It is common practice for ruminant livestock to be supplemented with minerals, either in compound feeds or as mineral blocks. Crosby et al. (Reference Crosby, Boland, Brophy, Quinn, Callan and Joyce2004) noted that when housed pregnant ewes were individually penned and given access to mineral blocks, intakes were in excess of 100 g/d. Importantly, it was also noted that the concentration of immunoglobulin (Ig) G in the serum of the lambs at 24 h of age was only 6·8 g/l, relative to 18·8 g/l for lambs from ewes that had received no block in late pregnancy. Adequate Ig absorption is critical for the subsequent health of the neonate, and a deficiency is associated with an increased incidence of pneumonia, septicaemia, infections of the navel and diarrhoea, all of which lead to increased mortality (Christley et al. Reference Christley, Morgan, Parkin and French2003). When the elements that comprised the block were separately omitted and the remaining elements were fed to pregnant sheep, only I was associated with the depression in plasma IgG concentration (Boland et al. Reference Boland, Brophy, Callan, Quinn, Nowakowski and Crosby2004).
In these experiments, the voluntary intake of I by the pregnant ewes was approximately 43 mg/d, whereas the I requirement of late pregnant ewes is thought to be less than 1 mg/d (Agricultural Research Council, 1980; National Research Council, 1985). Excessive I intake by pregnant ewes possibly causes a change in the physiology of the lambs prior to birth that lowers circulating concentrations of Ig, as there is no effect of treatment with I on the yield or quality of the dams' colostrum (Boland et al. Reference Boland, Guinan, Brophy, Callan, Quinn, Nowakowski and Crosby2005). Furthermore, when the progeny of mineral-supplemented ewes were fed either their own dam's colostrum or that of a control ewe, the concentration of Ig in the lambs' plasma was equally low (Boland et al. Reference Boland, Guinan, Brophy, Callan, Quinn, Nowakowski and Crosby2005).
To date, experiments investigating this phenomenon have allowed lambs to suckle their own dams. The yield of colostrum and its concentration of IgG are highly variable between ewes (Pattinson & Thomas, Reference Pattinson and Thomas2004). Accordingly, the aim of the present study was to establish the dose–response relationship between I in the diet of pregnant ewes and the concentration of plasma IgG after a controlled amount of colostrum fed to neonatal lambs. Plasma concentrations of hormones and metabolites were also measured in order to assess the likely mechanism by which excessive I inhibits Ig uptake by the neonate.
Materials and methods
Animals, feeds and experimental design
All experimentation was approved by the University of Wales, Aberystwyth, Ethical Review Committee. Using raddled Texel rams, approximately 200 Mule (Blueface Leicester cross Hardy Speckleface) ewes were predicted to lamb between 21 and 27 March 2005. Later in pregnancy, approximately eighty of these ewes were identified as carrying twin lambs; sixty ewes were selected at random in order to be assigned to the experimental groups. Lambs born between 19 and 29 March 2005 were used in the experiment.
Six weeks before lambing was predicted to begin, the ewes were allocated to one of five groups (twelve ewes per group). Each group was balanced for age, weight and condition score of the ewe. Body condition was assessed on a scale of 0 to 5 (Cooper, Reference Cooper and Soffe2003). The average age, weight and condition score of the ewes was 3·5 (se 0·1) years, 69·4 (se 1·0) kg and 3·1 (se 0·1), respectively; these values were not significantly different between the groups. The five groups received a concentrate meal supplemented with 0, 5·0, 9·9, 14·8 or 19·7 mg I/kg DM (Target Feeds, Whitchurch, UK), incorporated as calcium iodate. The measured concentrations of I in the feeds were 0·2, 5·5, 9·9, 14·8 and 21·0 mg/kg DM. Throughout this paper, reference is made only to these measured concentrations. The ingredients of the meal were ground barley (34 %), cooked ground maize (100 g/kg), rolled oats (100 g/kg), wheat feed (100 g/kg), hipro-soya extract (100 g/kg), sugar beet pulp (100 g/kg), prairie meal (50 g/kg), full-fat soya (50 g/kg), molasses (25 g/kg), vitamins and minerals (without supplemental I; 28·5 g/kg), and soya oil (6·5 g/kg). The ewes were group-fed.
