In the 1970s, studies on Greenland Inuit populations established that, despite consuming high-lipid diets, cardiac disease was virtually absent in these populations and that this was due to their high fish intake(Reference Dyerberg, Bang and Hjorne1). Subsequently, the beneficial effects of n-3 highly unsaturated fatty acids (HUFA), especially EPA (20 : 5n-3) and DHA (22 : 6n-3), have been shown to have cardioprotective activity(Reference Dewailly, Blanchet and Gingras2, Reference Dewailly, Blanchet and Lemieux3). More recently, the benefits of increased EPA and DHA intake have been shown for a wide range of lifestyle disorders with an associated inflammatory pathology(4, Reference Yaqoob5) as well as improving the symptoms of certain neurological disorders(Reference Young and Conquer6). Due to the proven benefits of n-3 HUFA, numerous governmental and non-governmental organisations across the world currently advise increased fish intake as a means of improving the health of their citizens(7, 8).
Atlantic salmon (Salmo salar) can store large quantities of oil in their flesh and this makes them a rich source of n-3 HUFA(Reference Bell, Henderson and Tocher9, Reference Torstensen, Bell and Sargent10). However, the oil-rich flesh can also accumulate lipophilic undesirable compounds, including polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) (collectively known as dioxins or PCDD/F), dioxin-like non-ortho and mono-ortho polychlorinated biphenyls (PCB), known collectively as dioxin-like (DL) PCB and polybrominated diphenyl ethers (PBDE), among others(Reference Hites, Foran and Carpenter11–Reference Friesen, Ikonomou and Higgs15). For these reasons there is currently considerable interest to investigate the transfer of persistent organic pollutants (POP) from feed to farmed fish as well as assessing the potential for transfer to humans and any consequences for human health(Reference Foran, Good and Carpenter16, Reference Mozaffarian and Rimm17).
There are 210 dioxin (seventy-five PCDD and 135 PCDF congeners) and 209 PCB congeners, of which seventeen PCDD/PCDF congeners and twelve DL-PCB have been assigned toxic equivalency factors (TEF) by the WHO, according to their relative toxicity to 2,3,7,8 tetra chlorinated dibenzo dioxin (TCDD). The concentration of PCDD/F and DL-PCB is expressed as toxic equivalents (TEQ), where a TEF is applied to the concentration of the individual congeners and summed to generate the total TEQ in a mixture of the twenty-nine PCDD/F and DL-PCB congeners. The TEF established in 1997 by the WHO have been most widely used for human risk assessment by government bodies(Reference Van den Berg, Birnbaum and Bosveld18); these TEF were re-evaluated in 2005(Reference Van den Berg, Birnbaum and Denison19). Application of these factors to individual PCDD/F and DL-PCB congener concentrations in a feed or fish can be used to determine the level of TEQ present. PCDD/F and PCB have originated either by natural means, such as forest fires and incomplete combustion (dioxins), or by industrial activity (PCDD/F and PCB) and both have half-lives of several decades and are highly persistent in the marine biota(20).
In addition to PCDD/F and PCB, the PBDE are of more recent origin, having been introduced as flame retardants in household furnishings, computers and electrical circuits since the 1970s(Reference Hites, Foran and Schwager12). As with PCDD/F and PCB, PBDE are lipophilic and accumulate in the aquatic biota and humans(Reference Hites, Foran and Schwager12, Reference De Wit21) although at the present time no TEQ have been assigned for any PBDE congeners. Although there are 209 possible PBDE congeners, only a few are present in commercial mixtures and those are represented in tissues by seven principal congeners, namely PBDE 28, 47, 99, 100, 153, 154 and 183(Reference De Wit21).
Within the European Union (EU), limit values for PCDD/F were established in 2001, which were revised in 2006 to include concentrations of all twenty-nine PCDD/F and DL-PCB congeners assigned WHO TEF(22, 23). These limit values cover raw materials, feeds and fish products. The current EU limit values are 24, 4·5, 7 and 8 ng TEQ/kg, for fish oil (FO), fish meal, fish feed and fish products, respectively. The main contributor to POP in fish feeds and fish is FO derived from pelagic marine fish(Reference Jacobs, Ferrario and Byrne24). In recent years a number of studies have been conducted where FO was replaced by terrestrial vegetable oils (VO) in salmon feeds(Reference Bell, Henderson and Tocher9, Reference Torstensen, Bell and Sargent10, Reference Berntssen, Lundebye and Torstensen14, Reference Friesen, Ikonomou and Higgs15). The fish produced using diets with a high VO content contained significantly lower levels of POP compared with fish grown on diets with a high marine FO content(Reference Berntssen, Lundebye and Torstensen14, Reference Bell, McGhee and Dick25). However, the reduction in POP was accompanied by reductions in EPA and DHA of 50–65 %, which would obviously reduce the health benefits of this product to the consumer(Reference Berntssen, Lundebye and Torstensen14, Reference Friesen, Ikonomou and Higgs15). With the introduction of the revised EU limits for PCDD/F and DL-PCB in 2006, some of the FO previously used in aquafeeds would no longer comply with these new limits and so would have to be removed from the food chain. The loss of valuable n-3 HUFA in this way, at a time when FO have reached their sustainable production limits and prices are rising rapidly, is a major dilemma and has increased the desire to develop technology to clean FO of POP so that they might be used in aquafeeds(Reference Maes, De Meulenaer and Van Heerswynghels26, Reference Breivik and Thorstad27).
The aim of the present study was to investigate the effects on tissue POP concentrations of replacing northern FO, containing high levels of POP, with either the same oil which had been cleaned using a decontamination protocol or a blend of southern FO, soya and rapeseed oils (4:3:3, by vol.). The three diets were fed to triplicate groups of Atlantic salmon in sea cages for a period of 11 weeks. The effects of these three treatments on PCDD/F, DL-PCB, PBDE and n-3 HUFA concentrations in fish feed and flesh are presented.
