA/EPA (2:1) and alkylglycerols on survival to Pseudomonas aeruginosa pulmonary infection. (a) Data represent the percentage of the survival of mice in each group. P. aeruginosa infection was instilled at day 0 (5 × 107 colony-forming units/mouse). * Survival was significantly increased in mice fed with the DHA/EPA (2:1) diet compared with the control diet (P< 0·05). (b) Body mass (BM) after the onset of P. aeruginosa lung infection in mice fed the control, DHA/EPA or alkylglycerol diet for 5 weeks. Data represent the percentage of initial BM. Control group, n 31 (–●–); alkylglycerol group, n 26 (–■–); DHA/EPA (2:1) group, n 34 (···▲···).
The alkylglycerol- and DHA/EPA (2:1)-enriched diets did not affect body mass before or after the onset of P. aeruginosa lung infection. A significant body mass loss was measured 2 and 3 d after the instillation of P. aeruginosa infection in each group. However, the loss did not exceed 15 % of the initial body mass (Fig. 1(b)). Of those mice that survived the infection, body mass started to increase at day 4, leading to the full recovery of the initial body mass 7 d after infection in the alkylglycerol and DHA/EPA (2:1) groups and 9 d after infection in the control group (Fig. 1(b)).
DHA/EPA (2:1) accelerate bacterial clearance in the lungs
None of the diets affected the bacterial load in the lungs during the first 8 h of infection (106·73 (sem 100·14) v. 106·02 (sem 100·37) and 106·94 (sem 100·16) bacteria/lung in the control, alkylglycerol and DHA/EPA (2:1) groups, respectively; Fig. 2). Mice fed with the control and alkylglycerol-enriched diets exhibited a similar bacterial load at all time points of the experiment (106·73 (sem 100·14), 106·24 (sem 100·67) and 105·81 (sem 100·55) bacteria/lung in the control group; 106·02 (sem 100·37), 104·91 (sem 100·62) and 106·23 (sem 100·33) bacteria/lung in the alkylglycerol group at 8, 24 and 36 h after infection, respectively; Fig. 2). Interestingly, mice fed with the DHA/EPA (2:1)-enriched diet exhibited accelerated bacterial clearance compared with the control mice. At 24 h after infection, the lung concentration of P. aeruginosa was significantly lower when compared with the control mice (104·52 (sem 100·63) v. 106·24 (sem 100·67) bacteria/lung in the DHA/EPA (2:1) and control groups, respectively; P< 0·05; two-way ANOVA; Fig. 2).
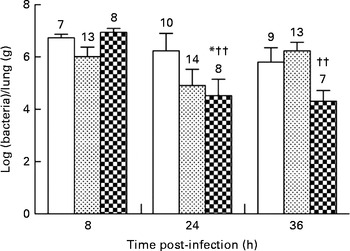
Fig. 2 Impact of diets enriched in DHA/EPA (2:1) and alkylglycerols on bacterial clearance following the onset of Pseudomonas aeruginosa pulmonary infection. Data represent the ratio between the number of bacteria collected and the weight of the lungs from each mouse. Briefly, the lungs excised from mice in each group at 8, 24 and 36 h after the onset of the infection were homogenised in 0·9 % sterile saline solution. Serial dilutions of the homogenates were inoculated on bromocresol agar plates for 24 h at 37°C in order to determine the number of P. aeruginosa colony-forming units. Values are means (n 7–14 mice per group), with standard errors represented by vertical bars. * Mean value was significantly lower 24 h after P. aeruginosa infection in the lungs of mice fed with DHA/EPA (2:1) compared with the control mice at the same time of infection (P< 0·05; two-way ANOVA). †† Mean value was significantly different from that at 8 h in the DHA/EPA (2:1) group (P< 0·01; two-way ANOVA). The numbers on top of the bars are the number of mice per group. ![]() , Control;
, Control; ![]() , alkylglycerols;
, alkylglycerols; ![]() , DHA/EPA (2:1).
, DHA/EPA (2:1).
