White adipose tissue (WAT) has become an important target for the treatment of metabolic complications associated with obesity, since either the impairment of its capacity to buffer excess lipids or the dysregulation of its endocrine function has been related to the development of a chronic pro-inflammatory state and insulin resistance typical of the onset of obesity. It has been found that adipokines are closely related to the maintenance of different metabolic processes such as the regulation of energy homeostasis, insulin sensitivity and lipid/carbohydrate metabolism(Reference Galic, Oakhill and Steinberg1), and are also linked to the regulation of some immune factors(Reference Trayhurn and Wood2). Some pro-inflammatory and atherothrombotic adipokines/cytokines such as leptin, resistin, IL-6, TNF-α and plasminogen activator inhibitor-1 (PAI-1) are increased in obesity, whereas the anti-inflammatory adipokine adiponectin tends to be decreased(Reference Brochu-Gaudreau, Rehfeldt and Blouin3).
Obesity also disturbs the equilibrium existing between the different cell types that constitute WAT(Reference Gesta, Tseng and Kahn4–Reference Subramanian and Ferrante6), and favours a hypoxic environment derived from the scarce vascularisation of this tissue, which is able to induce macrophage infiltration(Reference Trayhurn, Wang and Wood7, Reference Murdoch, Muthana and Lewis8). In fact, high-fat diet (HFD)-induced obesity has been extensively linked to a chronic pro-inflammatory state, in which cytokine/adipokine expression is disrupted, addressing macrophage activation as a key factor in the development of insulin resistance(Reference Arkan, Hevener and Greten9, Reference Shi, Kokoeva and Inouye10). Indeed, some inflammatory factors such as TNF-α(Reference Hotamisligil, Shargill and Spiegelman11) induce the serine phosphorylation of insulin receptor substrate 1, inhibiting its action. Therefore, inflammation has become a crucial factor in the progression of obesity, since the accumulation of activated macrophages in adipose tissue and, subsequently, the secretion of pro-inflammatory factors contribute to the development of insulin resistance(Reference Feral, Neels and Kummer12).
Defective mitochondrial function has also been reported in different tissues of insulin-resistant animals(Reference Bonnard, Durand and Peyrol13–Reference Schrauwen, Schrauwen-Hinderling and Hoeks15). However, the relationship between mitochondrial dysfunction and the development of insulin resistance is only beginning to be understood in WAT. Mitochondrial biogenesis, a process that involves mitochondrial proliferation (increase in the number of mitochondria) and differentiation (increase in mitochondrial function capacity), is directed to counteract mitochondrial dysfunction and is also related to the improvement of insulin sensitivity(Reference Lee and Wei16, Reference Wilson-Fritch, Burkart and Bell17). In addition, mitochondria constantly fuse (fusion process) and divide (fission process), and an imbalance of these two processes dramatically alters the overall mitochondrial morphology. Several proteins have been seen to be involved in these processes, such as mitofusin 1 and 2 (Mfn1 and 2)(Reference Ishihara, Eura and Mihara18) and mitochondrial fission 1 (Fis1)(Reference Yoon, Krueger and Oswald19). A HFD can induce an increase in oxidative capacity due to the high amount of oxidative substrates available, although, in turn, the accumulation of oxidative intermediates such as ceramides as well as reactive oxygen species production has been related to the development of insulin resistance(Reference Summers20, Reference Nishikawa, Kukidome and Sonoda21). In fact, a close association between HFD-induced obesity and oxidative stress has also been described(Reference Milagro, Campion and Martinez22), which leads to an irregular production of adipokines, contributing to the development of the metabolic syndrome(Reference Esposito, Ciotola and Schisano23).
Therefore, detailed knowledge of the relationship between mitochondrial dysfunction and the development of insulin resistance in WAT is of great interest. Taking this background into account and the evidence achieved by our group in tissues such as liver(Reference Nadal-Casellas, Amengual-Cladera and Proenza14, Reference Justo, Boada and Frontera24), muscle(Reference Gomez-Perez, Amengual-Cladera and Catala-Niell25, Reference Colom, Alcolea and Valle26), brown adipose tissue(Reference Nadal-Casellas, Proenza and Gianotti27–Reference Justo, Oliver and Gianotti29) and brain(Reference Guevara, Santandreu and Valle30), where female rats have greater mitochondrial function, lower oxidative damage and also higher insulin sensitivity than males, the aim of the present study was to determine whether sexual dimorphism found in the systemic insulin sensitivity profile is related to sex differences in a HFD response of gonadal WAT at mitochondrial function and inflammatory profile levels.
Materials and methods
Animals and diets
A total of sixteen male and sixteen female Wistar rats of 10 weeks of age (Charles River) were each divided into two dietary groups with a similar mean body weight (332 (sem 4) g for males and 217 (sem 2) g for females). For 26 weeks, a control group (n 8) was fed a standard pelleted diet (Panlab) containing 19 % of total energy (14 162 kJ/kg diet; 2·9 % fat, w/w) as protein, 73 % as carbohydrate and 8 % as lipid, and a cafeteria diet group (n 8) was fed a HFD to generate a diet-induced obesity model. The cafeteria diet was composed of cookies, pork liver pâté, fresh bacon, chocolate and ensaïmada (a typical Majorcan pastry), reaching an energy content of 16 217 kJ/kg diet, where protein, carbohydrate and lipids represent 13, 33 and 54 %, respectively. Rats were fed ad libitum with the standard diet (control group) and with the standard diet plus cafeteria-diet (HFD group). The amount consumed of each high-fat food and its nutrient composition is given in Table 1. The animals were weighed monthly and energy intake was measured at 22 weeks of dietary treatment, as reported previously(Reference Llado, Rodriguez-Cuenca and Pujol31). The rats were killed by decapitation at 36 weeks of age and serum was collected and frozen in liquid N2 and stored at − 70°C until analysed. Gonadal fat depots were removed and a piece of tissue was used to isolate adipocytes. The rest of the tissue was frozen at − 70°C until analysed. The animals were housed two per cage and always kept at 22°C on a 12 h light–12 h dark cycle, and the experiments were performed in accordance with the general guidelines approved by our institutional ethics committee and European Union regulations (2003/65/CE and 86/609/CEE).