Materials and methods
Fish, husbandry and diets
Three 9 mm extruded diets were prepared with the same basal composition, but were top coated with three different oils and were prepared at the BioMar Tech Centre, Brande, Denmark (Table 1). The diets were formulated to satisfy the nutritional requirements of salmonid fish(28), and contained 33 % protein and 34 % lipid. The three diets contained either (a) 100 % northern FO (cNFO) as control, (b) 100 % decontaminated northern FO (deNFO), or (c) a blend of southern FO and rapeseed and soya oil in a ratio of 40:30:30 (SFO/RO/SO). The deNFO was the same product and batch as the cNFO following the decontamination process. The cNFO was selected to contain high levels of POP and this oil was subjected to a two-stage decontamination process (FF Skagen, Skagen, Denmark) that involved an initial adsorption using activated carbon that was designed to remove about 90 % of the PCDD/F. The second step was a thin-film deodorisation step that should remove up to 95 % of PCB as well as pesticides and other contaminants, NEFA and peroxides. The deNFO was representative of decontaminated oil currently commercially available in Europe. Atlantic salmon of initial mean weight 0·78 ± 0·01 kg were fed one of the three experimental diets for 11 weeks in nine pens (each 5 m3) containing 120 fish/pen at the Fjord Research Station, Dønna, Norway. The experiment was conducted between July and October 2006 under ambient photoperiod and the mean seawater temperature was 11·5 ± 2·7°C and salinity 31·9 ± 0·8 parts per trillion. Feed was supplied manually to apparent satiation with waste feed collection provided by an uplift system. Experimental procedures complied with the Norwegian code of practice for the care and use of animals for scientific purposes and there are no aspects of this trial that would cause aggravated or unnecessary harm or stress to the fish involved.
Table 1 Diet formulations, proximate compositions (g/kg), energy (kJ/g) and major fatty acid compositions (% total fatty acids) of the three experimental diets fed to Atlantic salmon (Salmo salar) for 11 weeks

cNFO, control northern fish oil; deNFO, decontaminated northern fish oil; SFO/RO/SO, southern fish oil, rapeseed oil and soyabean oil.
* Includes 14 : 0, 15 : 0, 18 : 0, 20 : 0 and 22 : 0.
† Includes 16 : 1n-7, 16 : 1n-9, 20 : 1n-11, 20 : 1n-7, 18 : 1n-7, 20 : 1n-9, 22 : 1n-11, 22 : 1n-9 and 24 : 1n-9.
‡ Includes 18 : 3n-6, 20 : 2n-6, 20 : 3n-6, 20 : 4n-6, 22 : 4n-6 and 22 : 5n-6.
§ Includes 18 : 4n-3, 20 : 4n-3 and 22 : 5n-3.
Sample collection
Samples of the three feeds were collected at the start of the trial and wrapped in foil before placing in sealable polythene bags and storing at − 20°C. Samples of fish were collected at the start and end of the trial with three fish per pen (nine per dietary treatment) anaesthetised by metacaine (50 mg/l) and then killed by a blow to the head. Samples of flesh from the Norwegian quality cut (NQC) region were collected, wrapped in foil and immediately frozen at − 20°C and transported to the laboratory where they were stored at − 70°C until analysed. The samples were selected from fish that were close to the average fish weight in each pen. The three NQC samples from each pen were then pooled, to provide three samples per treatment group. Before analysis, the pooled steaks were homogenised in a food processor after removal of skin and bones. Samples of these homogenates were used for POP and fatty acid analyses.
Analysis of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans, dioxin-like polychlorinated biphenyls and polybrominated diphenyl ethers
Homogenised samples of wet flesh (about 50 g) were freeze-dried for minimum of 12 h before extraction. Samples of diet (about 10 g) were weighed as above and ground in a coffee grinder before extraction. The entire flesh and diet samples were extracted using isohexane in an accelerated solvent extractor (ASE™; Dionex, Camberley, Surrey, UK). Each sample was accurately weighed, mixed using Hydromatrix™ (Varian Inc., Palo Alto, CA, USA) before the addition of 1 ml of a 2 ng/ml 13C-labelled PCDD/F and PCB internal standard (EPA-1613-LCS; Wellington Laboratories, Guelph, Canada). Samples were extracted by ASE™ for 20 min for two cycles with isohexane under pressure (1500 psi; 10 342 kPa) and temperature (125°C). Fat and organic matter were removed from the 120 ml ASE™ extract by sulfuric acid treatment. A quantity of 50 ml of 95–97 % sulfuric acid was added to the lipid extract in a separation funnel and left for 72 h. The sulfuric acid was drained off and the organic phase washed with 50 ml nanopure water (Millipore UK Ltd, Watford, Herts, UK) and left for 3 h.
Clean-up of the extracted lipid samples was then performed using the automated multi-column Power-Prep™ System (Fluid Management Systems, Waltham, MA, USA) using a series of disposable Teflon columns of multi-layered silica (4 g acidic, 2 g basic and 1·5 g neutral), basic alumina (8 g) and carbon (2 g). For high-lipid samples, high-capacity silica columns (28 g acidic, 16 g basic and 6 g neutral) were used. The total run time was 150 min followed by a 40 min preventative decontamination program. The mono-ortho PCB were collected in 120 ml isohexane–dichloromethane (1:1, v/v) and the PCDD/F and non-ortho PCB eluted in 120 ml toluene. The purified fractions were evaporated to about 5 ml by rotary evaporation (Rotavapor® R-200; Büchi Ltd, Oldham, UK) in a heated water-bath set at 25°C and 320 mbar pressure for mono-ortho PCB and 40°C and 70 mbar pressure for PCDD/F and non-ortho PCB. The mono-ortho PCB fraction was evaporated to dryness under N2 (N-Evap™ 111; Organomation Associates Inc., Berlin, MA, USA) at room temperature before 5 ml isohexane and 5 ml concentrated sulfuric acid were added, vortex mixed, and centrifuged (5 min; 478 g). The organic phase was transferred to a clean conical tube and evaporated to approximately 1 ml under N2 before the addition of 1 ml isooctane and further evaporation to 500 μl. The sample was finally transferred to a conical GC vial containing 150 μl nonane as the keeper and evaporated to 100 μl before analysis by GC–MS/MS.