For the first week that the ewes were housed, the concentrate allocation was 0·22 kg DM/d for each ewe. This amount was then increased by 0·22 kg DM/d per ewe at the start of each of the following 3 weeks. Then, from 3 weeks before lambing was expected to begin and until each ewe lambed, the allocation of concentrate per ewe was maintained at 0·87 kg DM/d. The ewes also had access ad libitum to grass hay and molasses (SVG Intermol, Liverpool, UK). Intakes of hay and molasses were not recorded. Fresh water was freely available at all times. Samples of feed were taken each week and bulked. The crude protein, acid detergent fibre, neutral detergent fibre, ether extract and ash contents of the feeds were determined (Association of Official Analytical Chemists, 1996). The in vitro digestibility of the feeds was determined according to Tilley & Terry (Reference Tilley and Terry1963). The I content of the feeds was determined according to Vanhoe et al. (Reference Vanhoe, Vanallemeersch, Versieck and Dams1993). The average analysis for each feed is shown in Table 1.
Table 1 Feed analyses (g/kg DM, unless stated otherwise)

Lambing
When each ewe lambed, the ewe and lambs were immediately removed to an individual pen. The lambs were then weighed, their navels were disinfected, and they were given an oral dose of a prophylactic antibiotic (Orojet; Willows Francis Veterinary, Crawley, UK). Small pens were constructed in the corner of the dam's pen in order to prevent the lambs from suckling but still allow contact between the ewe and dam. Thirty minutes after lambing, a jugular vein blood sample was taken from each lamb. Following this, the lambs were bottle fed 10 g artificial ewe colostrum powder/kg live weight (Ovicol; Farmsense, Lytham, UK), made up according to the manufacturer's instructions. The colostrum replacer contained 39 g IgG/kg. Any refusals were noted, and the bottles were reoffered to the lambs 30 min later. Further meals of artificial colostrum, fed at a higher rate (13·3 g/kg live weight) were offered to the lambs at 6, 12 and 18 h after birth. Accordingly, a 5 kg lamb received 9·7 g IgG over the first 24 h of life. This is lower than the amounts of IgG fed in other experiments (Boland et al. Reference Boland, Brophy, Callan, Quinn, Nowakowski and Crosby2004, Reference Boland, Guinan, Brophy, Callan, Quinn, Nowakowski and Crosby2005; Crosby et al. Reference Crosby, Boland, Brophy, Quinn, Callan and Joyce2004) and reflects the lower concentration of IgG in commercial artificial colostrums relative to that of the ewe. The artificial colostrum was manufactured from bovine colostrum as artificial colostrum containing ovine antibodies was not available. Bovine IgG administered orally to newborn lambs is absorbed and has been shown to confer passive immunity (Quigley et al. Reference Quigley, Carson and Polo2002).
Excessive pressure in the ewe's udder during the first 24 h after lambing was relieved by milking. At 24 h after birth, a further jugular vein blood sample was taken from the lambs, which were then were returned to their dams and allowed to suckle freely. The ewes and lambs spent a further 24–48 h in the pen in order to ensure that the lambs were suckling properly and that maternal bonding had taken place before turnout to pasture.
