Obesity negatively influences overall health, adds significantly to societal and economic burdens, and shows no signs of slowing(Reference Orukwowu1). Globally, millions of individuals maintain a sedentary lifestyle and adhere to nutrient-poor and energy-dense diets, which contributes to overweightness/obesity and increases the risk for many non-communicable chronic diseases(Reference Hutchesson, Gough and Müller2,Reference Wang, McPherson and Marsh3) . Therefore, the identification of alternative adiposity-reducing strategies with the potential to prevent or alleviate the negative consequences of obesity is warranted. Apart from lifestyle modification, often considered the cornerstone of a weight management programme(Reference Ashtary-Larky, Bagheri and Bavi4–Reference Ashtary-Larky, Bagheri and Abbasnezhad6), a wide range of supplements are now available touting anti-obesity properties(Reference Asbaghi, Naeini and Ashtary-Larky7–Reference Bagheri, Negaresh and Motevalli10). Among those, conjugated linoleic acid (CLA) has shown promise as a food supplement to reduce adiposity in preventing overweightness and obesity(Reference Chang, Gan and Liao11,Reference Mądry, Malesza and Subramaniapillai12) .
CLA have been investigated for various beneficial effects, including cancer, atherosclerosis and obesity(Reference Basak and Duttaroy13–Reference O’Reilly, Lenighan and Dillon16). Major isomers of CLA are cis-9, trans-11 CLA (9, 11 CLA) and trans-10, cis-12 CLA (10, 12 CLA)(Reference Asbaghi, Ashtary-Larky and Naseri17), which are found naturally in ruminant animal food products(Reference Haghighat, Shimi and Shiraseb18) and are primary components of widely consumed CLA weight-loss supplements(Reference Ashwell, Ceddia and House19,Reference Churruca, Fernández-Quintela and Zabala20) . Although humans can produce endogenous CLA, the blood and tissue levels of CLA in non-supplemented individuals are less(Reference Sato, Shinohara and Honma21,Reference Turpeinen, Mutanen and Aro22) . According to prior investigations, isomer 10, 12 CLA seems to elicit the greatest beneficial effect on promoting weight loss in animals and humans(Reference Chen, Lin and Huang23–Reference Mądry, Chudzicka-Strugała and Grabańska-Martyńska25). One proposed explanation behind CLA action may be stimulating apoptotic mechanisms and regulating lipolytic pathways, both of which positively affect body composition and weight loss in humans(Reference Baddini Feitoza, Fernandes Pereira and Ferreira da Costa26). A substantial body of evidence also indicates that CLA promotes weight loss by reducing fat cells’ size and altering fat cells’ evolution(Reference Brown and McIntosh27). While future research needs to elucidate further the physiological or other mechanisms behind CLA-induced altered fat cells, the vast majority of literature on the role of CLA in managing obesity utilises common measures for anthropometrics and body composition, including body mass (BM), BMI, waist circumference (WC) and body fat percentage (BFP)(Reference Joseph, Wasir and Misra28,Reference Pi-Sunyer29) . To this, a series of recent well-controlled pharmacological investigations have demonstrated conflicting results on the effectiveness of CLA supplementation on these outcomes in adults(Reference Chang, Gan and Liao11,Reference Abedi, Aref-Hosseini and Khoshbaten30) .
As noted, investigations on the association between CLA supplementation with anthropometric and body composition outcomes have sometimes been in agreement, which may be due to various factors, including supplementation dosages, the length of intervention and the health status of participants. Although prior meta-analyses exist, such investigations targeted special populations, including overweight and obese individuals(Reference Namazi, Irandoost and Larijani31,Reference Onakpoya, Posadzki and Watson32) and those with metabolic syndrome(Reference Kim, Lim and Lee33). To the best of our knowledge, two prior meta-analyses have been conducted to determine the pooled effects of CLA supplementation on fat and fat-free mass (FFM) in the general adult population(Reference Whigham, Watras and Schoeller34,Reference Schoeller, Watras and Whigham35) . However, these meta-analytic investigations, in particular, were performed over a decade ago, and numerous relevant randomised controlled trials (RCT) have been published since. Therefore, we performed a comprehensive systematic review and meta-analysis of the literature to date on the effects of CLA supplementation on anthropometric and body composition markers in adults.
Methods
This investigation was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol(Reference Moher, Shamseer and Clarke36).
Search strategy
A comprehensive literature search was performed for RCT that investigated the efficacy of CLA supplementation on anthropometric measurements and body composition indicators using online databases, including PubMed/MEDLINE, Scopus, Web of Science, and Cochrane Library up to March 2022. The following MeSH and non-MeSH terms were applied in the search strategy: (‘Conjugated linoleic acid’ OR ‘conjugated fatty acid’ OR ‘bovic acid’ OR ‘rumenic acid’ OR ‘CLA’ AND Intervention OR ‘Intervention Study’ OR ‘Intervention Studies’ OR ‘controlled trial’ OR ‘randomized’ OR ‘randomized’ OR ‘random’ OR ‘randomly’ OR ‘placebo’ OR ‘clinical trial’ OR ‘Trial’ OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR ‘RCT’ OR ‘blinded’ OR ‘double blind’ OR ‘double blinded’ OR ‘trial’ OR ‘clinical trial’ OR ‘trials’ OR ‘Pragmatic Clinical Trial’ OR ‘Cross-Over Studies’ OR ‘Cross-Over’ OR ‘Cross-Over Study’ OR ‘parallel’ OR ‘parallel study’ OR ‘parallel trial’).