Fish oils attenuate the influx of neutrophils towards the lungs
The neutrophil granulocyte is a major participant in the early phases of acute inflammatory response in tissues( Reference Phillipson and Kubes 17 ). Accordingly, neutrophils were the predominant cell population observed in the BALF collected from mice in each group at all time points of infection. P. aeruginosa induced a continuous and significant influx of neutrophils in the BALF of mice fed the control and alkylglycerol-enriched diets during the course of infection (3·5 × 105 (sem 4·6 × 104) and 1·5 × 106 (sem 2·2 × 105) cells/ml; 2·4 × 105 (sem 4·5 × 104) and 1·2 × 106 (sem 2·4 × 105) cells/ml in the control and alkylglycerol groups at 8 and 36 h, respectively; P< 0·001 in the control group and P< 0·01 in the alkylglycerol group; two-way ANOVA; Fig. 3(a)). Interestingly, despite a similar pattern of recruitment between these two groups, mice fed the alkylglycerol-enriched diet exhibited attenuated infiltration of the lungs by neutrophils at 36 h after the onset of the infection when compared with the control mice (1·2 × 106 (sem 2·4 × 105) v. 1·9 × 106 (sem 4·2 × 105) cells/ml in the alkylglycerol and control groups, respectively; P< 0·05; two-way ANOVA; Fig. 3(a)). Similarly, the DHA/EPA-enriched diet was associated with a significant decrease in neutrophils detected in the lungs at 36 h after the onset of the infection (1·1 × 106 (sem 1·9 × 105) cells/ml in the DHA/EPA (2:1) group v. 1·9 × 106 (sem 4·2 × 105) cells/ml in the control group; P< 0·01; two-way ANOVA; Fig. 3(a)). As a result, only a modest non-significant increase in neutrophils was observed in the infected lungs of these mice during the course of infection (Fig. 3(a)).
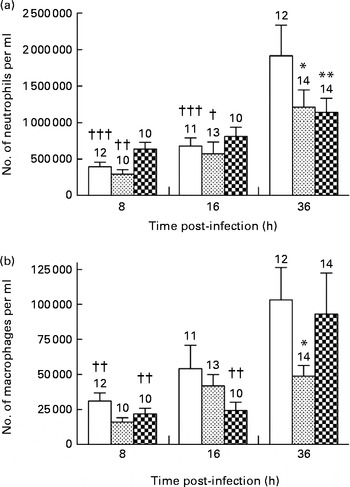
Fig. 3 Impact of diets enriched in DHA/EPA (2:1) and alkylglycerols on the recruitment of immune cells following the onset of Pseudomonas aeruginosa pulmonary infection. Data represent the number of neutrophils (a) and macrophages (b) in the bronchoalveolar lavage fluid (BALF) collected at 8, 16 and 36 h after the onset of the infection from mice in each group. Briefly, bronchoalveolar lavages were performed with a blunt 20 G needle by injecting/reaspirating 0·5 ml followed by 1 ml of 0·9 % sterile saline solution in the trachea of mice from each group at 8, 16 and 36 h after the onset of the infection. The cells collected were organised in monolayers with a cytocentrifuge and stained with Wright–Giemsa solutions in order to determine and count the morphotypes. Values are means, with standard errors represented by vertical bars. Mean value was significantly lower 36 h after P. aeruginosa infection compared with the control mice: * P< 0·05, ** P< 0·01 (two-way ANOVA). Mean value was significantly lower than that at 36 h after P. aeruginosa infection in the same group: † P< 0·05, †† P< 0·01, ††† P< 0·001 (two-way ANOVA). The numbers on top of the bars are the number of mice per group. ![]() , Control;
, Control; ![]() , alkylglycerols;
, alkylglycerols; ![]() , DHA/EPA (2:1).
, DHA/EPA (2:1).