Table 1 Food and nutrient composition* (Mean values with their standard errors)
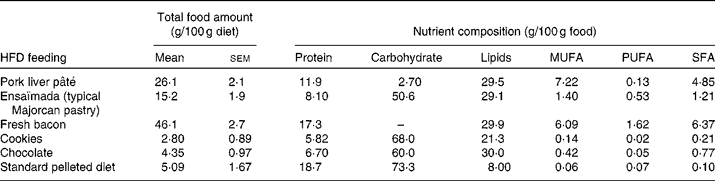
HFD, high-fat diet.
* Of the animals used in the study, twelve animals per group were chosen to determine energy intake. Total food amount is calculated as the amount of each HFD component consumed expressed per 100 g of diet consumed. As no sex differences were found in the consumption of the HFD components, the total food amount values are expressed as mean values with their standard errors taking into account the rats of both sexes.
Adipocyte isolation and culture
Epididymal and periovarian fat depots were removed under aseptic conditions from the control and HFD male and female Wistar rats, respectively, after decapitation. Adipocyte isolation was performed using collagenase digestion according to Landt et al. (Reference Landt, Gingerich and Havel32), with minor modifications. Briefly, fat pads were cut into pieces with scissors in buffer (pH 7·4) containing 5 mm-d-glucose, bovine serum albumin (20 g/l), 135 mm-NaCl, 2·2 mm-CaCl2, 1·25 mm-MgSO4, 0·45 mm-KH2PO4, 2·17 mm-Na2HPO4 and 10 mm-HEPES. Digestion was carried out with 2·5 mg type II collagenase in 2 ml of buffer/g tissue (specific activity, 760 × 10− 5 katal/g; Sigma Chemical Company), at 37°C with gentle shaking at sixty cycles per min for 45 min. A 600 μm nylon mesh (Spectrumlabs) was used to separate isolated adipocytes from the undigested tissue. After washing three times with buffer, adipocytes were resuspended in Dulbecco's modified Eagle's medium with glucose (4·5 g/l), 10 % fetal bovine serum and 1 % penicillin and streptomycin, and then incubated for 30 min at 37°C. Finally, six-well culture plates that had been previously covered with 500 μl of Matrigel (BD Biosciences) were used to keep the adipocytes in culture. Then, 150 μl of the adipocyte suspension (2:1 ratio of packed cells to media) were plated in each well with 2 ml of Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum in 5 % CO2 in air at 37°C.
Isolated adipocytes were treated with H2O2 to induce an acute oxidative stress. H2O2 (100 μm) was added to Dulbecco's modified Eagle's medium supplemented with bovine serum albumin instead of fetal bovine serum to avoid interference. After 18 h of treatment, the media were collected and cells were harvested and frozen at − 80°C until analysed.
The effect of the H2O2 treatment on adipocyte viability was tested using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega), a colorimetric method for measuring lactate dehydrogenase activity in the culture medium as a result of its release upon cell lysis. All reagents were prepared according to the manufacturer's instructions. In response to the H2O2 treatment, no differences between the groups were observed in cellular viability, which was 96 % as a mean value of the four groups studied.
Oral glucose tolerance test
The oral glucose tolerance test was performed on the week before killing. Rats were fasted for a 12 h period and then glucose (2 g/kg body weight) was given orally. Blood was collected from the tail vein before glucose administration and after 15, 60 and 120 min. Glucose concentrations were measured using the Accutrend® system (Roche Diagnostics).
Serum parameters, sample preparation and tissue composition
Serum glucose levels were measured by the Accutrend® GCT system (Roche Diagnostics). Insulin (Mercodia) and the adipokines leptin, total and high-molecular-weight adiponectin and resistin (Biovendor) were determined using enzyme immunoassay kits. In order to establish insulin resistance, the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated, as described previously(Reference Pickavance and Wilding33), as follows:
Briefly, 350 mg of the remaining epididymal or periovarian WAT, respectively, were homogenised in 1 ml of (STE) buffer (250 mm-sucrose, 5 mm-Tris–HCl and 2 mm-EGTA, pH 7·4). An aliquot of each homogenate was frozen ( − 20°C) with protease and phosphatase inhibitors (10 μm-leupeptin, 10 μm-pepstatin, 0·2 mm-phenylmethylsulfonyl fluoride (PMSF) and 0·2 mm-ortovanadate) for Western blot analysis.
The total DNA of gonadal fat pads and isolated adipocytes(Reference Thomas and Farquhar34) as well as the total protein content(Reference Lowry, Rosebrough and Farr35) in gonadal WAT were assessed. Mitochondrial DNA (mtDNA) extraction and semi-quantification was carried out in tissue homogenates according to a previously described method(Reference Koekemoer, Downing and Oelofsen36), with some modifications(Reference Justo, Oliver and Gianotti29). Primer sequences are shown in Table 2.