No restrictions were placed in database searches for the date of publication. Reference lists of all relevant studies were cross-checked against database search results for overlooked publications. All references were included in the Endnote software (EndNote X21, Thomson Reuters, New York) for screening, and duplicate citations and unpublished manuscripts were removed.
Study selection and eligibility criteria
Titles and abstracts of all records from the initial search were evaluated independently by two investigators. Studies were selected for further analysis if they met the following criteria: (a) original RCT with either parallel or crossover designs; (b) studies that were done on adult participants (≥18 years old); (c) trials investigating the impact of CLA supplementation on anthropometric measurements (BM, BMI and WC) and body composition indicators (fat mass (FM), BFP and FFM) in both intervention and placebo groups; (d) studies that reported means and standard deviations for each outcome or any other effect sizes by which the calculation of means and standard deviation was possible. Conversely, studies were excluded if: (a) the duration of intervention was less than 4 weeks; (b) inadequate data on the selected outcomes in intervention or control groups was presented; (c) they were observational, case reports, reviews, letters to an editor, editorial articles, and in vitro studies and animal experiments; (d) children or adolescents were enrolled; and (e) no control group was apparent.
Data extraction
The data extraction was independently performed using a pre-designed standardised electronic form (Excel, Microsoft Office). The following information from each study were extracted: first author’s name, year of publication, study location, total sample size, numbers of cases (those who received CLA) and controls, participant’s demographic data (sex, mean age and BMI), the health status of participants, study design, the intervention dose, length of follow-up, and outcomes measured as mean and standard deviation of selected end points at study baseline, post-intervention, and/or changes between baseline and post-intervention.
Quality and certainty assessment
A systematic bias assessment of the included studies was performed using the Cochrane criteria(Reference Higgins, Thomas and Chandler37). The quality of all eligible studies was evaluated based on the following items: random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias and other possible causes of bias. Based on the Cochrane Handbook recommendation, studies were ranked as low (L), high risk of bias (H) or unclear (U) regarding each field of bias(Reference Higgins, Thomas and Chandler37). In addition, the overall certainty of evidence across the studies was evaluated based on the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group guidelines. Subsequently, the quality of evidence was classified into four categories: high, moderate, low and very low(Reference Guyatt, Oxman and Vist38).
Data synthesis and statistical analysis
Data analysis was performed using STATA® version 14.0 (StataCorp.), and mean (sd) changes of outcomes were used to estimate the overall effect size. Effect sizes for all variables are reported as weighted mean differences (WMD) and 95 % CI derived from random-effects models. A random-effects model was selected to address significant heterogeneity between studies for methodology, outcome measures and participant characteristics. The I 2 index was used as an index of statistical heterogeneity of RCT. If the sd change following intervention was not reported in studies, it was calculated based on the formula provided by the Cochrane Collaboration(Reference Higgins39) such that: sd = square root [(sd baseline) 2 + (sd final) 2 – (2R × sd baseline × sd final)], where the correlation coefficient (R) = 0·8. Statistical heterogeneity between studies was assessed by Cochrane’s Q test (significance point at P < 0·1) and the I 2 index (significance point at I 2 > 40 %).
The publication bias was assessed using visual inspection of funnel plots and statistically using Egger’s regression and Begg’s tests(Reference Egger, Davey Smith and Schneider40). Subgroup analyses were performed to find probable sources of heterogeneity based on several predefined variables, including duration of follow-up (≥12 v. <12 weeks), intervention dosage (≥3 v. <3 g/d), participants’ health condition (healthy v. unhealthy), baseline values of BMI (normal v. overweight v. and obese), sex (female v. male v. combined) and the quality of studies (high v. moderate, v. high quality). Sensitivity analysis was applied to detect if an overall effect size relied on the outcomes of a particular RCT. To determine the non-linear dose–response and linear meta-regression effects of CLA dosage (g/d) as well as the duration of intervention on each marker, fractional polynomial modelling was used. A P-value ≤ 0·05 was considered to be a statistically significant outcome.
Results
Study selection
The study selection process is illustrated in Fig. 1. A total of 8351 publications were found in the initial search. Of those, 2419 were duplicates and thus removed from further consideration. After a review of the remaining 5932 titles and abstracts, 104 publications were advanced for full-text examination. An additional thirty-four studies were removed following full-text scrutiny according to inclusion/exclusion criteria. Finally, sixty-nine RCT with ninety-five effect sizes and 4159 participants met the inclusion criteria for quantitative and qualitative analyses.