Approximately 3 weeks before lambing, the ewes fed the concentrate without I supplementation began to lose their appetite for it. During the following weeks, two of the ewes in the 5·5 mg I/kg concentrate also began to stop eating the meal. All of these ewes were removed from the experiment and transferred onto a commercial pelleted pregnant ewe concentrate. The other ewes continued to eat the meal each time it was offered, throughout the experiment. During the period of data collection, eight, twelve, ten and nine ewes lambed from the 5·5, 9·9, 14·8 and 21·0 mg/kg groups, respectively. The remaining ewes from each group did not lamb within the period of data collection. The ewes that lambed produced sixteen, twenty-four, twenty and eighteen lambs, respectively. Of these, two lambs died shortly after birth (one each from the 5·5 and 14·8 mg/kg groups). No data from these lambs were used in the statistical analysis. One lamb was removed from a ewe because she did not have enough milk (14·8 mg/kg group). A further lamb died at 4 weeks of age through misadventure (9·9 mg/kg group). For these lambs, the data collected in the first 24 h of life were used in the statistical analysis, but further data were not used. The weights of the remaining lambs were recorded at 5, 10 and 14 weeks of age.
Plasma sample analysis
All blood samples were immediately mixed with heparin and were centrifuged at 1500 g for 30 min within 30 min of collection. The samples were then frozen at − 20°C until further analysis. Using commercially available kits and according to the respective manufacturers' instructions, the plasma samples were analysed for insulin by RIA (MP Biochemicals, New York, USA; intra-assay CV 0·055, interassay CV 0·061), free tri-iodothyronine (T3) by RIA (MP Biochemicals; intra-assay CV 0·111, interassay CV 0·113), total (bound and free) thyroxine (T4) by RIA (MP Biochemicals; intra-assay CV 0·063, interassay CV 0·097) and bovine IgG by ELISA (Alpha Diagnostic, San Antonio, TX, USA; intra-assay CV 0·127, all samples assayed in the same assay). The plasma samples were also analysed for cortisol by RIA (ICN Pharmaceuticals, New York, USA; intra-assay CV 0·045, interassay CV 0·075), glucose by colorimetric assay (Sigma-Aldrich, Gillingham, UK; intra-assay CV 0·042, interassay CV 0·039) and NEFA by colorimetric assay (Wako Chemicals, Richmond, VA, USA; intra-assay CV 0·035, interassay CV 0·026).
Calculations and statistics
The following equation was used to calculate the apparent efficiency of IgG appearance in blood plasma as a proportion of that fed:Efficiency (g/kg) =
This calculation assumes that the plasma of blood in newborn lambs is 75 ml/kg, as for calves (reviewed by Quigley et al. Reference Quigley, Hammer, Russel, Polo and Garnsworthy2005).
The plasma concentrations of insulin were not normally distributed, and data were normalised using base ten logarithmic transformation. The data were analysed by fitting general linear models, using Genstat statistical software (version 8.1; Lawes Agricultural Trust, Rothamsted, UK) to evaluate the fixed effects of treatment group, sex of the lamb and their interaction. The effects of sex were not significant (P>0·05) for any trait. Least squares means are presented for models that fitted only the fixed effect of group. Polynomial (linear, quadratic, cubic) effects of the level of I in the ewe concentrate on the parameters measured were tested. In all cases, the quadratic and cubic effects were not significant and are not shown in the results. When there was a significant (P < 0·05) linear effect of dietary treatment, differences between individual treatments were determined using the least significant difference test, and these are presented in the text.
Results
The mean plasma concentration of IgG in the lambs at 24 h of age is shown in Table 2. As the concentration of I in the concentrate increased, the concentration of IgG in the plasma significantly decreased (P < 0·001). Plasma IgG values in the 14·8 and 21·0 mg/kg supplementation groups were approximately half of those seen in the 5·5 mg/kg group. The values for the 5·5 and 9·9 mg/kg groups were both significantly higher than those for the 14·8 and 21·0 mg/kg groups (P < 0·05; least significant difference test). Similarly, the proportion of the Ig fed that appeared in the blood plasma (efficiency of IgG appearance) for the 5·5 mg/kg I supplementation concentrate was approximately twice as great as those for the 14·8 and 21·0 mg/kg groups. Again, the values for the 5·5 and 9·9 mg/kg groups were both significantly higher than those for the 14·8 and 21·0 mg/kg groups (P < 0·05; least significant difference test).