Table 2 Oligonucleotide primer sequences and conditions used in real-time PCR amplification
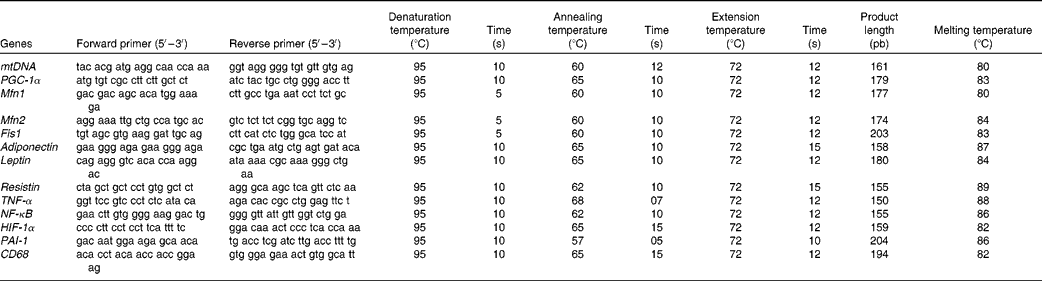
mtDNA, mitochondrial DNA (gene: NADH dehydrogenase); PGC-1α, PPAR-γ coactivator-1α; Mfn1, mitofusin 1; Mfn2, mitofusin 2; Fis1, mitochondrial fission 1; HIF-1α, hypoxia-inducible factor 1α; PAI-1, plasminogen activator inhibitor-1; CD68, cluster of differentiation 68.
Western blot analysis for markers of mitochondrial biogenesis and function, antioxidant enzymes and markers of insulin and adiponectin signalling pathways
Equal amounts of protein (40 μg) from homogenates, which had been frozen with protease and phosphatase inhibitors, were fractionated on 8, 10, 12 and 15 % SDS-PAGE gels and electrotransferred onto a nitrocellulose filter. Membranes were blocked in 5 % non-fat dry milk in PBS–Tween for 1 h and incubated overnight with a primary antibody. Rabbit antisera against mitochondrial transcription factor A were kindly provided by Inagaki(Reference Inagaki, Hayashi and Matsushima37). Primary antibodies were supplied by Santa Cruz Biotechnology (anti-cytochrome c oxidase subunit II (anti-COXII) and GLUT4, ref. sc-23 984 and sc-7938, respectively), MitoSciences (anti-cytochrome c oxidase subunit (anti-COXIV), ref. MS407), Alpha Diagnostic International (anti-carnitine palmitoyltransferase I, 4-hydroxy-2-nonenal and adiponectin receptor 1 (AdipoR1), ref. CPT1M11-A, HNE-11S and ADIPOR12-A, respectively), BD Biosciences (anti-insulin receptor β, ref. 611277), Cell Signaling (anti-IRS-1, phosphorylated AMP-activated protein kinase (pAMPK), AMP-activated protein kinase (AMPK), phosphorylated Ser/Thr kinase A (pAkt) and Ser/Thr kinase A (Akt), ref. 10/2008, 2531, 01/2009, 9272 and 9271, respectively) and Calbiochem (anti-catalase, mitochondrial superoxide dismutase, cytosolic superoxide dismutase and glutathione peroxidase, ref. 219010, 574596, 574597 and ST1000, respectively). Afterwards, incubation with a secondary antibody was performed for 1 h. Development of immunoblots was performed using an enhanced chemiluminescence kit from Bio-Rad. Bands were obtained using the Chemidoc XRS system (Bio-Rad), and quantification was done with Quantity One software (Bio-Rad). Autoradiograms revealed an apparent molecular mass of 16 kDa for COXIV and cytosolic superoxide dismutase, and 60 kDa for Akt, pAkt, AMPK and pAMPK. Moreover, a molecular weight of 21, 22·5, 25, 28, 42·5, 54, 65, 88, 95 and 180 was observed for COXII, glutathione peroxidase, mitochondrial superoxide dismutase, mitochondrial transcription factor A, AdipoR1, insulin-responsive GLUT4, catalase, carnitine palmitoyltransferase I, insulin receptor β and insulin receptor substrate 1, respectively.
Measurement of carbonyl group content and 4-hydroxy-2-nonenal content
As a marker of protein oxidation, protein carbonyl group content was determined in homogenates of gonadal fat depots by immunoblot analyses using the Oxyblot™ Protein Oxidation Detection Kit (Chemicon International) according to the manufacturer's instructions with minor modifications(Reference Guevara, Santandreu and Valle30). 4-Hydroxy-2-nonenal content was assessed by Western blot as a marker of lipid peroxidation(Reference Ali, Matragoon and Pillai38).
Real-time RT-PCR
Total RNA was obtained from 200 mg of WAT using TriPure® isolation reagent and quantified using a spectrophotometer set at 260 nm. RNA purity was assessed by the 260:280 nm ratio.
Briefly, 1 μg of the total RNA was reverse transcribed to complementary DNA for 60 min at 42°C, with 25 U MuLV RT in a 10 μl volume of retrotranscription reaction mixture containing 10mm-Tris–HCl, 50mm-KCl, 0·1 % Triton X®-100, 2·5 mm-MgCl2, 2·5 μm-random hexamers, 10 U RNase inhibitor and 500 μm of each dNTP in a Gene Amp 9700 thermal cycler (Applied Biosystems). Each complementary DNA solution was diluted (1:10) and aliquots were frozen ( − 20°C) until the PCR were performed.
Real-time PCR was carried out for the mRNA of PPAR-γ coactivator-1α, a regulator of mitochondrial biogenesis, as well as for those of adiponectin, leptin and resistin, the inflammatory markers NF-κB, TNF-α and CD68, the markers of hypoxia and impaired fibrinolysis, such as hypoxia-inducible factor 1α (HIF-1α) and PAI-1, and the markers of mitochondrial fusion, such as Mfn1 and Mfn2, and mitochondrial fission such as Fis1. Oligonucleotide primer sequences (Table 1) were obtained from Primer3 and tested with IDT OligoAnalyser 3.0. Finally, a basic local alignment search tool (NCBI Blast) revealed that the primer sequence homology was obtained only for the target genes. Real-time PCR was performed using the LightCycler SYBR Green technology on a LightCycler rapid thermal cycler (Roche Diagnostics). Each reaction contained 1 μl LightCycler-FastStart DNA Master SYBR™ Green I (containing FastStart Taq DNA polymerase, dNTP mix, reaction buffer and SYBR Green I dye), 0·5 μm of the sense and antisense specific primers, 2 mm-MgCl2 and 3 μl of the complementary DNA dilution in a final volume of 10 μl. The amplification programme consisted of a pre-incubation step for denaturation of template complementary DNA (95°C, 10 min), followed by forty cycles consisting of a denaturation, an annealing and an extension step in the conditions shown in Table 2. After each cycle, fluorescence was measured at 72°C. Product specificity was confirmed in the initial experiments by agarose gel electrophoresis and routinely by melting curve analysis.