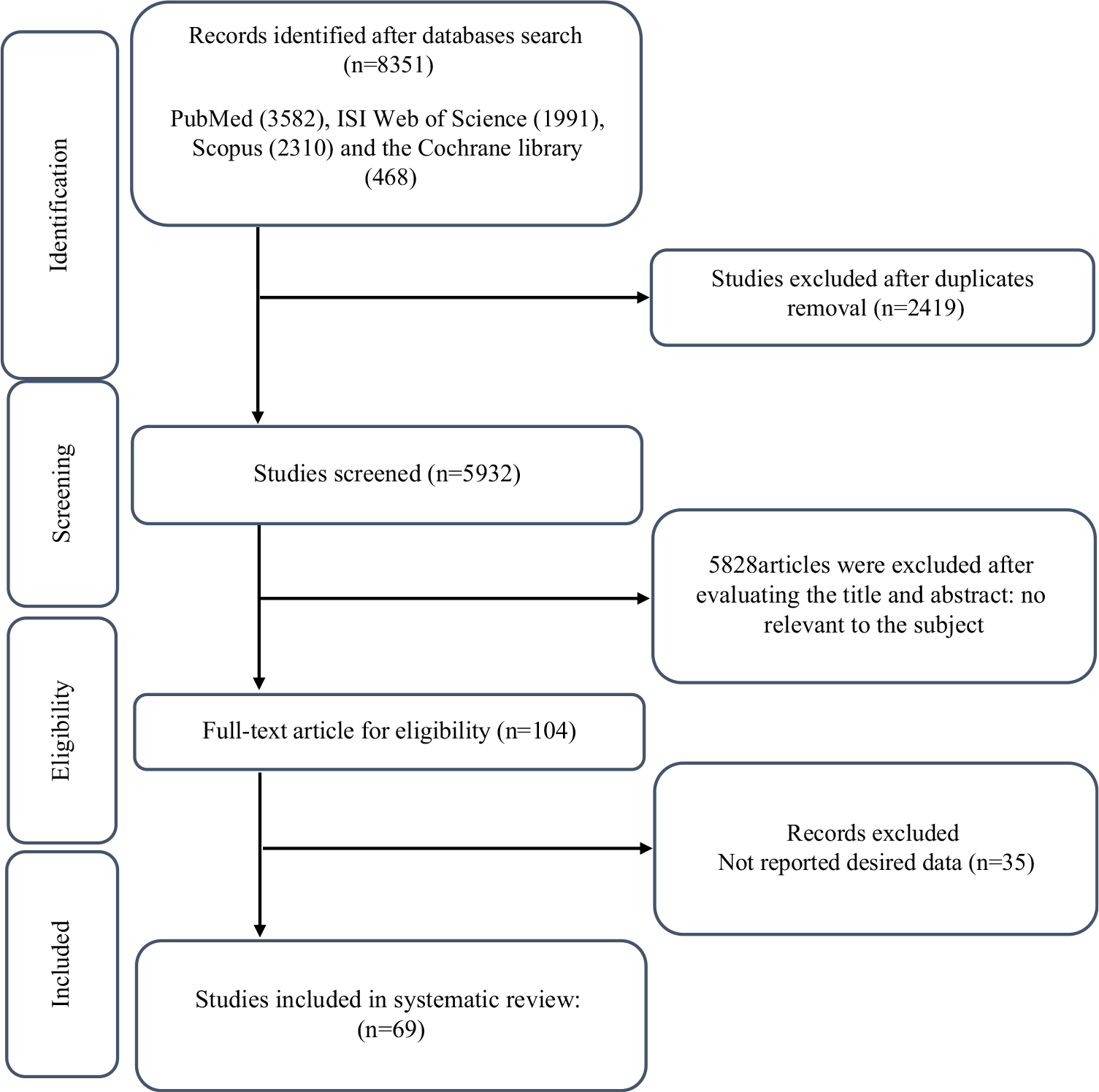
Fig. 1. Flow chart of study selection for inclusion trials in the systematic review.
Study characteristics
The characteristics of the included RCT are outlined in Table 1. Investigations were published between 2000 and 2020 and were carried out in Europe(Reference Mądry, Chudzicka-Strugała and Grabańska-Martyńska25,Reference Berven, Bye and Hals41–Reference Thom, Wadstein and Gudmundsen68) , Asia(Reference Chen, Lin and Huang23,Reference Abedi, Aref-Hosseini and Khoshbaten30,Reference Aryaeian, Shahram and Djalali69–Reference Zh, Rastmanesh and Hehayati87) , America(Reference Adams, Hsueh and Alford88–Reference Plourde103), Africa(Reference Goedecke, Rae and Smuts104–Reference Lambert, Goedecke and Bluett106) and Oceania(Reference Attar-Bashi, Weisinger and Begg107). Of the sixty-nine RCT, six were randomised crossover design(Reference Rubin, Herrmann and Much63,Reference Sneddon, Tsofliou and Fyfe65,Reference Desroches, Chouinard and Galibois92,Reference Joseph, Jacques and Plourde93,Reference Venkatramanan, Joseph and Chouinard98,Reference Plourde103) , and the remaining were of parallel design. Intervention dosages of CLA varied between 1·0 and 6·8 g/d, and follow-up durations ranged from 4 to 104 weeks. Selected studies enrolled participants with different health conditions; three studies enrolled patients with diabetes(Reference Norris, Collene and Asp55,Reference Shadman, Taleban and Saadat78,Reference Zh, Rastmanesh and Hehayati87) ; three investigated the effects of CLA supplementation in individuals with hyperlipidemia(Reference Joseph, Jacques and Plourde93,Reference Venkatramanan, Joseph and Chouinard98,Reference Plourde103) , two recruited patients with metabolic syndrome(Reference Risérus, Arner and Brismar59,Reference Carvalho, Uehara and Rosa90) , and individual studies investigated the following health conditions: rheumatoid arthritis(Reference Aryaeian, Shahram