The PCDD/F and non-ortho PCB fraction was evaporated to 500 μl under N2 and transferred to a conical GC vial containing 60 μl nonane before further evaporation to 10 μl where 400 μl isohexane and 10 μl recovery standard (EPA-1613-ISS for PCDD/F and 68A-IS for PCB; Wellington Laboratories, Guelph, Canada) were added and vortex mixed. Sulfuric acid (35 μl) was added to the vial, vortex mixed and left for 1 h before centrifugation (5 min; 1328 g). The organic phase was transferred to a clean conical GC autosampler vial containing 10 μl nonane as the keeper and evaporated to 10 μl or 50 μl before analysis by GC–MS for PCDD/F or non-ortho PCB analysis, respectively.
Analysis of the twenty-nine PCDD/F and DL-PCB congeners with WHO TEF was conducted using GC–MS/MS on a Trace GC 2000 coupled to a Polaris Q ion trap MS/MS (Thermo Finnegan, Hemel Hempstead, Herts, UK). The chromatographic separations were conducted on a Rxi-5®ms (5 % phenyl–95 % dimethyl polysiloxane) fused silica column (Thames Restek UK Ltd, Saunderton, Bucks, UK), 30 m × 0·25 mm internal diameter × 0·25 mm film thickness, with He as the carrier gas at 0·8 ml/min. The injector temperature was kept at 250°C and samples and standards were injected in the splitless mode. MS conditions were in the positive electron ionisation (EI+) mode using automatic gain control with electron energy of 70 eV and emission current of 250 μA. The transfer line and ion source were kept at 305 and 250°C, respectively. Xcalibar version 1.3 software (Xcalibar Software, Inc., Herndon, VA, USA) was used for data acquisition and processing of results. The limit of quantification (LOQ) was determined by using nine times the background level (three times the limit of detection; LOD) in the blanks of each congener. Procedural blanks were run for each batch of up to ten samples. Each batch of samples was analysed with one procedural blank, one external quality-control (QC) sample and one internal QC sample. The external QC was cod liver oil (Food Analysis Performance Assessment Scheme®; CSL, York, UK; T0623) and the internal QC was internally validated salmon flesh. Percentage recoveries were in the range 78–114 %. Quantification of each congener is based on the isotope dilution method of the United States Environmental Protection Agency (USEPA) methods 1613 and 1668(29, 30). The range of LOQ for whole fish samples were 0·03–0·18 pg/g wet weight for PCDD/Fs, 0·03–0·06 pg/g wet weight for non-ortho PCB and 0·03–0·06 pg/g wet weight for mono-ortho PCB. The congeners analysed included the seventeen PCDD/Fs and twelve DL-PCB, for which the WHO has established TEF from 1997(Reference Van den Berg, Birnbaum and Bosveld18) and recently re-evaluated TEF(Reference Van den Berg, Birnbaum and Denison19) (Table 2).
Table 2 Dietary concentrations (ng toxic equivalents/kg wet weight) of dioxin and dioxin-like polychlorinated biphenyl (PCB) congeners in the control northern fish oil (cNFO), decontaminated northern fish oil (deNFO) and southern fish oil, rapeseed oil and soyabean oil (SFO/RO/SO) diets

TCDD, 2,3,7,8 tetra chlorinated dibenzo dioxin; PCDD, polychlorinated dibenzo-p-dioxins; nd, not detected; TCDF, 2,3,7,8 tetra chlorinated dibenzofuran; PCDF, polychlorinated dibenzofurans.
Analysis of polybrominated diphenyl ethers
For PBDE analysis, 25 g homogenised wet flesh were freeze-dried as described above. The dried flesh sample or 10 g diet was ground by mortar and pestle or coffee grinder, respectively, and 2·5 g flesh or diet was extracted using the ASE™ extraction cell containing 19 g silica gel–sulfuric acid mixture (1:1, w/w) and hydromatrix before addition of 20 μl of a 500 ng/ml PBDE-119 internal standard (Wellington Laboratories, Guelph, Canada). Samples were extracted by ASE™ for 20 min × two cycles with dichloromethane–isohexane (4:1, v/v) under pressure (1500 psi; 10 342 kPa) and temperature (40°C). The extract from the ASE™ was reduced to about 5 ml by rotary evaporation at 30°C and 320 mbar and then reduced to 1 ml under N2 at room temperature. Isohexane (5 ml) and concentrated sulfuric acid (2 ml) were added, vortex mixed and centrifuged (15 min at 1328 g). The organic phase was transferred to a clean tube and the procedure repeated. The combined organic phases were reduced to 1 ml under N2 and 100 μl concentrated sulfuric acid added, mixed and centrifuged (4 min at 212 g). The isohexane layer was then transferred to a silanised GC autosampler vial containing 500 μl nonane as the keeper and the solvent evaporated under N2 to the nonane mark before analysis by GC–MS.
Analysis of the seven PBDE congeners (28, 47, 99, 100, 153, 154 and 183) was conducted using a Thermo Finnegan Trace GC Ultra equipped with a ZB5-MS column (30 m × 0·25 mm internal diameter × 0·25 μm phase; Phenomenex, Macclesfield, Cheshire, UK) using He as the carrier gas coupled to a Trace DSQ MS (Bremen, Germany) in the negative chemical ion (CI − ) mode and methane as the reagent gas at a flow rate of 2·0 ml/min. The mass spectrometer was operated in the selective ion monitoring mode at m/z 79 and 81. Procedural blanks were the same as described for PCDD/F+DL-PCB above. The external QC was supplied by the Norwegian Institute of Public Health and the internal QC was internally validated salmon flesh. Xcalibar version 1.4 software (Xcalibar Software, Inc., Herndon, VA, USA) was used for data acquisition and processing of results. The range of limits of quantification (LOQ) for whole fish samples was 2–63 pg/g wet weight for PBDE.