Statistical analysis
All data are expressed as mean values with their standard errors of eight animals per group. C t values of real-time PCR were analysed taking into account the efficiency of the reaction and referring the results to the total DNA amount, using GenEx Standard software (MultiDAnalises). Statistical differences between the groups were analysed by two-way ANOVA to study tissue or sex, and diet effects. Additionally, when there was an interactive effect between tissue and diet or between sex and diet, a post hoc comparison by Student's t test was applied. A P value less than 0·05 was considered statistically significant. A statistical software package (SPSS 17.0 for Windows; SPSS, Inc.) was used to perform all statistical analyses.
Results
Energy intake, body and white adipose tissue weight and tissue composition
The HFD induced an increase in energy intake (Table 3) in both sexes which led to a rise in both body weight and adiposity index, especially in female rats. In fact, although the same percentage of adiposity was reached both in male and female rats, the increase was higher in females (2·4- v. 1·7-fold).
Table 3 Body weight, energy intake and adiposity index (Mean values with their standard errors, n 8)
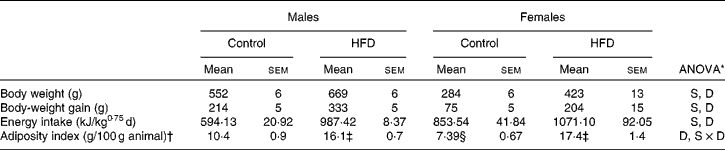
HFD, high-fat diet; S, sex effect; D, diet effect; S × D, sex and diet interactive effect.
* P< 0·05.
† Adiposity index is the sum of inguinal, gonadal, mesenteric and retroperitoneal depot weights relative to 100 g of body weight.
‡ Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
§ Mean value was significantly different for females from that of males (P< 0·05; Student's t test).
Both epididymal and periovarian WAT weight (Table 4) relative to body weight increased with dietary treatment, reaching higher values in the periovarian depot compared with the epididymal one. The total tissue weight was modified with dietary treatment, showing an increase by 160 % in the periovarian depot and by 57 % in the epididymal one.
Table 4 Gonadal white adipose tissue (WAT) composition (Mean values with their standard errors, n 8)
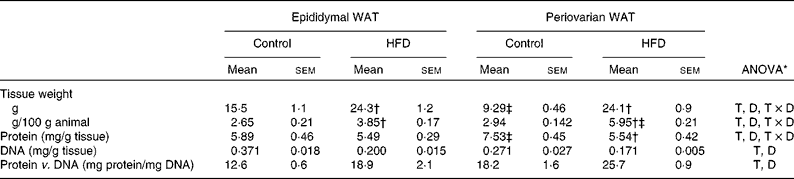
HFD, high-fat diet; T, tissue effect; D, diet effect; T × D, tissue and diet interactive effect.
* P< 0·05.
† Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
‡ Mean values were significantly different for periovarian WAT from those of epididymal WAT (P< 0·05; Student's t test).
Protein content was significantly higher in the periovarian depot of the control animals than in the epididymal one, whereas HFD feeding matched the levels of both depots. The total DNA levels were higher in the epididymal depot compared with the periovarian one, and decreased in both depots after HFD feeding. The protein content per cell was higher in the periovarian depot and increased after HFD feeding in both sexes.
Oral glucose tolerance test
The present results (Fig. 1) indicate that there is an increase in blood glucose levels in all the experimental groups at 15 min after glucose administration, reaching higher levels in male rats than in female rats. In the control rats, a decrease in glucose levels was observed after the peak found at 15 min, whereas, in the HFD-fed rats, glucose levels at 60 and 120 min were higher than those in the controls, especially in males.

Fig. 1 Oral glucose tolerance curves. Values are means for six animals per group, with their standard errors represented by vertical bars. Mean values were significantly different (P< 0·05; ANOVA). ![]() , Control males;
, Control males; ![]() , high-fat diet (HFD) males;
, high-fat diet (HFD) males; ![]() , control females;
, control females; ![]() , HFD females; S, sex effect; D, diet effect.
, HFD females; S, sex effect; D, diet effect.
Serum glucose, hormone levels and homeostasis model assessment-insulin resistance values
No differences between the groups were found in serum glucose levels, either between sexes or in response to the HFD (Table 5). Compared with females, higher systemic insulin values were found in male rats, and a trend to increase after HFD feeding was observed in both sexes, although this did not reach statistical significance. Male control rats exhibited a higher HOMA-IR compared with females, whereas HFD feeding induced an increase in this parameter only in female rats, although this increase was not enough to reach the values observed in males. Male rats showed greater serum resistin levels than females and in response to the HFD, only females showed an increase. The levels of total adiponectin were lower in control female rats than in male rats, increasing after HFD feeding only in females. However, levels of high-molecular-weight adiponectin were higher in female rats compared with male rats, and these did not change with the HFD. Leptin levels were higher in control male rats than in female rats and increased in both sexes after HFD feeding.
Table 5 Serum glucose, hormone levels and homeostasis model assessment-insulin resistance (HOMA-IR)* values (Mean values with their standard errors, n 8)
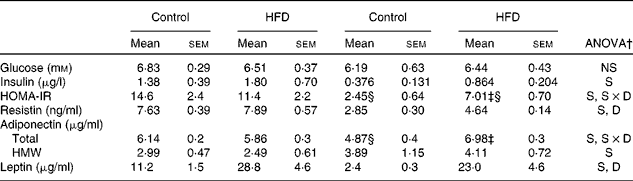
HFD, high-fat diet; S, sex effect; S × D, sex and diet interactive effect; D, diet effect; HMW, high molecular weight.