Alkylglycerols decrease macrophage colonisation in the lungs
Alveolar macrophages play an important role in resolving inflammation( Reference Krysko, Vandenabeele and Krysko 18 ). P. aeruginosa infection induced a continuous recruitment of macrophages in the BALF of the control and DHA/EPA (2:1) groups during the course of infection (Fig. 3(b)). Moreover, according to their role, pulmonary colonisation reached a maximum 36 h after the onset of the infection in these two groups (1·03 × 105 (sem 2·3 × 104) v. 3·11 × 104 (sem 5·7 × 103) cells/ml and 9·30 × 104 (sem 2·9 × 104) v. 2·19 × 105 (sem 4·0 × 103) cells/ml in the control and DHA/EPA (2:1) groups at 36 v. 8 h, respectively; P< 0·01 in the control and DHA/EPA (2:1) groups; two-way ANOVA; Fig. 3(b)). Interestingly, the number of macrophages in the BALF of the alkylglycerol group remained comparable at all time points of infection (1·60 × 104 (sem 3·12 × 103), 4·18 × 104 (sem 8·13 × 103) and 4·88 × 104 (sem 7·70 × 103) cells/ml in the alkylglycerol group at 8, 16 and 36 h, respectively; Fig. 3(b)). This pattern of colonisation led to a significant decrease in macrophage infiltration in the lungs of the alkylglycerol group when compared with the control group at a later stage of infection (4·88 × 104 (sem 7·70 × 103) and 1·03 × 105 (sem 2·30 × 104) cells/ml in the control and alkylglycerol groups, respectively, at 36 h; P< 0·05; two-way ANOVA; Fig. 3(b)).
DHA/EPA (2:1) increase TNF-α secretion in the lungs
In the control and alkylgycerol groups, P. aeruginosa infection was associated with the similar levels of TNF-α at 8 and 16 h after the onset of the infection (1516 (sem 256) and 2516 (sem 516) pg/ml; 2145 (sem 241) and 2030 (sem 488) pg/ml in the control and alkylglycerol groups at 8 and 16 h, respectively; Fig. 4(a)). Then, TNF-α secretion decreased in both groups but without reaching significance in the alkylglycerol group (866 (sem 81) v. 2030 (sem 488) and 522 (sem 144) v. 2516 (sem 516) pg/ml at 36 and 16 h in the alkylglycerol and control groups, respectively; P< 0·01 in the control group; Fig. 4(a)). Interestingly, P. aeruginosa infection triggered a peak secretion of TNF-α at 16 h after the onset of the infection in the DHA/EPA (2:1) group (3951 (sem 671) v. 1791 (sem 283) and 1162 (sem 123) pg/ml at 16, 8 and 36 h, respectively; P< 0·001; two-way ANOVA; Fig. 4(a)). Moreover, at this time point, the TNF-α level was significantly higher than that of the control group (3951 (sem 671) v. 2515 (sem 516) pg/ml at 16 h in the DHA/EPA (2:1) and control groups, respectively; P< 0·05; two-way ANOVA; Fig. 4(a)).
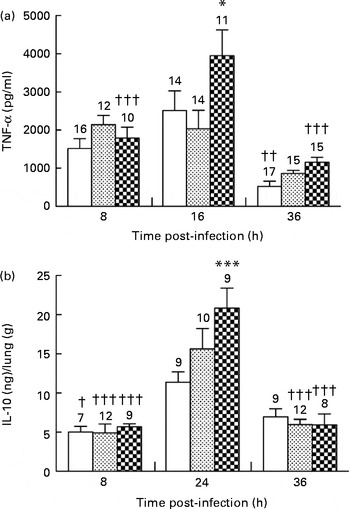
Fig. 4 Inflammatory status after the onset of Pseudomonas aeruginosa infection in mice fed the control (![]() ), alkylglycerol- (
), alkylglycerol- (![]() ) or DHA/EPA (2:1)-enriched (
) or DHA/EPA (2:1)-enriched (![]() ) diets. Cytokines were evaluated in the bronchoalveolar lavage fluid (BALF) for TNF-α and in lung homogenates for IL-10 using ELISA kits. (a) Data represent the concentration of TNF-α in the BALF collected from mice in each group. Values are means (n 7–17 mice per group), with standard errors represented by vertical bars. * Mean value was significantly higher 16 h after P. aeruginosa infection compared with the control mice (P< 0·05; two-way ANOVA). Mean value was significantly lower than that at 16 h after P. aeruginosa infection in the same group: †† P< 0·01, ††† P< 0·001 (two-way ANOVA). (b) Data represent the concentration of IL-10 extracted from the lungs of mice in each group. Values are means (n 7–17 mice per group), with standard errors represented by vertical bars. *** Mean value was significantly higher 24 h after P. aeruginosa infection compared with the control mice (P< 0·0001; two-way ANOVA). Mean value was significantly lower than that at 24 h after P. aeruginosa infection in the same group: † P< 0·05, ††† P< 0·0001 (two-way ANOVA). The numbers on top of the bars are the number of mice per group.