Lipid content and fatty acid compositions
Total lipid was extracted from 1 g diet or flesh homogenates by homogenising in 20 volumes of ice-cold chloroform–methanol (2:1, v/v) using an Ultra-Turrax tissue disrupter (Fisher Scientific, Loughborough, Leics, UK). The total lipid fraction was prepared according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley31) with non-lipid impurities removed by washing with 0·88 % (w/v) KCl. The lipid weight was determined gravimetrically after evaporation of solvent under N2 and desiccation in vacuo for at least 16 h.
Fatty acid methyl esters (FAME) were prepared from diet and flesh total lipid by acid-catalysed transesterification according to the method of Christie(Reference Christie and Christie32). Extraction and purification of FAME were performed as described by Tocher & Harvie(Reference Tocher and Harvie33). FAME were separated and quantified by GC (Carlo Erba Vega 8160; Milan, Italy) using a 30 m × 0·32 mm internal diameter × 0·25 mm film thickness capillary column (CP Wax 52CB; Chrompak, London, UK) and cold on-column injection. H2 was used as the carrier gas and temperature programming was from 50°C to 150°C at 40°C per min and then to 230°C at 2·0°C per min. Individual methyl esters were identified by comparison with known standards and by reference to published data(Reference Tocher and Harvie33, Reference Ackman and Connell34). Data were collected and processed using the Chromcard for Windows (version 2.01) computer package (Thermoquest Italia S.p.A., Milan, Italy).
Statistical analysis
Significance of differences (P < 0·05) between dietary treatments was determined by one-way ANOVA. Differences between means were determined by Tukey's test. Data identified as non-homogeneous, using Bartlett's test, were subjected to log or arcsin transformation before applying the ANOVA. ANOVA was performed using a GraphPad Prism (version 4.0) statistical package (GraphPad Software, San Diego, CA, USA).
Results
The fish grew from an initial weight of 0·78 kg to 2·19 kg over the 11-week trial and there were no significant differences in final weights between the three treatments. Specific growth rate (%/d) values were in the range 1·34–1·35 and thermal growth coefficient (TGC) between 3·86 and 3·92 while the feed conversion ratio values were in the range 0·96–0·98. There were no differences between treatments and all these values were comparable with normal commercial practice for salmon of this size and culture conditions.
The cNFO diet contained 17·4 ng TEQ/kg of the twenty-nine WHO PCDD/F and DL-PCB congeners with assigned TEF (Table 2). This value is significantly higher than the EU limit for fish feeds of 7 ng TEQ/kg(22). However, following the two-step decontamination of the cNFO, this value was reduced to 0·45 ng TEQ/kg in the deNFO diet that was comparable with the value of 0·53 ng TEQ/kg in the SFO/RO/SO blend diet (Table 2). These values represent 6·3 and 7·6 % of the EU limit, respectively(22), and indicate that the decontamination process was successful. When comparing the POP concentrations in the three diets, a reduction of PCDD/F of 97 % was observed for both the deNFO and SFO/RO/SO diets compared with the cNFO diet. Similarly, an overall 98 % reduction of both mono-ortho and non-ortho PCB was noted compared with the cNFO diet, with individual congeners being reduced by 71–99 and 93–99 %, respectively (Table 2).
The sum concentrations of the seven PBDE congeners in diets were 5·9, 1·9 and 0·31 ng/g wet weight, for the cNFO, deNFO and SFO/RO/SO diets, respectively (Table 3). The sum of PBDE was reduced by 68 % for the deNFO compared with the cNFO diet, and 95 % for the SFO/RO/SO, compared with the cNFO diet. The individual PBDE congeners were reduced by 35–94 % in the deNFO diet and by 93–100 % in the SFO/RO/SO diet compared with the cNFO diet (Table 3).
Table 3 Dietary concentrations (ng/g wet weight) of seven polybrominated diphenyl ether (PBDE) congeners in the control northern fish oil (cNFO), decontaminated northern fish oil (deNFO) and southern fish oil, rapeseed oil and soyabean oil (SFO/RO/SO) diets
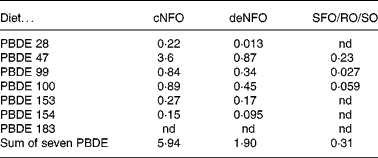
nd, Not detected.
In the initial fish at the start of the trial, the sum of flesh PCDD/F+DL-PCB was 1·06 ng TEQ/kg (Table 4). In fish fed the cNFO diet for 11 weeks this value increased significantly to 6·42 ng TEQ/kg while in fish fed the deNFO and SFO/RO/SO diets the concentrations were significantly reduced to 0·34 and 0·41 ng TEQ/kg, respectively, compared with the initial fish (Table 4). The flesh concentration for the fish fed cNFO was, however, below the EU limit value of 8 ng TEQ/kg while the deNFO and SFO/RO/SO flesh represented 4·3 and 5·1 % of the EU limit value, respectively(23).
Table 4 Flesh concentrations (ng toxic equivalents/kg wet weight) of dioxin and dioxin-like polychlorinated biphenyl (PCB) congeners in salmon (Salmo salar) at the start of the trial and after feeding the control northern fish oil (cNFO), decontaminated northern fish oil (deNFO) and southern fish oil, rapeseed oil and soyabean oil (SFO/RO/SO) diets for 11 weeks
(Mean values (n 3) and standard deviations)

TCDD, 2,3,7,8 tetra chlorinated dibenzo dioxin; PCDD, polychlorinated dibenzo-p-dioxins; nd, not detected; TCDF, 2,3,7,8 tetra chlorinated dibenzofuran; PCDF, polychlorinated dibenzofurans.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
In the initial fish flesh at the start of the trial, the sum concentration of the seven PBDE congeners was 0·26 ng/g wet weight. After feeding the experimental diets for 11 weeks the flesh value in fish fed the cNFO diet increased significantly to 0·94 ng/g, while fish fed the deNFO diet had similar levels to the initial fish of 0·25 ng/g and fish fed the SFO/RO/SO diet had a significantly reduced concentration of 0·095 ng/g, compared with the initial flesh (Table 5). The sum of PBDE was reduced by 73 % for the deNFO compared with the cNFO diet and by 90 % for the SFO/RO/SO compared with the cNFO diet.