and Djalali69), hypertension(Reference Zhao, Zhai and Wang80), atherosclerosis(Reference Hassan Eftekhari, Aliasghari and Babaei-Beigi76), chronic obstetric pulmonary disease(Reference Ghobadi, Matin and Nemati75) and benign breast disease(Reference Rezvani, Montazeri and Baradaran84). All remaining studies were performed on apparently healthy individuals. The vast majority of RCT were performed on both sexes, except sixteen investigations that were conducted exclusively on females(Reference Mądry, Chudzicka-Strugała and Grabańska-Martyńska25,Reference Mądry, Malesza and Subramaniapillai50,Reference Norris, Collene and Asp55,Reference Colakoglu, Colakoglu and Taneli72,Reference Kim, Kim and Ha82,Reference Rezvani, Montazeri and Baradaran84–Reference Tavakkoli Darestani, Hosseinpanah and Tahbaz86,Reference Brown, Trenkle and Beitz89,Reference Carvalho, Uehara and Rosa90,Reference Medina, Horn and Keim95,Reference Ribeiro, Pina and Dodero97,Reference Zambell, Keim and Van Loan100–Reference Pina, Ribeiro and Dodero102,Reference Lambert, Goedecke and Bluett106) and seventeen on males(Reference Pfeuffer, Fielitz and Laue57–Reference Rubin, Herrmann and Much63,Reference Sneddon, Tsofliou and Fyfe65,Reference Bulut, Bodur and Colak70,Reference Tajmanesh, Aryaeian and Hosseini79,Reference Adams, Hsueh and Alford88,Reference Deguire, Makarem and Vanstone91–Reference Joseph, Jacques and Plourde93,Reference Plourde103,Reference Kreider, Ferreira and Greenwood105,Reference Lambert, Goedecke and Bluett106) . The mean age of individuals was between 18 and 63·3 years, with BMI ranging from 19 to 37·1 kg/m2. All sixty-nine included trials had an appropriately controlled design, with the sole difference between control and treatment groups being the CLA intervention.
Table 1. Characteristics of the included studies
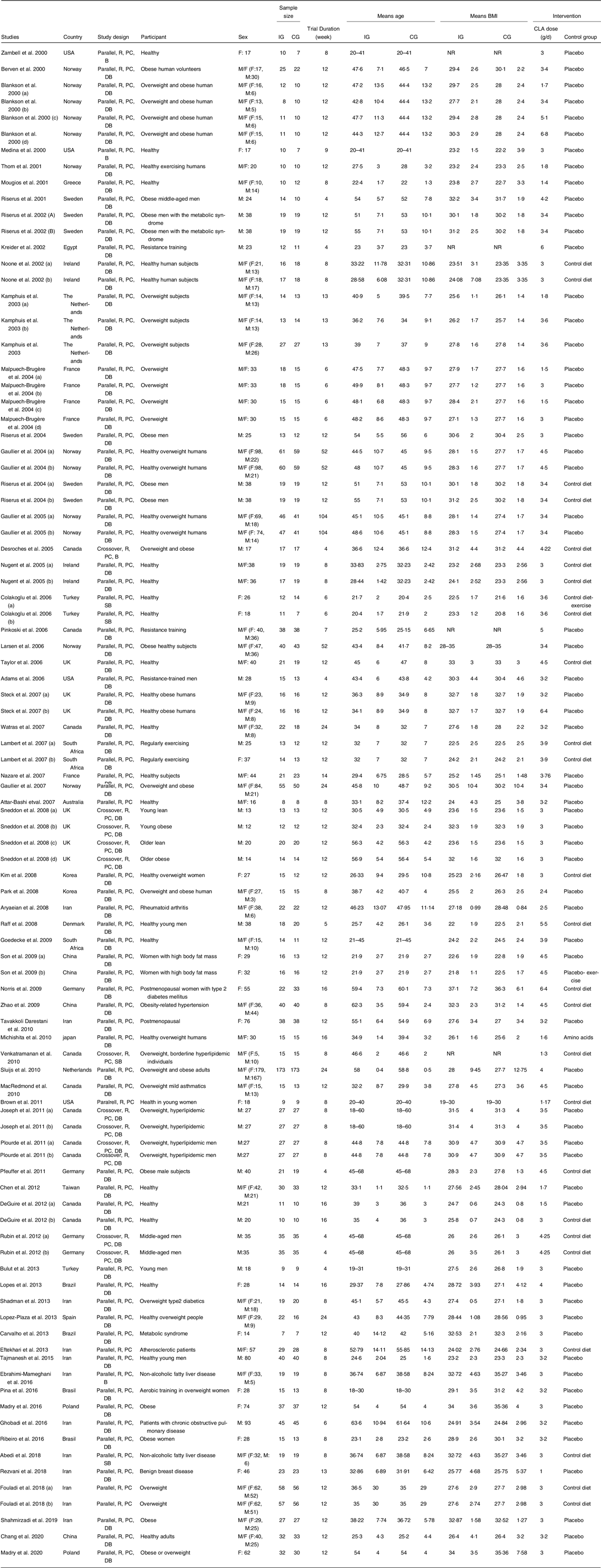
R, randomised; PC, placebo-control; DB, double-blind; M, male; F, female.