) diets. Cytokines were evaluated in the bronchoalveolar lavage fluid (BALF) for TNF-α and in lung homogenates for IL-10 using ELISA kits. (a) Data represent the concentration of TNF-α in the BALF collected from mice in each group. Values are means (n 7–17 mice per group), with standard errors represented by vertical bars. * Mean value was significantly higher 16 h after P. aeruginosa infection compared with the control mice (P< 0·05; two-way ANOVA). Mean value was significantly lower than that at 16 h after P. aeruginosa infection in the same group: †† P< 0·01, ††† P< 0·001 (two-way ANOVA). (b) Data represent the concentration of IL-10 extracted from the lungs of mice in each group. Values are means (n 7–17 mice per group), with standard errors represented by vertical bars. *** Mean value was significantly higher 24 h after P. aeruginosa infection compared with the control mice (P< 0·0001; two-way ANOVA). Mean value was significantly lower than that at 24 h after P. aeruginosa infection in the same group: † P< 0·05, ††† P< 0·0001 (two-way ANOVA). The numbers on top of the bars are the number of mice per group.
DHA/EPA (2:1) increase IL-10 secretion in the lungs
The anti-inflammatory cytokine IL-10 plays an important role in the resolution of inflammation promoted by P. aeruginosa infection( Reference Armstrong, Jordan and Millar 19 , Reference Cox 20 ). None of the diets affected IL-10 secretion in the lungs of mice in each group 8 h after the onset of the infection. The control, alkylglycerol and DHA/EPA (2:1) groups exhibited a significant increase in IL-10 secretion in the lungs 24 h after the onset of P. aeruginosa infection (11·3 (sem 1·2) v. 5·0 (sem 0·7) ng/g of lungs; 15·6 (sem 2·6) v. 4·9 (sem 1·1) ng/g of lungs and 20·8 (sem 2·5) v. 5·7 (sem 0·4) ng/g of lungs in the control, alkylglycerol and DHA/EPA (2:1) groups at 24 and 8 h, respectively; P< 0·05 in the control group and P< 0·001 in the alkylglycerol and DHA/EPA (2:1) groups; two-way ANOVA; Fig. 4(b)). Interestingly, the peak observed in mice fed with the DHA/EPA (2:1)-enriched diet was significantly higher than that observed in the control group (20·8 (sem 2·5) v. 11·3 (sem 1·2) ng/g of lungs; P< 0·001; two-way ANOVA; Fig. 4(b)). At 36 h after the onset of P. aeruginosa infection, alkylglycerol- and DHA/EPA (2:1)-fed mice exhibited a significant decrease in IL-10 secretion, leading to values comparable to those measured at 8 h after the onset of the infection (5·97 (sem 0·66) v. 4·90 (sem 1·14) ng/g of lungs and 5·92 (sem 1·4) v. 5·67 (sem 0·41) ng/g of lungs in the alkylglycerol and DHA/EPA (2:1) groups at 36 and 8 h, respectively; P< 0·001; two-way ANOVA; Fig. 4(b)).
Fish oils do not affect lung permeability
Alveolar–capillary barrier permeability was used as a marker of pulmonary injuries in mice infected with P. aeruginosa. The diets did not affect alveolar–capillary barrier permeability in the lungs during the first 8 h of infection (3·5 (sem 0·3), 3·3 (sem 0·5) and 4·5 (sem 0·4) % in the control, alkylglycerol and DHA/EPA (2:1) groups, respectively; Fig. 5). A significant decrease in alveolar–capillary barrier permeability was observed at 16 h after the onset of the infection in the DHA/EPA (2:1) group, but without reaching significance when compared with the control group (2·5 (sem 0·5) v. 4·5 (sem 0·4) % in the DHA/EPA (2:1) group at 16 and 8 h, respectively; P< 0·05; two-way ANOVA; 2·5 (sem 0·5) v. 3·5 (sem 0·4) % in the DHA/EPA (2:1) and control groups at 16 h after the onset of the infection, respectively; Fig. 5).