* HOMA-IR was calculated as (blood fasting glucose (mm) × blood fasting insulin (μU/ml))/22·5.
† P< 0·05.
‡ Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
§ Mean values were significantly different for females from those of males (P< 0·05; Student's t test).
mRNA expression of adipokines and inflammatory markers
Adiponectin expression levels were higher in the periovarian WAT of the control rats (Table 6), and increased after HFD feeding only in the epididymal depot. mRNA levels of leptin and resistin were lower in the periovarian fat compared with the epididymal one and increased after HFD feeding in both depots (Table 6). TNF-α, NF-κB and CD68 expression levels did not show differences between the epididymal and periovarian fat, but increased in both depots in response to the HFD, although to a greater extent in the epididymal one. The periovarian depot of the control rats showed greater levels of HIF-1α and PAI-1, which increased after HFD feeding, although to a lesser extent than those in the epididymal depot (Table 6).
Table 6 mRNA expression of adipokines and inflammatory markers* (Mean values with their standard errors, n 8)
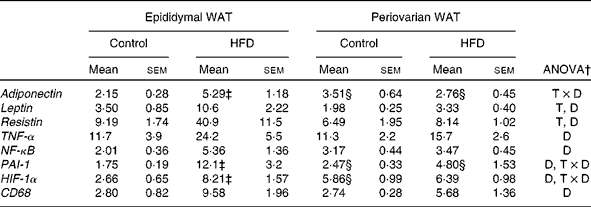
WAT, white adipose tissue; HFD, high-fat diet; T × D, tissue and diet interactive effect; T, tissue effect; D, diet effect; PAI-1, plasminogen activator inhibitor-1; HIF-1α, hypoxia-inducible factor 1α; CD68, cluster of differentiation 68.
* Levels of the control male rats were set at 100 %.
† P< 0·05.
‡ Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
§ Mean values were significantly different for periovarian WAT from those of epididymal WAT (P< 0·05; Student's t test).
Protein levels of insulin and adiponectin signalling pathway elements of epididymal and periovarian white adipose tissue
Periovarian fat showed higher expression levels of insulin receptor β than epididymal WAT (Table 7), whereas no sex differences were observed in any other element of the insulin signalling pathway. Consumption of the HFD induced an important increase in insulin receptor substrate 1(IRS1), total Akt and GLUT4 protein levels in both depots. No differences were found in either pAkt protein levels or the pAkt:total Akt ratio.
Table 7 Protein levels of insulin and adiponectin signalling pathway elements of gonadal white adipose tissue (WAT)* (Mean values with their standard errors referred to DNA, n 8)
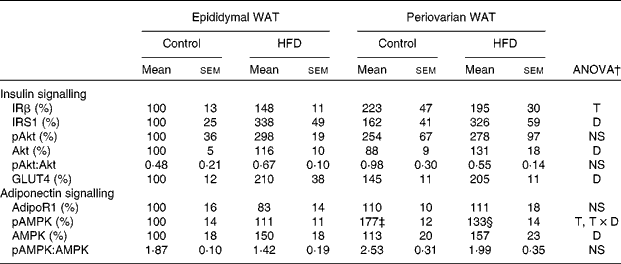
HFD, high-fat diet; IRβ, insulin receptor β; T, tissue effect; IRS1, insulin receptor substrate 1; D, diet effect; pAkt, phosphorylated Akt; Akt, Ser/Thr kinase A; adipoR1, adiponectin receptor 1; AMPK, T × D, tissue and diet interactive effect; pAMPK, phosphorylated AMPK; AMP-activated protein kinase.
* Protein levels of the control male rats were set at 100 %.
† P< 0·05.
‡ Mean value was significantly different for periovarian WAT from that of epididymal WAT (P< 0·05; Student's t test).
§ Mean value was significantly different for the HFD group from that of the control group (P< 0·05; Student's t test).
No differences between the groups were observed in protein levels of adiponectin receptor 1 (Table 7). pAMPK protein levels were higher in the periovarian depot than in epididymal fat and decreased after HFD feeding in the former. Consumption of the HFD induced an increase in the levels of total AMPK in both depots and no depot-related differences were observed at baseline. Compared with epididymal fat, the ratio of pAMPK:total AMPK was greater in the periovarian depot and decreased after HFD feeding, although this decrease did not reach statistical significance (P= 0·058).
mRNA and protein levels of gonadal white adipose tissue mitochondrial biogenesis and function markers
Greater expression levels of PPAR-γ coactivator-1α (Table 8) were observed in the periovarian depot of the control animals compared with the epididymal fat. However, these values increased after HFD feeding only in the latter. This profile agrees with the higher mitochondrial transcription factor A protein levels found in the periovarian depot of the control animals compared with the epididymal one, while the HFD induced an increase in its levels especially in the latter. Both the nuclear-encoded protein COXIV and the mitochondrial-encoded protein COXII levels in each cell were higher in the periovarian depot compared with the epididymal one, despite the increase in both sexes after HFD feeding. In the control situation, no significant differences were found between the depots in mtDNA content, whereas it increased after HFD feeding, especially in epididymal fat (Fig. 2). To estimate mitochondrial dynamics, the markers of mitochondrial fusion and fission were determined. Although Mfn1 and Mfn2 mRNA expression tended to be higher in female rats than in male rats, these differences did not reach statistical significance. The HFD induced an increase in mRNA levels of both factors only in males, reaching higher values than those observed in obese females. Female control rats showed greater expression levels of Fis1 than male rats, increasing after HFD feeding only in males. Interestingly, the Mfn1:Fis1 ratio increased in males after HFD feeding, reaching higher levels than those observed in their female counterparts. No statistical differences were found between the groups in the Mfn2:Fis1 ratio values.