Risk of bias assessment
Cochrane risk of bias results of included studies is shown in Supplementary 1 and indicated that thirty-three(Reference Mądry, Chudzicka-Strugała and Grabańska-Martyńska25,Reference Abedi, Aref-Hosseini and Khoshbaten30,Reference Berven, Bye and Hals41,Reference Gaullier, Halse and Høye44,Reference Kamphuis, Lejeune and Saris46,Reference Kamphuis, Lejeune and Saris47,Reference Malpuech-Brugère, Verboeket-Van de Venne and Mensink51,Reference Nazare, de la Perrière and Bonnet53,Reference Pfeuffer, Fielitz and Laue57,Reference Risérus, Arner and Brismar59–Reference Risérus, Vessby and Arnlöv62,Reference Sneddon, Tsofliou and Fyfe65–Reference Bulut, Bodur and Colak70,Reference Colakoglu, Colakoglu and Taneli72,Reference Zhao, Zhai and Wang80,Reference Fouladi, Peng and Mohaghehgi81,Reference Brown, Trenkle and Beitz89–Reference Joseph, Jacques and Plourde93,Reference Medina, Horn and Keim95,Reference Venkatramanan, Joseph and Chouinard98–Reference Zambell, Keim and Van Loan100,Reference Plourde103,Reference Lambert, Goedecke and Bluett106) trials were considered to be at high risk for bias, nineteen(Reference Blankson, Stakkestad and Fagertun42,Reference Gaullier, Halse and Høivik43,Reference Mądry, Malesza and Subramaniapillai50,Reference Mougios, Matsakas and Petridou52,Reference Noone, Roche and Nugent54,Reference Nugent, Roche and Noone56,Reference Raff, Tholstrup and Basu58,Reference Rubin, Herrmann and Much63,Reference Hassan Eftekhari, Aliasghari and Babaei-Beigi76–Reference Tajmanesh, Aryaeian and Hosseini79,Reference Kim, Kim and Ha82,Reference Park, Kim and Kim83,Reference MacRedmond, Singhera and Attridge94,Reference Pinkoski, Chilibeck and Candow96,Reference Pina, Ribeiro and Dodero102,Reference Goedecke, Rae and Smuts104,Reference Kreider, Ferreira and Greenwood105) were deemed the moderate risk of bias and the remaining eighteen(Reference Chen, Lin and Huang23,Reference Gaullier, Halse and Høye45,Reference Larsen, Toubro and Gudmundsen48,Reference López-Plaza, Bermejo and Koester Weber49,Reference Norris, Collene and Asp55,Reference Sluijs, Plantinga and de Roos64,Reference Chang, Gan and Liao71,Reference Ebrahimi-Mameghani, Jamali and Mahdavi73–Reference Ghobadi, Matin and Nemati75,Reference Rezvani, Montazeri and Baradaran84–Reference Adams, Hsueh and Alford88,Reference Ribeiro, Pina and Dodero97,Reference Lopes, Silvestre and Silva101,Reference Attar-Bashi, Weisinger and Begg107) were at low risk of bias.
Meta-analysis results
Effects of conjugated linoleic acid supplementation on anthropometric measurements
Combining eighty-three effect sizes where CLA supplementation was compared with a placebo control revealed a significant lowering effect of CLA supplementation on BM (WMD: −0·34 kg, 95 % CI (−0·54, −0·15), P < 0·001). However, there was a significant between-study heterogeneity (I 2 = 42·7 %, P < 0·001) (Fig. 2(a)). The findings from subgroup analyses showed that CLA consumption was associated with decreased BM irrespective of the health condition of participants and follow-up length. In addition, BM was only reduced in overweight and obese individuals (as defined by BMI) and female and both sexes and in those who ingested 3 g/d or more of CLA. Weight-lowering effects of CLA were shown in both high- and low-quality studies but not in low-quality studies (Table 2).

Fig. 2. Forest plot detailing weighted mean difference and 95 % CI for the effect of CLA supplementation on: (a) body weight (kg); (b) BMI (kg/m2); (c) WC (cm); (d) FM (kg); (e) BFP (%); and (f) FFM (kg). CLA, conjugated linoleic acid; WC, waist circumference; FM, fat mas; BFP, body fat percentage; FFM, fat-free mass.
Table 2. Subgroup analyses of CLA supplementation on anthropometric indices and body composition

CLA, conjugated linoleic acid; WMD, weighted mean differences; WC, waist circumferenc; FM, fat mass; BFP, body fat percentage; FFM, fat-free mass.
Bold value: significant effect (p<0.05).
Eighty effect sizes from seventy-seven included RCT reported the effect of CLA supplementation on BMI and revealed a significant reduction in BMI (WMD: −0·15 kg/m2, 95 % CI (−0·24, −0·06), P = 0·001) albeit with a significant between-study heterogeneity (I 2 = 70·6 %, P < 0·001) (Fig. 2(b)). Subgroup analyses demonstrated BMI values were significantly reduced following CLA supplementation regardless of participant health status and follow-up duration. As with BM, BMI was only reduced in overweight and obese individuals and those who ingested 3 g/d or more CLA. However, BMI only decreased in high-quality studies subgroups (Table 2).
Overall, thirty-nine arms of included clinical trials investigated the effect of CLA supplementation on WC, and pooled effect size showed a significant reduction in WC (WMD: −0·67 cm, 95 % CI (−1·10, −0·23), P = 0·002) with significant between-study heterogeneity (I 2 = 76·0 %, P < 0·001) (Fig. 2(c)). Subgroup analyses revealed that CLA supplementation resulted in a significant reduction in WC among healthy participants, in those with baseline BMI > 30 kg/m2, upon supplementing with 3 g/d or more of CLA, and in cases where follow-up length was less than 12 weeks. Regarding the quality of studies, CLA was the cause of the WC decrease in moderate-quality studies (Table 2).