Table 2 Plasma concentrations of immunoglobulin G in lambs at 24 h of age, and tri-iodothyronine (T3), thyroxine (T4), insulin and cortisol in lambs at 30 min (birth) and at 24 h of age

*P < 0·05; **P < 0·01; ***P < 0·001.
† Maximum standard error of the difference between treatment groups.
‡ Values in brackets are back-transformed from the logarithm values.
The mean plasma concentrations of hormones and metabolites in the lambs at 30 min after birth and at 24 h of age are shown in Table 2. The plasma concentration of T3 in the lambs at 24 h after birth was not significantly affected by the dietary treatments. However, the plasma concentrations of T3 at birth, and the plasma concentrations of T4 at birth and at 24 h after birth, were significantly higher in the lambs from ewes given the greater I inclusion rates (P < 0·05, P < 0·001 and P < 0·01, respectively). The values for the 5·5 and 9·9 mg/kg groups were not significantly different (P>0·05), but they were significantly lower than the values for the 14·8 mg/kg group (P < 0·05, least significant difference test) for T3 at birth and for T4 at birth and 24 h after birth. There were no significant effects of dietary I treatment on the plasma concentrations of insulin, cortisol, glucose or NEFA in the lambs, at either 30 min or 24 h of age (P>0·05).
The mean weights and growth rates of the lambs are shown in Table 3. The lambs' birth weights significantly increased (P < 0·01) with an increasing inclusion rate of I. This was largely a function of a relatively low mean birth weight for lambs from the 5·5 mg/kg supplementation group. There was no statistical difference in the mean live weight of the lambs between the groups during the following weeks (P>0·05). There was also no difference between the groups in the mean daily live weight gain of the lambs.
Table 3 Mean weights of the lambs at birth and at 5, 10 and 14 weeks of age
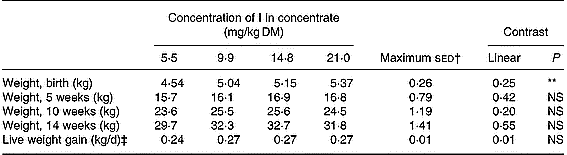
** P < 0·01.
† Maximum standard error of the difference between treatment groups.
‡ Average daily live weight gain between birth and 14 weeks of age.
Discussion
In this study, late-pregnant ewes were fed with concentrate containing 5·5, 9·9, 14·8 or 21·0 mg I/kg DM, which corresponded to an average consumption of 4·8, 8·6, 12·9 or 18·3 mg I/d per ewe during the last 3–4 weeks of pregnancy, respectively. The data demonstrate that the amount of IgG that appeared in the blood plasma of the lambs decreased with increasing I in the diet; this effect was particularly marked between I supplementation rates of 9·9 and 14·8 mg/kg DM. There were insufficient lambs in the present experiment to determine whether the lower IgG concentrations of the high-I treatments were associated with an increased incidence of disease in the subsequent weeks. Nevertheless, low Ig levels in the neonate are firmly associated with impaired health in all classes of mammalian livestock (Korhonen et al. Reference Korhonen, Marnila and Gill2000).