Table 8 mRNA and protein levels of gonadal white adipose tissue markers of mitochondrial biogenesis and function* (Mean values with their standard errors referred to DNA, n 8)
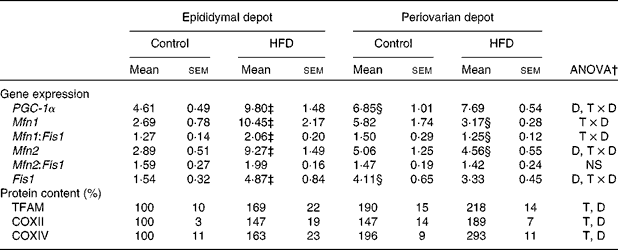
HFD, high-fat diet; PGC-1α, PPAR-γ coactivator-1α; D, diet effect; T × D, tissue and diet interactive effect; Mfn1, mitofusin 1; Fis1, mitochondrial fission 1; Mfn2, mitofusin 2; TFAM, transcription factor A; T, tissue effect; COXII and COXIV, cytochrome c oxidase subunits II and IV, respectively.
* Levels of the control male rats were set at 100 %.
† P< 0·05.
‡ Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
§ Mean values were significantly different for the periovarian depot from those of the epididymal depot (P< 0·05; Student's t test).
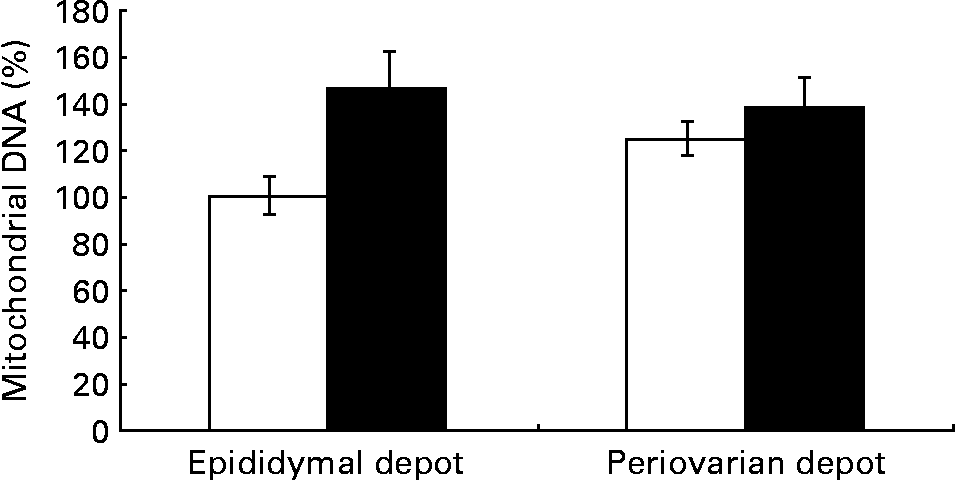
Fig. 2 Gonadal white adipose tissue mitochondrial DNA levels in control animals (⋄) and high fat diet-fed animals (♦). Values are means for eight animals per group, with their standard errors represented by vertical bars. Levels of the control male rats were set at 100 %. Mean values were significantly different (P< 0·05; ANOVA: diet effect).
Gonadal white adipose tissue levels of antioxidant enzymes and oxidative damage markers
Higher levels of catalase, mitochondrial superoxide dismutase and cytosolic superoxide dismutase (Table 9) were observed in periovarian WAT compared with epididymal fat. HFD feeding induced an increase in the levels of antioxidant enzymes in both depots, except in the case of mitochondrial superoxide dismutase. Carbonyl protein content was higher in the epididymal fat compared with the periovarian WAT, and no HFD effects were observed in this parameter. However, no differences between the depots were observed in 4-hydroxy-2-nonenal levels, although they increased after HFD feeding in both epididymal and periovarian fat.
Table 9 Gonadal white adipose tissue (WAT) levels of antioxidant enzymes and oxidative damage markers* (Mean values with their standard errors referred to DNA, n 8)
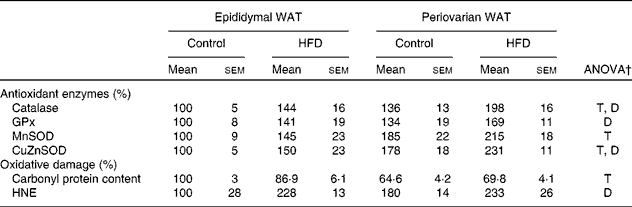
HFD, high-fat diet; T, tissue effect; D, diet effect; GPx, glutathione peroxidase; MnSOD and CuZnSOD, mitochondrial and cytosolic superoxide dismutase; HNE, 4-hydroxy-2-nonenal.
* Levels of the control male rats were set at 100 %.
† P< 0·05.
‡ Mean values were significantly different for the HFD group from those of the control group (P< 0·05; Student's t test).
§ Mean values were significantly different for females from those of males (P< 0·05; Student's t test).
Discussion
The HFD induces a greater increase in gonadal tissue weight gain and adiposity index in female rats compared with male rats (2- v. 1·5-fold increase, respectively). The decreased levels of DNA per g tissue associated with the HFD in both depots suggest adipocyte hypertrophy, thus contributing to the buffering of excess lipids from the diet by WAT. The greater increase in serum leptin levels found in females also supports the greater fat accumulation observed in the periovarian depot, as this adipokine correlates with the amount of fat accumulated. As a result, the higher expandability capacity pointed out in periovarian WAT, along with the lower lipid accumulation found in peripheral tissues such as liver(Reference Nadal-Casellas, Amengual-Cladera and Proenza14) and muscle(Reference Gomez-Perez, Amengual-Cladera and Catala-Niell25) of HFD-fed female rats, is in accordance with the capacity of adipose tissue expansion to disrupt lipotoxicity and the development of insulin resistance(Reference Virtue and Vidal-Puig39).