Effects of conjugated linoleic acid supplementation on body composition indicators
Meta-analysis of forty-nine effect sizes revealed a significant change in FM values after CLA intervention (WMD: −0·46 kg, 95 % CI (−0·68, −0·23), P < 0·001) despite a significant heterogeneity between studies (I 2 = 51·6 %, P < 0·001) (Fig. 2(d)). The findings of the subgroup analyses showed that CLA consumption reduced FM regardless of the intervention dosage and duration. However, CLA supplementation was associated with decreased FM only in healthy individuals and those with a baseline BMI categorised as overweight or obese. Furthermore, FM decreased in low- and moderate-quality subgroups (Table 2).
A total of forty-three effect sizes (835 cases and 808 controls) investigated the effect of CLA supplementation on BFP. Pooled data analysis indicated that BFP was significantly reduced following CLA supplementation compared with placebo (WMD: −0·76 %, 95 % CI (−1·08, −0·44), P < 0·001) albeit with a significant degree of heterogeneity between RCT (I 2 = 66·6 %, P < 0·001) (Fig. 2(e)). Subgroup analyses revealed that CLA supplementation significantly reduced BFP irrespective of participants’ health condition, baseline BMI values, and intervention dosages and duration. BFP-lowering effects of CLA are only seen in low-quality studies (Table 2).
Forty-five effect sizes (975 cases and 939 controls) were assessed for the effect of CLA supplementation on FFM. Meta-analysis indicated that CLA supplementation significantly increased FFM values in study participants (WMD: 0·27 kg, 95 % CI (0·09, 0·45), P = 0·003). A significant between-studies degree of heterogeneity was observed (I 2 = 47·6 %, P < 0·001) (Fig. 2(f)). Findings from subgroup analyses showed that CLA consumption was associated with increased FFM in healthy participants and in those with normal baseline BMI values upon supplementing with 3 g/d or more of CLA and when the trial duration was 12 weeks or more. Finally, FFM increases in high- and low-quality studies (Table 2).
Publication bias
There was no evidence of publication bias in RCT examining the effect of CLA supplementation for all outcomes, including BM (P = 0·142 Egger’s test), BMI (P = 0·201 Egger’s test), WC (P = 0·107 Egger’s test), FM (P = 0·055 Egger’s test), BFP (P = 0·059 Egger’s test) and FFM (P = 0·601 Egger’s test). Funnel plots further indicated no evidence of asymmetry for the effects of CLA consumption on each outcome analysed in this meta-analysis (online Supplementary 2).
Dose–response and meta-regression analyses
Dose–response analyses showed that CLA supplementation significantly altered BFP based on the intervention duration (r = −1·41, P-non-linearity = 0·04) in a non-linear manner. No other significant non-linear dose–response associations were observed for the remaining outcomes (online Supplementary 3 and 4). A meta-regression analysis was performed to assess the presence of any correlation between intervention duration (weeks) and dose of CLA supplementation with BM, BMI, WC, FM, BFP and FFM values. However, the meta-regression results demonstrated no significant linear relationship between changes in BM, BMI, WC, FM, BFP and FFM with the dose and duration of the intervention (online Supplementary 5 and 6).
Grading of evidence
An evaluation of the quality of evidence using the GRADE approach is presented in Table 3. For BM and FFM, the quality of evidence was high since included RCT had a low to moderate risk of bias with low statistical and clinical heterogeneity and narrow CI. Moreover, moderate quality of evidence was detected for BMI, WC, FM and BFP because of existing very serious limitations for inconsistency (I 2 = 69·5 %, I 2 = 75·0 %, I 2 = 51·8 %, and I 2 = 66·6 % for heterogeneity, respectively).
Table 3. GRADE profile of CLA supplementation for on anthropometric indices and body composition
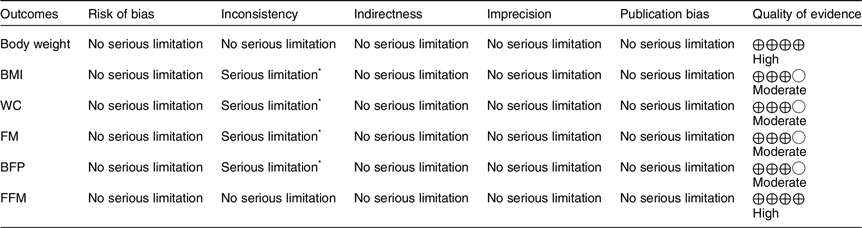
GRADE, Grading of Recommendations Assessment, Development, and Evaluation; CLA, conjugated linoleic acid; WC, waist circumference; FM, fat mass; BFP, body fat percentage; FFM, fat-free mass.
* There is significant heterogeneity for BMI (I 2 = 70·6 %), WC (I 2 = 76·0 %), FM (I 2 = 51·6 %) and BFP (I 2 = 67·2 %).
Sensitivity analysis
Sensitivity analysis revealed that no particular RCT significantly influenced outcomes (BM, BMI, WC, FM, BFP and FFM) compared with others in a given data set (pooled effect).