A number of studies have recently demonstrated the negative effect of I on plasma Ig concentrations in lambs, albeit at supplementation rates substantially higher than those of the present experiment (Boland et al. Reference Boland, Brophy, Callan, Quinn, Nowakowski and Crosby2004; Crosby et al. Reference Crosby, Boland, Brophy, Quinn, Callan and Joyce2004; Guinan et al. Reference Guinan, Harrison, Boland and Crosby2005). In agreement with the present study, Boland et al. (Reference Boland, Guinan, Brophy, Callan, Quinn, Nowakowski and Crosby2005) demonstrated that I fed at 17·7 mg/d to pregnant ewes in the lead-up to lambing suppressed the plasma concentration IgG in the lambs after colostrum feeding, but that when the I was fed at 8·9 mg/d, there was no significant effect, relative to a basal diet assumed to provide less than 1 mg I/d. When lambs are fed their own dam's colostrum, however, the yield of IgG is highly variable between ewes (Pattinson & Thomas, Reference Pattinson and Thomas2004), and it is unclear how much impact this might have had on the results obtained. The lambs in the present experiment were fed a consistent amount of artificial colostrum, and the results clearly show a significant effect of elevated levels of I supplementation on IgG concentrations, particularly when supplemental I was fed at 14·8 mg I/kg DM (or 12·9 mg I/d per ewe) or more, relative to the two lowest rates of addition. Assuming that the total DM intake of the late pregnant ewes was 1·3 kg DM/d (AFRC, 1993), the I intake from the hay and molasses components of the diet would have been approximately 0·1 mg/d, and thus trivial relative to the amount of I in the concentrates.
A survey of three feed manufacturers indicated that I is typically included in concentrates for pregnant ewe at approximately 9 mg/kg DM, whereas the requirements of sheep for I are said to be in the region of 1 mg/d (Agricultural Research Council, 1980; National Research Council, 1985). The consumption of I will be higher still when sheep are also offered mineral blocks, which tend to include a high level of I (Boland et al. Reference Boland, Guinan, Brophy, Callan, Quinn, Nowakowski and Crosby2005), or in locations near the sea, where forages tend to have greater concentrations of I (Fuge, Reference Fuge, Selinus, Alloway, Centeno, Finkleman, Fuge, Lindh and Smedley2005). Therefore, given that the concentration of I that we identify as the upper safe limit in the present experiment was only marginally greater than commercial practice, there is possibly a need to consider a revision of the I inclusion rate in commercial concentrates to provide a greater safety margin in order to avoid a reduction in the absorption of IgG.
In cattle (the species from which the artificial colostrum fed was derived), IgG accounts for approximately 80 % of the total Ig present. The remaining antibodies are largely of the IgA and IgM types (Butler, Reference Butler, Mestecky, Bienenstock, Lamm, Strober, McGhee and Mayer1999); these were not measured in the present experiment. In neonatal farm livestock species, a plasma concentration of total Ig of less than 10 g/l after 24 hours of age is widely considered to indicate impaired passive immunity (Quigley et al. Reference Quigley, Hammer, Russel, Polo and Garnsworthy2005). Crucially, all of the lambs that had derived total Ig levels (IgG concentration divided by 0·8) of more than 10 mg/kg were in the two lowest I supplementation groups (five of the sixteen lambs in the 5·5 mg/kg group, and four of the twenty-four lambs in the 9·9 mg/kg group). However, even though the amount of colostrum fed over the first 24 h of life was similar to that normally consumed by lambs suckling naturally (Boland et al. Reference Boland, Brophy, Callan, Quinn, Nowakowski and Crosby2004), most of the lambs had relatively low concentrations of IgG. This was probably due to the lower IgG concentration of the artificial colostrum used in the present experiment relative to ovine colostrum (Boland et al. Reference Boland, Brophy, Callan, Quinn, Nowakowski and Crosby2004). The lower plasma IgG concentration of the lambs in the present experiment was also possibly due to delayed feeding of much of the artificial colostrum until after the period of most efficient Ig absorption. A second feed of colostrum at 4 h after birth, and then every 4 h (rather than feeds at 6-hourly intervals, as in the present experiment) might have ensured that more of the antibody was consumed by the lambs during the period of maximal antibody absorption.
The only known physiological role of I in animals is as a constituent of the thyroid hormones T3 and T4 (Underwood & Suttle, Reference Underwood and Suttle1999). T3 is the more active of the two hormones, although both hormones are thought to have a very wide range of functions as regulators of cell activity and proliferation (Stanbury, Reference Stanbury, Ziegler and Filer1996). The peripheral concentration of T3 largely depends on the local deiodination of T4 and consequently on the activity of T4 deiodinase enzymes (Goglia et al. Reference Goglia, Moreno and Lanni1999). In the present experiment, the higher-I treatments were associated with higher levels of T3 and T4 in both the ewes (data not shown) and lambs.