A HFD induces an increase in the production of adipokines, especially in the epididymal depot, which could have important implications in the systemic and WAT pro-inflammatory(Reference Bokarewa, Nagaev and Dahlberg40, Reference Fantuzzi41) state and in sex differences found in the insulin sensitivity profile(Reference Perez, Fernandez-Galaz and Fernandez-Agullo42, Reference Rodriguez, Catalan and Becerril43). In fact, as a consequence of adipose tissue expansion, a hypoxic environment could develop in both depots, since the scarce vascularisation of WAT fails to meet the demand of adipose tissue growth when challenged by a HFD(Reference Ye44). Noticeably, despite the fact that the periovarian depot accumulates more fat than the epididymal one, HIF-1α levels, major mediators of hypoxia(Reference Semenza45), are higher in the latter. In this sense, the 3-fold increase in HIF-1α mRNA levels observed in the epididymal WAT in response to the HFD would trigger the activation of angiogenic factors in response to hypoxia(Reference Forsythe, Jiang and Iyer46), and would explain the greater increase in PAI-1 levels observed in this depot, as it is one of the downstream targets of HIF-1α(Reference Trayhurn, Wang and Wood7). Indeed, despite its role in providing the stromal matrix necessary to allow the ingrowth of new vascularisation(Reference Kietzmann, Samoylenko and Roth47), PAI-1 would also have negative effects, since the elevated levels of this factor and therefore the accumulation of fibrin would favour the development of metabolic complications(Reference Juhan-Vague and Alessi48) in HFD-fed male rats to a greater extent than in their female counterparts. In fact, although further investigation is still needed to elucidate the specific contribution of each depot to systemic adipokine concentration, gonadal fat could be reflecting what occurs in other depots. In this sense, the mesenteric depot is the most closely related to metabolic complications during obesity due to its portal vein drainage; however, gonadal fat has been seen to contribute to systemic adipokine secretion more than other depots such as subcutaneous and perirenal fat(Reference Gabriely, Ma and Yang49–Reference Harris, Ramsay and Smith51). HFD-induced hypoxia also contributes to the development of a mild pro-inflammatory state that becomes particularly evident in epididymal WAT. The greater macrophage infiltration of epididymal fat, suggested by its higher CD68 mRNA levels, is supported by the higher expression levels of TNF-α and NF-kB, pro-inflammatory molecules mainly produced by macrophages, and is also supported by the greater levels of leptin expression that acts as a chemotactic factor(Reference La Cava and Matarese52). Therefore, the lower expandability capacity and the higher pro-inflammatory state developed in the epididymal depot would indicate that this depot is more prone to generate metabolic complications at a systemic level than the periovarian fat.
Beyond the effects already described, hypoxia can also induce mitochondrial biogenesis to cope with hypoxic stress(Reference Eells, Henry and Gross53, Reference Nisoli, Clementi and Moncada54). Thus, HFD feeding leads to an increase in mitochondrial proliferation and differentiation in epididymal WAT and to mitochondrial differentiation in periovarian WAT, since the increase in mitochondrial machinery content (COXII and COXIV) was not accompanied by an increase in mtDNA levels in the latter. Moreover, the increase in epididymal expression levels of the markers of mitochondrial fusion, Mfn1 and Mfn2(Reference Ishihara, Eura and Mihara18, Reference Palmer, Osellame and Stojanovski55), as well as in those of PPAR-γ coactivator-1α, an upstream regulator of Mfn(Reference Palmer, Osellame and Stojanovski55, Reference Cartoni, Leger and Hock56), suggest that changes are happening in this depot not only in mitochondrial machinery content but in mitochondrial morphology as well. In fact, mitochondrial fusion could be protecting the mitochondrial function of the epididymal depot in obese males by promoting mitochondrial oxidative function, as reported previously(Reference Pich, Bach and Briones57), and also the rapid mixing of membranes and cytosolic components of damaged mitochondria in order to avoid the permanent loss of materials, which would lead to cell dysfunction(Reference Chen, Detmer and Ewald58). Indeed, mitochondrial fusion has been related to an increase in mtDNA levels(Reference Hori, Yoshida and Ling59–Reference Chen, Vermulst and Wang61), which agrees with the higher mtDNA content observed in HFD-fed male rats. On the other hand, the unaltered levels of Mfn1 and Mfn2 observed in obese female rats compared with their control counterparts might indicate that no changes occur in the mitochondrial dynamics of HFD-fed female rats, and are in accordance with the profile of PPAR-γ coactivator-1α. Reinforcing this idea, the Mfn1:Fis1 and Mfn2:Fis1 ratio values in the control and obese female rats suggest an equilibrium existing between mitochondrial fusion and fission in response to the HFD that is altered in males by the enhancing effect of the HFD on the Mfn1:Fis1 ratio values. This is in agreement with the idea that Mfn1 is the most efficient Mfn to induce mitochondrial fusion(Reference Ishihara, Eura and Mihara18). These results suggest the existence of mitochondrial dysfunction in males but not in females, which promotes changes in mitochondrial dynamics (enhanced fusion) and biogenesis (proliferation and differentiation) in epididymal tissue and this would be in agreement with a better metabolic profile of periovarian fat, probably derived from its greater expandability capacity and its lower pro-inflammatory state developed in response to the HFD.