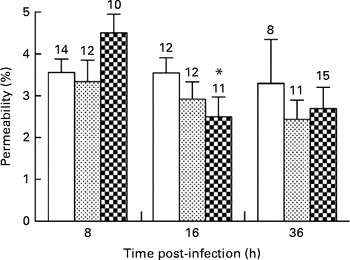
Fig. 5 Impact of diets enriched in DHA/EPA (2:1) and alkylglycerols on pulmonary injuries following the onset of Pseudomonas aeruginosa infection. Data represent the proportion of fluorescence detected in the bronchoalveolar lavage fluid v. serum collected by cardiac puncture 2 h after intraperitoneal injection of albumin-fluorescein isothiocyanate (FITC) (0·2 mg/mouse) in mice from each group. Values are means (n 8–14 mice per group), with their standard errors represented by vertical bars. * Mean value was significantly lower 16 h after P. aeruginosa infection in mice fed with the DHA/EPA (2:1)-enriched diet compared with 8 h in the same group (P< 0·05; two-way ANOVA). The numbers on top of the bars are the number of mice per group. ![]() , Control;
, Control; ![]() , alkylglycerols;
, alkylglycerols; ![]() , DHA/EPA (2:1).
, DHA/EPA (2:1).
Discussion
The present study showed that DHA/EPA supplementation in a 2:1 ratio improved the outcomes of P. aeruginosa infection in a more efficient manner than supplementation with alkylglycerols. Thus, supplementation of the DHA/EPA (2:1)-enriched diet for 5 weeks improves the survival rate in our mouse model of acute P. aeruginosa pulmonary infection via a specific pattern of cytokine secretion and neutrophil influx. Indeed, DHA/EPA (2:1) supplementation induces a high level of the pro-inflammatory cytokine TNF-α and a high secretion of the anti-inflammatory cytokine IL-10. In addition, supplementation with DHA/EPA (2:1) limits the influx of neutrophils in the lungs in response to P. aeruginosa infection. Together, these events are associated with significant bacterial clearance, leading to an improvement of survival.
We previously demonstrated in wild-type and cftr − / − mice that 5 weeks of supplementation with the same quantity of DHA/EPA but with an inverted 1:2 ratio is enough to modulate fatty acid incorporation in phospholipid membranes and to improve the outcomes of acute and chronic lung infection with P. aeruginosa ( Reference Pierre, Husson and Le Berre 7 , Reference Tiesset, Bernard and Bartke 21 ). A growing number of studies have suggested that DHA demonstrates immunomodulatory and anti-inflammatory functions in a wide variety of inflammatory diseases( Reference Lee, Lim and Kim 22 – Reference Cleland, Caughey and James 25 ), besides its well-documented action in the maturation of the nervous system (for a review, see Miles & Calder( Reference Miles and Calder 6 ) and Larque et al. ( Reference Larque, Gil-Sanchez and Prieto-Sanchez 26 )). However, divergences in the anti-infectious actions of these compounds in the outcomes of infections by influenza virus or Citrobacter rodentium raised the question of the extraction/purification of the lipids, as well as the doses and optimal ratio of EPA and DHA needed in a mixture of fatty acids to reach a significant anti-infectious effect( Reference Schwerbrock, Karlsson and Shi 27 – Reference Hekmatdoost, Wu and Morampudi 29 ). These aspects of the impact of EPA and DHA on the immune system mainly remain unclear because of the lack of dose–response studies (for a review, see Calder( Reference Calder 30 )). One other important finding in the present study is that the fatty acid mixture naturally extracted from rays and enriched in DHA/EPA (2:1) demonstrates strong anti-infectious properties associated with a global anti-inflammatory effect, opening the field for a future human clinical trial.