Table 5 Flesh concentrations (ng/g wet weight) in salmon (Salmo salar) of seven polybrominated diphenyl ether (PBDE) congeners at the start of the trial and after feeding the control northern fish oil (cNFO), decontaminated northern fish oil (deNFO) or southern fish oil, rapeseed oil and soyabean oil (SFO/RO/SO) diets for 11 weeks
(Mean values (n 3) and standard deviations)
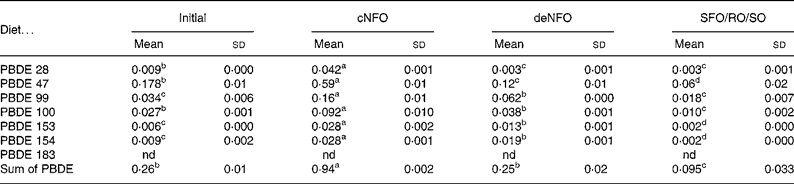
nd, Not detected.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Flesh total lipid content increased from 9·0 (sd 0·9) % in initial fish to 17·0 (sd 1·6) % in the final fish. There were no differences between treatments. Flesh fatty acid levels were closely correlated to dietary values and DHA values were similar for fish fed the cNFO and deNFO diets, although EPA was significantly higher in the latter (Fig. 1). By contrast, the DHA and EPA values in the flesh of fish fed the SFO/RO/SO diet were significantly lower than in the two FO groups, being reduced by 50 and 30 %, respectively (Fig. 1). At the same time, fish fed the SFO/RO/SO diet had increased levels of linoleic (18 : 2n-6) and linolenic acids (18 : 3n-3) of 3·6- and 2-fold, respectively (Fig. 1).

Fig. 1 Concentrations (% total fatty acids) of 18 : 2n-6, 18 : 3n-3, 20 : 5n-3 and 22 : 6n-3 in the flesh of salmon (Salmo salar) fed either the northern fish oil (■), decontaminated northern fish oil (□) or southern fish oil, rapeseed and soyabean oil (![]() ) diet for 11 weeks. Values are means, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) diet for 11 weeks. Values are means, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Data from the present study show that consumption of two 140 g portions of cNFO salmon would represent 171 % of the weekly limit for PCDD/F+DL-PCB for girls and women of reproductive age(35) (UK Food Standards Agency (FSA); www.food.gov.uk) (Fig. 2). Portions of 140 g are the standardised portion sizes used by the FSA. However, this value assumes that salmon is the only dietary input of PCDD/F and DL-PCB while in practice the two 140 g portions would contribute to more than 171 % of the weekly limit due to the consumption of other food products that also contain POP, albeit at a lower level than that seen in the cNFO salmon. In the UK, as in most countries in the developed world, the largest contribution to weekly POP intake is derived from dairy products(4). Consuming four 140 g portions would represent 86 % of the FSA weekly limit value for boys, men and women over reproductive age. By comparison, consuming two 140 g portions of salmon fed the deNFO or SFO/RO/SO diets represents 9·0 and 10·8 %, respectively, of the weekly limit for girls and women of reproductive age, while consuming four 140 g portions represents 4·5 and 5·4 %, respectively, of the weekly limit for PCDD/F+DL-PCB for boys, men and women over reproductive age (Fig. 2). Over the years many advisories have been issued on guideline intakes for EPA and DHA in humans(4, 7, 8). The intake advice of the International Society for the Study of Fatty Acids and Lipids (ISSFAL) is for a daily intake of EPA+DHA of 500 mg or 3·5 g/week(8). Thus, two 140 g portions of salmon fed the cNFO, deNFO and SFO/RO/SO diets provide 4·81, 5·14 and 2·83 g EPA+DHA, respectively, while four 140 g portions provide 9·62, 10·29 and 5·67 g EPA+DHA, respectively (Fig. 3).
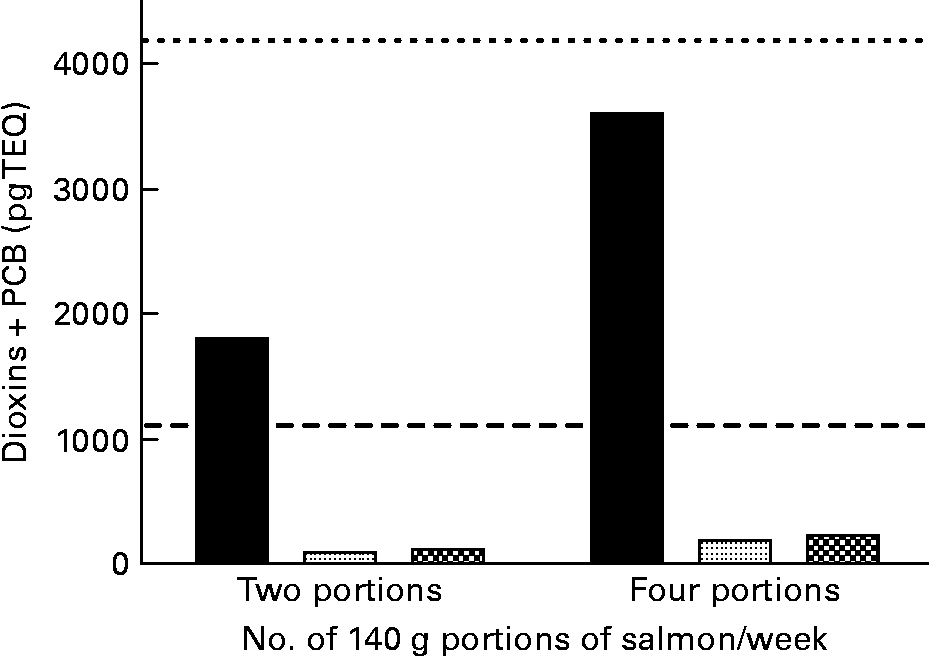
Fig. 2 Amount of dioxin+dioxin-like polychlorinated biphenyls (PCB) present in two or four 140 g portions of salmon (Salmo salar), produced using the northern fish oil (■), decontaminated northern fish oil (![]() ) and southern fish oil, rapeseed and soyabean oil
) and southern fish oil, rapeseed and soyabean oil ![]() diets. (- - -), UK Food Standards Agency (FSA) maximum for dioxin+PCB intake of 56 pg/kg per week for a 75 kg individual; (– – –), UK FSA maximum intake of 14 pg/kg per week for a 75 kg girl or woman of reproductive age. TEQ, toxic equivalents.
diets. (- - -), UK Food Standards Agency (FSA) maximum for dioxin+PCB intake of 56 pg/kg per week for a 75 kg individual; (– – –), UK FSA maximum intake of 14 pg/kg per week for a 75 kg girl or woman of reproductive age. TEQ, toxic equivalents.