It has long been recognised that increasing the supply of I to farm livestock increases the circulating concentrations of thyroid hormones (Aumont et al. Reference Aumont, Lamand and Tressol1989a, Reference Aumont, Levieux, Lamand and Tressolb). In the pregnant rat, subcutaneous T4 injections immediately prepartum have been shown to reduce the intestinal permeability to macromolecules in the postpartum period (Chan et al. Reference Chan, Daniels and Thomas1973). Furthermore, the injection of T4 into the amnios once every 7 d from 30 d before expected parturition in pregnant goats reduced the duration over which IgG could be absorbed (Cabello et al. Reference Cabello, Levieux and Lefaivre1980). In calves, however, T4 injections immediately post partum did not influence the absorption of IgG, although there was a significant negative correlation between the plasma level of T4 at birth (before the T4 injection) and the apparent efficiency with which Ig could be absorbed (Cabello & Levieux, Reference Cabello and Levieux1978, Reference Cabello and Levieux1982). Thus, although there are substantial differences in the mechanisms of Ig absorption between species (particularly between ruminants and the rat), it seems that it may be elevated levels of circulating T4 and T3 in the fetus immediately before parturition that is negatively associated with low concentrations of Ig in the blood after birth.
The absorption of Ig into the gut epithelial cells of sheep occurs non-selectively by pinocytosis (Butler, Reference Butler, Mestecky, Bienenstock, Lamm, Strober, McGhee and Mayer1999; Quigley et al. Reference Quigley, Hammer, Russel, Polo and Garnsworthy2005). Ig is then transported through the mucosal layer into the lymphatic system and then into the blood. Meanwhile, Ig of all classes are also selectively re-exported to the gut mucosal membrane, where they act to block the attachment of pathogenic micro-organisms to endothelial receptors. The site of maximal Ig absorption is in the lower ileum, although absorption can occur along the length of the small intestine (Fetcher et al. Reference Fetcher, Gay, McGuire, Barbee and Parish1983). It is not known whether the effect of I in the present experiment was to reduce the period of time during which the gut of the lamb was open to Ig transport, or to reduce the efficiency of Ig transport during the whole of the first 24 h of life. Alternatively, the effect of the I treatments might have been to affect the rate of IgG turnover rather than IgG absorption in the lamb.
Additionally, it remains to be established whether dietary I for the dam causes Ig to be redeposited to a greater extent on the gut wall or in the lymphatic system, rather than affecting the rate of Ig absorption from the lamb's gut. Whether or not there is an effect of ewe or sire breed on the effect of I in this context also remains to be determined. For example, lambs of breeds that have developed in close proximity to the sea, such as the North Ronaldsay, may have a greater resistance to excessive I levels in the diets of their dams. Further research, utilising genomic and proteomic approaches, will be required in order to answer these questions.
In conclusion, relative to feeding an additional 9·9 mg I/kg DM in pregnant ewe concentrate, 14·8 mg I/kg DM significantly reduced the concentration of IgG in the blood plasma of neonatal lambs following the consumption of colostrum. This was associated with elevated levels of T3 at birth and T4 at birth and at 24 h of age. The mechanism by which elevated levels of thyroid hormones may suppress the concentration of IgG in the plasma of neonatal lambs remains to be determined.
Acknowledgements
The authors thank Hybu Cig Cymru (Meat Promotion Wales) for their financial support of this project, and three anonymous feed manufacturers for divulging the I content of their pregnant ewe concentrates. The authors gratefully acknowledge the technical assistance given by Maddy Lewis, Joss Rees, Jenny Evans, Lucy Hill, Samara Johnstone, Trefor Roberts, Mina Davies-Morel and Brian Davies.