Indeed, we showed that a DHA/EPA (2:1)-enriched diet tends to increase the influx of neutrophils in the lungs at the early stage of infection, although this influx was significantly reduced during the later stage of infection. These cells represent the first line of defence against bacterial infections by orchestrating activities leading to the destruction of pathogens( Reference Lotz, Aga and Wilde 31 ). The prominence of P. aeruginosa sepsis in neutropenic patients highlights the critical role of neutrophils in defence against this pathogen( Reference Maschmeyer and Braveny 32 ). In contrast, it is also well known that patients with cystic fibrosis suffer from chronic inflammation because of persistent pulmonary colonisation by neutrophils( Reference Bonfield, Konstan and Berger 33 , Reference Chmiel and Davis 34 ). In this way, Freedman et al. ( Reference Freedman, Katz and Parker 35 ) showed that restoration of the DHA level in the cell membranes of cftr − / − mice by oral supplementation of DHA leads to the normalisation of neutrophil influx in the lungs after the onset of P. aeruginosa infection. This effect was not observed in wild-type mice, leading the authors to conclude that DHA could not directly act on the mediators of inflammation. Here, we show that even in the context of a balanced level of DHA in cell membranes, a DHA/EPA (2:1)-enriched diet could play a major role in preventing chronic inflammation by improving the influx of neutrophils towards the site of infection. The duration of the diet and/or the association with EPA, also known to improve the outcomes of P. aeruginosa infection( Reference Pierre, Husson and Le Berre 7 , Reference Tiesset, Pierre and Desseyn 8 ), could explain the differences observed. However, to provide isolipidic–isoenergetic diets to our three groups of mice, the SFA content was decreased in the DHA/EPA (2:1) diet compared with the control and alkylglycerol diets. Also, SFA are well known to promote inflammation (for a review, see Calder( Reference Calder 30 )). So, we cannot rule out the impact of the decreased level of SFA in our DHA/EPA (2:1) diet. However, Freedman et al. ( Reference Freedman, Katz and Parker 35 ) showed a significant and beneficial impact of DHA alone without modifications of SFA, suggesting that the limitation of neutrophil influx observed here results at least from the combination of DHA/EPA (2:1) and the decreased level of SFA, if not DHA/EPA (2:1) alone.
The DHA/EPA (2:1)-enriched diet is associated with a concomitant increase in IL-10 and TNF-α secretion during the infection. IL-10 decreases lung inflammation by limiting neutrophil influx and promoting their apoptosis( Reference Armstrong, Jordan and Millar 19 , Reference Cox 20 , Reference Dang, Elbim and Marie 36 – Reference Chmiel, Konstan and Saadane 38 ). Accordingly, in our model, DHA/EPA (2:1) enhance the peak secretion of IL-10 at 24 h after the onset of the infection followed by attenuation of neutrophil influx in the lungs at 36 h after the onset of P. aeruginosa infection. Concomitantly, we measured a high level of TNF-α secretion in DHA/EPA (2:1)-fed mice. We previously observed a concomitant and equivalent rise of TNF-α and IL-10 secretion in mice fed with DHA/EPA with an inverted 1:2 ratio and subjected to acute P. aeruginosa infection( Reference Tiesset, Pierre and Desseyn 8 ). In the same way, the increased level of TNF-α was not associated with increased bacterial load or neutrophil influx in our protocol. In that study( Reference Tiesset, Pierre and Desseyn 8 ), despite notable changes in the inflammatory and immune responses of mice fed with the DHA/EPA (1:2)-enriched diet, no significant improvement of survival was observed. However, the pattern of cell recruitment was not improved with the DHA/EPA (1:2) diet as it is with our DHA/EPA (2:1) diet. These results suggest that DHA exhibits more potent anti-infectious actions than EPA, partly by optimising a coordinated cell immunity response at the site of infection. TNF-α has been shown to play a significant role in priming and activating neutrophils in patients with cystic fibrosis, enhancing lung injuries and leading to chronic inflammation( Reference Taggart, Coakley and Greally 39 ). However, inflammation is needed to eradicate the invading pathogens( Reference Schwerbrock, Karlsson and Shi 27 , Reference Ghosh, DeCoffe and Brown 40 ), so the anti-inflammatory effect of n-3 LC-PUFA could be deleterious. Here we show that besides its anti-inflammatory role, the DHA/EPA (2:1) diet triggers a high level of the pro-inflammatory cytokine TNF-α during P. aeruginosa infection, and this may participate in the beneficial effect of the diet on the outcomes of the infection. Indeed, Murray et al. ( Reference Murray, Barbara and Dunkley 41 ) showed that TNF-α is an important factor implicated in neutrophil apoptosis by binding to TNF-α receptors 55 and 75. In our model, we observed a decreased influx of neutrophils towards the end of the infection, following the significant increase in TNF-α secretion and concomitantly with an increase in macrophage influx towards the lungs. So, one could speculate that the high level of TNF-α combined with macrophage influx observed in DHA/EPA (2:1)-fed mice leads to neutrophil apoptosis and removal from the lungs. Ultimately, the high secretion of TNF-α promoted by the DHA/EPA (2:1) diet in our model compensates the anti-inflammatory impact of the diet, and leads to a decrease of the pathogen in the lungs and an increase in the survival rate.