Oxidative stress could be one of the factors involved in alterations caused by the HFD, as suggested by the results of an experiment performed in vitro. Treatment with H2O2 induces an increase in HIF-1α, PAI-1 and NF-κB expression levels in isolated epididymal and periovarian adipocytes from these obese animals, although statistically significant differences were not reached (data not shown). This is in agreement with the increase in these parameters in response to the HFD observed at the tissue level, thus pointing to reactive oxygen species as important effectors of HFD consequences and of the induction of angiogenesis(Reference Ushio-Fukai and Alexander62) through the regulation of HIF-1α expression(Reference Bonello, Zahringer and BelAiba63). Therefore, the increase in the antioxidant capacity of the gonadal WAT of HFD-fed rats could respond to the potential increase in reactive oxygen species production, underlying the rise in the amount of substrates available for oxidation. Actually, lipid peroxidation increased in both depots, especially in males, suggesting that antioxidant activity would not have been able to avoid oxidative damage.
In agreement with the lower hypoxic and pro-inflammatory state of periovarian fat, female rats manage to maintain a better systemic insulin sensitivity profile than do males, both under control and HFD feeding conditions. The higher serum levels of total adiponectin, an insulin-sensitising factor(Reference Brochu-Gaudreau, Rehfeldt and Blouin3), observed in obese female rats compared with their male counterparts could contribute to this profile. However, in response to HFD feeding, the adiponectin serum level profile does not parallel the tissue expression level profile, which suggests that the adiponectin secretion of the gonadal depot would not be the main contributor to the systemic levels. In this sense, results obtained in the same animal model(Reference Amengual-Cladera, Lladó and Gianotti64) point to the retroperitoneal depot as the greater contributor to systemic adiponectin secretion, in agreement with previous studies(Reference Romero, Fernandez-Lopez and Esteve65). Moreover, HFD-fed female rats also exhibit a better insulin sensitivity profile than males, in accordance with their faster clearance of postprandial serum glucose levels pointed out by the results obtained in the oral glucose tolerance test compared with males, thus indicating higher insulin resistance in obese males. However, this dimorphism is not reflected in the levels of key proteins of the insulin signalling pathway of gonadal WAT. This suggests that gonadal WAT is not as sensitive as other tissues or even other WAT depots to the negative effects of the diet, in accordance with what has been observed in previous studies(Reference Frontini and Cinti66) and in the retroperitoneal WAT of these animals(Reference Amengual-Cladera, Lladó and Gianotti64), in which insulin signalling increases in males and tends to decrease in females. On the other hand, the activation of the adiponectin signalling pathway was compromised by HFD feeding in both depots, as suggested by the decrease in the pAMPK:AMPK ratio (diet effect, P= 0·058). Moreover, a positive correlation between high-molecular-weight adiponectin values and the pAMPK:AMPK ratio was found only in males (Pearson's correlation 0·577, P= 0·05). In this sense, this could be another fact to support a possible impairment of this signalling pathway in response to the HFD, which suggests the development of adiponectin resistance in the periovarian depot. This idea is further supported by the statistically significant decrease in pAMPK values exhibited by female rats in response to HFD feeding. In fact, the higher levels of adiponectin observed in HFD-fed females did not induce a greater activation of its signalling pathway in gonadal WAT, which would favour the accumulation of lipids instead of their oxidation(Reference Gaidhu, Frontini and Hung67), and, subsequently, the greater expansion of the periovarian depot observed with the HFD. Moreover, given that AMPK has been described to inhibit inflammatory signalling cascades in different cell types(Reference Salminen, Hyttinen and Kaarniranta68, Reference Peairs, Radjavi and Davis69), the higher levels of pAMPK in the periovarian depot compared with the epididymal fat could contribute to the lower degree of inflammation observed compared with the epididymal depot.
Finally, based on the sex/depot-dependent profile observed in the present study, a potential implication of sex hormones in the existence of these differences is quite possible. In fact, oestrogens, specifically 17β-oestradiol, induce mitochondrial biogenesis and function(Reference Mattingly, Ivanova and Riggs70), as well as regulate insulin sensitivity through the oestrogen receptors α and β(Reference Barros, Machado and Gustafsson71, Reference Barros, Machado and Warner72). Indeed, while oestrogens protect from HFD-induced insulin resistance in mice(Reference Riant, Waget and Cogo73), testosterone inhibits adiponectin production(Reference Xu, Chan and Hoo74). However, further investigation is still needed to elucidate the specific role of these hormones in the overall sexual dimorphism observed in the gonadal WAT depot.
In summary, the present results point to the existence of a sex/depot-dependent response to the HFD in gonadal WAT, which implies that periovarian adipose tissue exhibits a greater expandability capacity than the epididymal depot, which would be related to a lower hypoxic environment and inflammatory profile without signs of mitochondrial dysfunction or changes in mitochondrial dynamics, as well as a better insulin sensitivity profile. This healthier serum and tissue profile in response to HFD feeding could be attributed to the stronger evolutionary pressures to which females have been subjected(Reference Hoyenga and Hoyenga75) and that have led them to develop WAT that is genetically programmed to store more lipids and in a safer way than that of males. In contrast, the epididymal WAT of HFD-fed male rats shows a lower expandability capacity and greater inflammation than female rats, thus resulting in a much more detrimental tissue metabolic profile, with changes in mitochondrial dynamics probably associated with HFD-induced mitochondrial dysfunction, thus explaining the worse serum insulin sensitivity profile of male rats. These results demonstrate that gonadal WAT highly reflects the more detrimental effects of HFD feeding on the systemic insulin resistance profile of male rats.
Acknowledgements
This study was supported by the Dirección General de Investigación y Gestión del Plan Nacional de I+D+i (SAF2010-21792) and by the Fondo de Investigaciones Sanitarias of the Spanish Government (PIO60293). E. A.-C. was funded by a grant from the Comunitat Autònoma de les Illes Balears. The authors are grateful to Dr Hidetoshi Inagaki for providing the antiserum against the mitochondrial transcription factor A. The contribution of the authors is as follows: I. L., A. M. P. and M. G. designed the study; E. A.-C. conducted the study and performed the data collection and analysis. All authors participated in data interpretation and manuscript writing. The authors declare that they have no conflict of interest.