Fig. 3 Amount of EPA+DHA provided by two or four 140 g portions of salmon (Salmo salar), produced using the northern fish oil (■), decontaminated northern fish oil (□) and southern fish oil, rapeseed and soyabean oil ![]() diets. (– – –), International Society for the Study of Fatty Acids and Lipids (ISSFAL) recommended EPA+DHA intake of 3·5 g/week.
diets. (– – –), International Society for the Study of Fatty Acids and Lipids (ISSFAL) recommended EPA+DHA intake of 3·5 g/week.
Discussion
The cNFO diet used a sprat oil from the Baltic Sea that was selected to be high in POP; this same oil was subjected to the two-step decontamination process at FF Skagen (Skagen, Denmark) to produce the deNFO. Previously, removal of > 90 % of PCDD/F from FO could be achieved using activated carbon treatment alone. However, removal of DL-PCB was less effective and required the use of high-temperature deodorisation that could cause oxidation of HUFA(Reference Maes, De Meulenaer and Van Heerswynghels26, Reference Hilbert, Lillemark and Balchen36). More effective removal of both PCDD/F and DL-PCB can be achieved using activated carbon coupled with short-path distillation as has been used in the present study(Reference Breivik and Thorstad27). Other methods for removal of POP from FO are being developed, including supercritical CO2 extraction along with activated carbon(Reference Kawashima, Watanabe and Iwakiri37). However, this methodology is equally efficient but has only been used in relatively small-scale extractions at the present time.
The cNFO diet in the present study contained 17·4 ng TEQ/kg of PCDD/F+DL-PCB and, as such, exceeds the EU maximum permitted value of 7·0 ng TEQ/kg for fish feeds(22). FO are generally considered to be the major source of PCDD/F and DL-PCB in high-energy fish diets, although a smaller contribution is derived from the fish meal component(Reference Berntssen, Lundebye and Torstensen14, Reference Friesen, Ikonomou and Higgs15, Reference Jacobs, Ferrario and Byrne24, 38). Despite there being considerable variation in contaminant concentrations in northern FO, due to season and location of capture, they are generally higher in PCDD/F and DL-PCB compared with those of Pacific origin(Reference Isosaari, Kiviranta and Lie39, Reference Lundebye, Berntssen and Lie40). As a result of high POP levels, a significant tonnage of FO from the North Atlantic, North and Baltic Seas would currently exceed EU permissible limits for PCDD/F+DL-PCB. Currently, oils such as the cNFO used in the present study could not be used in animal feeds and would be destroyed by high-temperature incineration. Given the nutritional value of n-3 HUFA as beneficial nutrients to attenuate a range of inflammatory disorders in humans(Reference Young and Conquer6, Reference Kris-Etherton, Harris and Appel41), and the fact that FO prices exceeded $1700/tonne in January 2008, it seems wasteful to dispose of valuable nutrients in this way. The decontamination of the cNFO was very successful, with the dietary concentration of PCDD/F+DL-PCB falling by 97 % in the deNFO diet, which was similar to the concentration of 0·53 ng TEQ/kg in the SFO/RO/SO diet. This reduction in PCDD/F and DL-PCB indicates that the efficiency of the decontamination process was consistent with reports in the literature(Reference Maes, De Meulenaer and Van Heerswynghels26, Reference Oterhals, Solvang and Nortvedt42).
Hites et al. (Reference Hites, Foran and Carpenter11) reported PCDD/F+DL-PCB in Scottish farmed salmon feeds in the range 2·5–7 ng TEQ/kg, with lowest values of about 1 ng TEQ/kg in feed from Canada. Both the deNFO and SFO/RO/SO diets in the present study had lower levels of PCDD/F+DL-PCB compared with the Canadian feeds sampled in 2003–4. Easton et al. (Reference Easton, Luszniak and Von der Geest43) sampled five feeds from North America and found concentrations for DL-PCB alone in the range 1·73–10·9 ng TEQ/kg. The highest concentration found by Easton et al. (Reference Easton, Luszniak and Von der Geest43) was similar to the DL-PCB concentration in the cNFO diet (8·71 ng TEQ/kg) while the concentrations in the deNFO and SFO/RO/SO diets were 83 % lower than the lowest diets sampled from North America. This clearly demonstrates the value of using either a decontaminated FO or a FO/VO blend as a means of reducing feed contaminant levels. The contaminant concentrations found in the present study, in the deNFO and SFO/RO/SO diets, are broadly similar to those seen in previous studies where FO has been substituted, partially or completely, with VO(Reference Berntssen, Lundebye and Torstensen14, Reference Friesen, Ikonomou and Higgs15, Reference Bell, McGhee and Dick25). In a study where salmon were fed diets containing either 17 or 34 % oil, which was a 100 % replacement of FO with a blend of rapeseed and linseed oils, the PCDD/F+DL-PCB concentrations in the diet were 0·95 and 0·78 ng TEQ/kg, respectively(Reference Bell, McGhee and Dick25). These values are about double those in the deNFO and SFO/RO/SO diets used in the present study. These differences probably reflect the use of northern fish meal in the study of Bell et al. (Reference Bell, McGhee and Dick25) compared with southern fish meal in the present study. Two similar studies using different levels of FO substitution were conducted by Berntssen et al. (Reference Berntssen, Giskegjerde and Rosenlund13, Reference Berntssen, Lundebye and Torstensen14). In the first trial, fish were fed 100 % northern FO or 100 % of a VO blend comprising rapeseed, linseed and palm oils for the whole production cycle. As FO content increased with pellet size, the PCDD/F+DL-PCB increased from 2·43 ng TEQ/kg feed in the 0·3 mm pellet to 4·74 ng TEQ/kg feed in the 9 mm pellet(Reference Berntssen, Lundebye and Torstensen14). In the VO diet, as the VO inclusion increased with pellet size, the PCDD/F+DL-PCB reduced from 1·07 ng TEQ/kg in the 0·3 mm pellet to 0·61 and 0·33 ng TEQ/kg in the 6 and 9 mm pellets. Thus, the values for PCDD/F+DL-PCB recorded in the present study, for the deNFO and SFO/RO/SO diets of 0·447 and 0·533 ng TEQ/kg, respectively, were broadly similar to the 100 % VO diets used by Berntssen et al. (Reference Berntssen, Lundebye and Torstensen14). In a second study where 100 % southern FO was replaced with 30 or 60 % rapeseed oil, the diet concentrations of PCDD/F+DL-PCB were 1·18, 0·93 and 0·86 ng TEQ/kg, respectively(Reference Berntssen, Giskegjerde and Rosenlund13). The value of 0·86 ng TEQ/kg is slightly higher than for the SFO/RO/SO diet of 0·53 ng TEQ/kg, which was also a 60 % replacement with VO.