We did not observe an improvement in resistance to acute P. aeruginosa pulmonary infection in mice fed the alkylglycerol-enriched diet. More importantly, our model did not confirm the anti-bacterial effect of alkylglycerols that was previously observed in vitro ( Reference Ved, Gustow and Mahadevan 14 , Reference Brissette, Cabacungan and Pieringer 15 ). However, previous studies emphasised high variability in the anti-bacterial effect of these compounds depending on the type of fatty acids linked to glycerol, the dose of alkylglycerols and the species of bacteria. In our protocol, the mix of alkylglycerols used to feed the mice was particularly enriched in long-chain fatty acids such as C18 (data not shown), which could explain the absence of the anti-bacterial effect observed in our model. In addition, although our laboratory previously demonstrated that dietary supplementation for 5 weeks is sufficient to increase the incorporation of n-3 PUFA in the plasma membrane( Reference Pierre, Husson and Le Berre 7 , Reference Tiesset, Pierre and Desseyn 8 ), the duration of the diet and the concentration of alkylglycerols needed to reach a significant incorporation of alkylglycerols in tissues have been poorly documented. Previously, similar doses of alkylglycerols (10 g/kg diet) have been shown to be highly incorporated in various tissues of mice, including lungs, after 4 weeks of diet( Reference Weber 42 ). Interestingly, we observed that the diet and the protocol used in the present study tended to modulate the immune system of mice subjected to acute P. aeruginosa infection, such as macrophage and neutrophil influx and TNF-α secretion, suggesting that higher doses and/or longer duration of the diet may modulate the outcomes of acute P. aeruginosa pulmonary infection in mice.
Conclusion
We demonstrate that DHA/EPA in a 2:1 ratio increase the survival rate in our mouse model of acute P. aeruginosa pulmonary infection. The DHA/EPA (2:1) diet triggers high levels of IL-10 and TNF-α secretion, preventing the persistent influx of neutrophils towards the site of infection. Altogether, these events lead to a significant decrease in bacterial load, increasing survival to the infection. Overall, we showed that the DHA/EPA (2:1)-enriched diet improves coordinated responses of the immune system to bacterial infection. In contrast, alkylglycerols did not significantly improve survival to P. aeruginosa infection at the dose and duration of the diet used. However, significant changes in macrophage and neutrophil influx as well as slight variations in TNF-α secretion were observed, suggesting that a higher dose and/or longer duration of the diet might have a significant impact on immune functions. Finally, DHA/EPA (2:1) and alkylglycerols do not seem to activate the same pathways, suggesting that a combination of both lipids warrants further research in the prevention of P. aeruginosa infection.
Acknowledgements
The present study was funded by a ‘seaminaroil’ grant – a project supported by FUI (Fonds Unique Interministériel) aiming at adding value to unused fish by-products – and financially supported by Nutricia Research.
The authors' contributions are as follows: E. C., M.-O. H. and F. G. conceived and designed the experiments; L. S. and N. B. contributed unpublished reagents/analytic tools; E. C. performed the experiments; E. C., J.-L. D., M.-O. H., A. D. and F. G. analysed the data; E. C. and F. G. wrote the paper. All authors read and approved the final manuscript.
The authors have no conflict of interest to declare.