Discussion
This meta-analysis showed that CLA supplementation decreased BM, BMI, WC, FM and BFP and increased FFM. It should be noted that the weight-loss properties of CLA were small and may not reach clinical importance. Based on subgroup analysis, CLA supplementation reduced BM and BMI only in overweight/obese individuals and those consuming more than 3 g/d of CLA. Moreover, CLA supplementation decreased WC in obese participants, dose ≥ 3 g/d and duration < 12 weeks. Regarding body composition indices, CLA supplementation only reduced FM in overweight/obese and in healthy participants. CLA intake as a dietary supplement increased FFM in healthy participants, normal BMI, dose ≥ 3 g/d and duration ≥ 12 weeks. Body composition improvement seems to be only statistically significant in females, not men. Meanwhile, a subgroup based on the quality of studies showed that high-quality studies failed to show the fat loss effects of CLA supplementation. However, high-quality studies showed a small but significant decrease in BM and BMI and an increase in FFM. The time–response model revealed that the optimal duration of CLA supplementation for reducing BFP was around 6 to 7 weeks. Variability in body fat distribution and susceptibility to obesity may explain this study’s failure to show a similar dose–response relationship of CLA supplementation with BFP, BM, BMI, WC, FM and FFM.
Numerous mechanisms of action in regulating body anthropometrics and composition following ingestion of CLA have been suggested, resulting from animal and human studies. For example, CLA seems to enhance fat mobilisation and oxidation, reduce the size of adipocytes, regulate lipolysis by adipocytes, increase apoptosis in preadipocytes and adipocytes, reduce adipocyte differentiation through interaction with PPAR-γ, and modulate cytokines-/adipokines-associated mechanisms(Reference Chen and Park108). Despite promising results in animal studies regarding an inverse relationship between CLA and obesity, evidence in humans supporting the role of CLA in reducing BM and improving repartitioning of body fat and FFM is limited.
As noted, early meta-analytic work by Whigham et al. (2007), having focused on the effects of CLA supplementation on body composition in the general adult population, indicated that by pooling effect estimates of eighteen RCT, CLA ingestion promoted moderate alterations in BF(Reference Whigham, Watras and Schoeller34). Moreover, Schoeller et al. (2009) conducted a meta-analytic study focused on eighteen trials, illustrating a small increase in FFM following CLA treatment(Reference Schoeller, Watras and Whigham35). Subsequent meta-analyses have focused on specific population outcomes, such as those who are overweight and/or obese, individuals with metabolic syndrome and postmenopausal women. For instance, Onakpoya et al. (2012) performed a meta-analysis on a select number of studies (n 7) and reported that consuming CLA for more than 6 months resulted in small yet significant reductions in BM, BMI and FM, along with no change in WC in overweight and obese and individuals(Reference Onakpoya, Posadzki and Watson32). Namazi et al. study (2019), working with thirteen trials utilising overweight and obese participants, showed that CLA slightly reduced BM, BMI, and FM and slightly increased lean body mass. Yet, these authors similarly reported no influence of CLA on WC measurements(Reference Namazi, Irandoost and Larijani31). A meta-analysis by Kim et al. (2016) conducted on nine RCT in metabolic syndrome patients showed BM and BMI improvements following CLA consumption. However, neither body composition nor WC was considered, thus limiting any direct comparisons between body composition and anthropometric alterations(Reference Kim, Lim and Lee33). Lastly, a recent meta-analysis (n 8) involving female participants performed by Hamdallah et al. (2020) illustrated that consuming CLA for between 6 and 16 weeks had moderate effects on BM, BMI and total body fat, particularly in those classified as overweight/obese and postmenopausal status(Reference Hamdallah, Ireland and Williams109).
In contrast with the meta-analyses mentioned above, the present study’s findings analysing an accumulation of seventy RCT demonstrated a small but significant efficacy for CLA supplementation to reduce WC. Moreover, some previous studies revealed that CLA could be a moderate anti-obesity agent without generating clinically relevant effects. This effect owes to CLA’s relatively limited reductions in BM (upwards of 5 %) and FM (approaching 8 %), as noted in prior investigations(Reference Chen and Park108,Reference Ryan and Yockey110) . Further, CLA administration might aid in targeted FM reduction (e.g. central abdominal fat pattern) rather than a more evenly distributed reduction of whole-body fat. Such modest effects of CLA in addressing obesity may also be advantageous when the risk of weight gain is heightened at particular times of the year (e.g. social occasions, holidays, etc.).
It should be noted that RCT in this study frequently used various types of vegetable oils as a placebo, including sunflower, olive, soyabean, paraffin, rapeseed and safflower. These oils are rich in MUFA and PUFA, like oleic acid, linoleic acid and α-linolenic acid. Biohydrogenation of linoleic acid into CLA may occur through the bacteria in the digestive tract and via the mediation of vaccenic acid. While it is assumed that these oils have supplementary or complementary effects which can influence human health(Reference Benjamin, Prakasan and Sreedharan111), overall effect size differences between CLA supplementation v. placebo may be muted in certain RCT, and care should be taken in future investigations to avoid such confounding variables. It is also worth noting that type of CLA supplement (isomer or mixture) as well varies in RCT where the trans-10 and cis-12 isomers of CLA are suggested to induce catabolic effects, including enhanced lipolysis and fat oxidation, while cis-9 and trans-11 are considered anabolic agent(Reference MacRedmond, Singhera and Attridge94).