The reduction of PBDE in the deNFO diet, compared with the cNFO diet, was 73 %, while the SFO/RO/SO diet had a 90 % reduction compared with the cNFO diet, which suggested less efficient removal of PBDE compared with dioxins and PCB. However, use of activated carbon alone did not reduce PBDE levels in FO(Reference Oterhals, Solvang and Nortvedt42) and so the addition of the short-path distillation step resulted in a significant reduction in PBDE in the deNFO diet. However, the decontamination method used in the present study is less efficient for PBDE than for PCDD/F and DL-PCB. In four samples of feed from Scotland, Hites et al. (Reference Hites, Foran and Schwager12) reported total PBDE concentrations in the range 2·44–10·9 ng/g wet weight while in six samples from Canada the range was 0·49–9·3 ng/g. By comparison, Easton et al. (Reference Easton, Luszniak and Von der Geest43) measured two North American feeds which contained 1·87 and 1·90 ng/g. It is noteworthy that the sum of PBDE for three of the Scottish diets and two of the Canadian diets(Reference Hites, Foran and Schwager12) were higher than the value of 5·94 ng/g observed in the cNFO diet. By comparison, the PBDE concentration in the deNFO diet of 1·93 ng/g is lower than nine of the feeds from Scotland, Canada and Chile analysed by Hites et al. (Reference Hites, Foran and Schwager12) and similar to the two feeds analysed by Easton et al. (Reference Easton, Luszniak and Von der Geest43). The sum of PBDE in the SFO/RO/SO diet was 0·31 ng/g, and was lower than any value reported by either Hites et al. (Reference Hites, Foran and Schwager12) or Easton et al. (Reference Easton, Luszniak and Von der Geest43).
In the present study, the PCDD/F and DL-PCB concentrations in flesh were closely correlated with diet concentrations, with the highest values in fish fed the cNFO diet (6·4 ng TEQ/kg). This value was increased compared with the initial flesh (1·1 ng TEQ/kg) but was much lower than the diet concentration. This was also observed in previous studies with salmon where the accumulation efficiency of PCDD/F was shown to be lower than for DL-PCB(Reference Berntssen, Giskegjerde and Rosenlund13, Reference Berntssen, Lundebye and Torstensen14). In comparison with the fish fed the cNFO diet, the PCDD/F+DL-PCB concentrations in flesh of fish fed the deNFO and SFO/RO/SO diets were decreased compared with the initial values to 0·34 and 0·41 ng TEQ/kg, respectively. Although there are no data in the literature on the effects of feeding decontaminated oils, values are reported for fish fed varying levels of VO, as well as different sources of FO. In the study by Bell et al. (Reference Bell, McGhee and Dick25) flesh PCDD/F+DL-PCB concentration in salmon fed a similar oil level to the present study, but where the VO was 50 % rapeseed oil and 50 % linseed oil, was 0·73 ng TEQ/kg, which was more than double the values seen in the fish fed deNFO and SFO/RO/SO in the present study. Despite having a higher level of VO, this higher value is probably due to the use of northern fish meal in the 2005 study(Reference Bell, McGhee and Dick25). The concentrations in the study by Bell et al. (Reference Bell, McGhee and Dick25) are similar to those found by Berntssen et al. (Reference Berntssen, Giskegjerde and Rosenlund13) who reported values of 0·95 and 0·86 ng TEQ/kg for fish fed either 30 % or 60 % rapeseed oil, respectively. The sum of ng TEQ/kg for fish fed the deNFO and SFO/RO/SO diets, in the present study, was 0·34 and 0·41, respectively. These values are similar to the results reported by Berntssen et al. (Reference Berntssen, Lundebye and Torstensen14), where salmon were fed a 100 % VO inclusion for 97 weeks. Although the fish in the present study were harvested at a smaller size than those produced by Berntssen et al. (Reference Berntssen, Lundebye and Torstensen14), it is likely that the values would decrease even more with continued culture on the deNFO and SFO/RO/SO diets. The present study lasted for only 11 weeks, while the half-life of PCDD/F and DL-PCB is estimated at approximately 5 months(Reference Berntssen, Giskegjerde and Rosenlund13), and an additional growth dilution would cause a continued lowering of POP loads. Thus, a further decrease in PCB levels, at least, would be expected with continued feeding on deNFO and SFO/RO/SO diets.