Furthermore, applying different body composition measurement methodologies in clinical trials (e.g. bioelectrical impedance analysis v. dual-energy X-ray absorptiometry v. skinfold calipers) may influence the interpretation and accuracy of results. To alleviate such concerns, further clinical trials comparing CLA supplement types and selecting gold standard body composition methodologies should be undertaken.
While rare, a few RCT analysed in this meta-analysis reported complications during or following CLA intervention, amongst which gastrointestinal disorders were the most common. However, such unwanted side effects were not serious in the CLA dosage range reported in RCT, and generally, CLA appears to be safe and well tolerated.
Of other note, CLA taken with other supplements, dietary restriction and increased physical activity may further promote the correction of anthropometric indices and body composition in obese individuals(Reference Akkila112). For example, combining CLA with γ-oryzanol significantly reduced body fat in overweight Korean female participants(Reference Kim, Kim and Ha82). Another investigation on well-trained young adults indicated that CLA along with creatine and whey protein consumption enhanced strength gains and lean mass following heavy resistance training(Reference Cornish, Candow and Jantz113). Therefore, CLA intake and other weight-reducing or body composition-modulating treatments may provide additional benefits.
Although there is evidence outlining the small but significant effects of CLA supplementations on body composition, little is known about the impact of gender differences on body composition changes induced following CLA consumption. The gender-specific effect of different dietary interventions is important because it is generally more difficult for females to lose BM(Reference Williams, Wood and Collins114). Females are also likely to lose less BM than males during a dietary intervention(Reference Williams, Wood and Collins114), although they are more likely to adopt and adhere to a diet initially(Reference Kashubeck-West, Mintz and Weigold115). Although the findings of the gender differences in body composition changes induced by CLA supplementation in humans are limited, our study showed that CLA supplementation may be more beneficial in women than men. Further studies are needed to evaluate the gender-specific effects of CLA supplementation on body composition.
Strengths of this meta-analysis include a relatively large number of RCT containing no observable publication bias, suggesting overestimation of the relationship between CLA and body composition indicators and/or anthropometric measurements were avoided. Moreover, findings from sensitivity analyses support the robustness of the results. Finally, the quality of evidence was moderate to high. Limitations that should be acknowledged include identifying the sources of heterogeneity for BFP needed to be elucidated, and individuals with varying degrees of health status and other characteristics were pooled for overall effect size analyses, thus contributing to a rather heterogeneous sample. Heterogeneity was encountered, perhaps due to various regimens, doses, types, duration, centre settings and populations enrolled. Significant heterogeneity is a serious limitation and should be included because it may significantly undermine the validity of the result. Subgroup analyses were performed to find probable sources of heterogeneity based on the duration of studies, intervention dosage, participants’ health condition, obesity status and sex. However, significant heterogeneity in all included variables remains a main limitation in our findings. Moreover, the varying risk of bias in the pool of studies is another main limitation of our analysis. Although we conducted a subgroup analysis based on the quality of studies to minimise the limitation, another drawback is the devices used for body composition analysis in the included studies. Different body composition assessment methods do not always similarly reflect changes in body composition associated with weight loss. Finally, the present study has not been registered in the PROSPERO; this could also be considered a limitation.
In conclusion, CLA supplementation significantly, albeit mildly, reduces obesity markers, including BM, BMI, WC and FM, while enhancing FFM in an adult population. More specifically, anthropometric measures (BM, BMI and WC) improved following 3 g/d or more of CLA regardless of intervention duration (except for WC, which favoured shorter dosage durations of <12 weeks). In contrast, body composition alterations (FM, FFM and BFP) improved regardless of intervention dosage or duration (except for FFM, which favoured CLA dosages 3 g/d or more and longer duration trials lasting >12 weeks). Certain additional participant characteristics such as being overweight/obese (BM, BMI, WC and FM), noted as having healthy status (FM and FFM) and having normal BMI (FFM) further delineated the significance of overall effect size results. It should be noted that the data from high-quality studies failed to show the body fat-lowering properties of CLA. Also, both overall effects and high-quality studies showed that CLA supplementation resulted in weight loss. However, it should be noted that the weight-loss properties of CLA were small and may not reach clinical importance. It has been mentioned that the minimal clinically important difference is classified as clinically important and is considered the smallest effect required to produce clinically important results(Reference Setayesh, Ashtary-Larky and Clark116). The data for minimal clinically important difference regarding body composition are limited; however, a wide range of studies have confirmed that the risk of metabolic disorders could be decreased whenever they saw reductions of 5 % of initial weight(Reference Ashtary-Larky, Ghanavati and Lamuchi-Deli117,Reference Nascimento, Amorim and Alves118) . Warkentin et al. showed that weight reductions to achieve minimal clinically important difference for most health-related quality-of-life instruments are markedly higher than the conventional threshold of 5 % to 10 %. Future investigations should also determine the best combination of CLA with other anti-obesity agents to promote additional benefits of health-related BM parameters. Finally, to improve the continuity of results, the composition of fatty acids in a placebo should be carefully considered when investigating the effects of CLA in various populations.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
O. A. contributed to the conception and design of the study; G. S. and M. Z. contributed to data extraction; S. S. and R. B. screened articles for inclusion criteria; O. A. contributed to data analysis, A. W., M. N., S. R., D. A. L. and K. N. contributed in manuscript drafting; O. A. and M. N. supervised the study. All authors approved the final version of the manuscript.
The authors declared that there is no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523001861