In the study of Hites et al. (Reference Hites, Foran and Carpenter11) PCDD/F+DL-PCB concentrations for Scottish salmon were about 2·9 ng TEQ/kg (n 30) while the concentrations in fish fed the deNFO and SFO/RO/SO diets were about 13 % of this value. In a more recent study Ikonomou et al. (Reference Ikonomou, Higgs and Gibbs44) recorded a maximum value of 1·85 (sd 0·27) ng TEQ/kg in Canadian salmon. By comparison, the fish fed the deNFO and SFO/RO/SO diets were about 20 % of this value. The mean feed PCDD/F+DL-PCB value in the Canadian diets was 3·74 ng TEQ/kg(Reference Ikonomou, Higgs and Gibbs44), which is 7·5 times higher than the deNFO and SFO/RO/SO diets in the present study. The PCDD/F+DL-PCB concentration for wild Pacific Chinook salmon was about 0·13 ng TEQ/kg(Reference Hites, Foran and Carpenter11), while the DL-PCB values alone for a different source of Chinook salmon were about 0·45 ng TEQ/kg(Reference Easton, Luszniak and Von der Geest43). Thus, the PCCD/F+DL-PCB concentrations in fish fed the deNFO and SFO/RO/SO diets are only slightly higher than those quoted by Hites et al. (Reference Hites, Foran and Carpenter11) and considerably lower than those quoted by Easton et al. (Reference Easton, Luszniak and Von der Geest43) for wild Pacific salmon.
The flesh PBDE concentrations for the fish fed the cNFO, deNFO and SFO/RO/SO diets were 0·94, 0·25 and 0·10 ng/g, respectively. These values are all significantly lower than the values recorded for wild Canadian Chinook (4·2 ng/g), Scottish farmed (3·9 ng/g) or wild Oregon Chinook (2·2 ng/g) by Hites et al. (Reference Hites, Foran and Schwager12). These values were also considerably lower than the average PBDE concentration of 5 ng/g reported by Jacobs et al. (Reference Jacobs, Covaci and Schepens45) in thirteen samples of European salmon. Excluding the nine wild Chinook salmon, the average value for the thirty-six wild Pacific salmon, including Pink, Coho, Sockeye and Chum, was 0·130 (sd 0·020) ng/g(Reference Hites, Foran and Schwager12). The PBDE concentrations in salmon fed the deNFO and SFO/RO/SO diets were slightly higher and lower than the wild Pacific salmon, respectively.
There is currently considerable interest in fish, and especially oily fish, as a means of increasing n-3 HUFA intake in populations that currently have low fish intake. However, while the benefits of consuming n-3 HUFA are widely known, there is also concern about the relative risks and benefits due to the presence of POP in oily fish(4, Reference Berntssen, Lundebye and Torstensen14, Reference Foran, Good and Carpenter16, 46). The current guideline of the UK FSA (www.food.gov.uk) suggests that we should consume up to four 140 g portions of oily fish per week. However, the FSA adds the caveat that girls and women of child-bearing age should only consume two 140 g portions of oily fish due to concerns that POP present in the fish might pose a risk to the developing fetus. Thus, for girls or women of child-bearing age, the FSA recommendation is in agreement with the EU Scientific Committee on Food that the maximum intake of PCDD/F+DL-PCB should be 2 pg/kg body weight per d(35). However, the FSA guideline suggests that boys, men and women over reproductive age can consume up to 8 pg/kg body weight per d. The salmon fed the cNFO diet in the present study would give 171 % of the weekly limit for girls and women of reproductive age or 86 % of the weekly limit value for boys, men and women over reproductive age, from two or four 140 g portions of cNFO salmon, respectively. However, salmon with these levels of POP would not be available for public consumption due to current EU limits on PCDD/F and DL-PCB in fish feeds(22). In a sample of standard production Scottish salmon analysed in our laboratory, the average PCDD/F+DL-PCB concentration in two 140 g portions was 493 pg TEQ. This represents 47 % of the value for girls and women of child-bearing age or 24 % of the limit for boys, men and women over reproductive age. By comparison, for the deNFO and SFO/RO/SO fish, consuming two or four 140 g portions represents only 9·0 and 10·8 % of the weekly limit for girls and women of reproductive age or 4·5 and 5·4 %, respectively, of the weekly limit for PCDD/F+DL-PCB for boys, men and women over reproductive age. While the POP concentrations are very low for both the deNFO and SFO/RO/SO salmon, the same two and four 140 g portions provide 147 and 81 % and 294 and 162 %, respectively, of the International Society for the Study of Fatty Acids and Lipids (ISSFAL) recommended weekly intake of EPA+DHA(8).
In summary, the present study has confirmed that FO with high POP concentrations can be successfully decontaminated with removal of more than 97 % of PCDD/F and DL-PCB using two-stage activated carbon and thin-film deodorisation. Removal of PBDE was less successful, with concentrations being reduced by about 70 %. Feeding the deNFO and SFO/RO/SO diets to 0·8 kg Atlantic salmon for 11 weeks resulted in excellent growth rates and feed conversion. Flesh PCDD/F and DL-PCB concentrations were reduced to very low levels in fish fed the deNFO and SFO/RO/SO diets, with values being similar to those seen in wild Pacific salmon, while PBDE concentrations were comparable or lower than Pacific salmon. Feeding the deNFO diet did not significantly alter the n-3 HUFA content of the fillet, as was observed in fish fed the SFO/RO/SO diet. This suggests that decontaminated FO can be used successfully to produce salmon that are both high in n-3 HUFA and low in POP, although further longer-term trials should be conducted to ensure that no detrimental effects on fish performance and health occur. These results could be of significant benefit in producing highly nutritious farmed salmon that are very low in undesirables as well as utilising valuable FO that would otherwise be destroyed by incineration.
Acknowledgements
J. P. was funded by a Royal Thai Government Scholarship.
J. G. B. wrote the manuscript with assistance from all other authors especially M. H. G. B. and D. R. T.; E.Å. B. was responsible for all aspects of the feeding trial including diet manufacture, fish production and sample collection; M. S., F. S. and J. R. D. were responsible for all aspects of contaminant analysis with technical advice supplied by M. H. G. B.; J. P. was responsible for lipid analysis; D. R. T. and J. G. B. were PhD supervisors of J. D. All authors read and approved the findings of the study.
None of the authors had a conflict of interest.










