CVD is the most common cause of death in the world( Reference Pagidipati and Gaziano 1 ). Approximately one third of deaths are related to CVD( Reference Pagidipati and Gaziano 1 ). In 2010, the prevalence of CVD among individuals over 65 years old was 19·8 % in the USA( 2 ).
Older people are at higher risk of obesity( Reference Guarner and Rubio-Ruiz 3 ). Obesity with insulin resistance is associated with hyperinsulinaemia( Reference Nagasaki, Hara and Ogawa 4 ) and hyperinsulinaemia can lead to dyslipidaemia( Reference Haffner, Fong and Hazuda 5 ), high blood pressure( Reference Fournier, Gadia and Kubrusly 6 ) and inflammation( Reference Dandona, Aljada and Bandyopadhyay 7 ). Moreover, obesity in the elderly can increase inflammatory parameters that lead to dyslipidaemia and insulin resistance( Reference Guarner and Rubio-Ruiz 3 ).
The potential use of diet to induce postprandial insulin secretion is likely to be critical for managing dyslipidaemia, weight gain and inflammation( Reference Hensrud 8 – Reference Setorki, Nazari and Asgary 12 ). Although evidence has been accumulating regarding specific dietary factors and insulin resistance( Reference Pereira, Jacobs and Pins 13 – Reference Zhang, Lu and Zheng 15 ), dietary indices that examine the overall dietary patterns may be more informative. Dietary insulin load (DIL) is an example of one such index( Reference Mirmiran, Esfandiari and Bahadoran 16 ).
The insulin index represents the insulin response to isoenergetic components of foods in comparison to a reference food (glucose or white bread)( Reference Holt, Miller and Petocz 17 ). Insulin index is based on postprandial insulin secretion that is evoked through mixed meals( Reference Holt, Miller and Petocz 17 ). This index takes into account not only carbohydrate-containing foods but also high-fat, high-protein foods and their interactions( Reference Bao, de Jong and Atkinson 18 ). Given that insulin index is based on insulin secretion, a link between insulin exposure and propensity to chronic diseases might exist( Reference Nimptsch, Brand-Miller and Franz 11 , Reference Bao, Nimptsch and Meyerhardt 19 ). DIL is another dietary index that is estimated through multiplying the reported insulin index value of each food by its energy content and the frequency of consumption of each food( Reference Mirmiran, Esfandiari and Bahadoran 16 ).
A Finnish study with 22 years of follow-up demonstrated that insulin was a suitable predictor of coronary disease( Reference Pyörälä, Savolainen and Kaukola 20 ). Limited research exists on the association between insulin indices and CVD risk factors, with existing literature lacking systematic evaluation across studies focusing on the same risk factors. Mirmiran et al. ( Reference Mirmiran, Esfandiari and Bahadoran 16 ) showed an inverse association between DIL and insulin resistance. Nimptsch et al. ( Reference Nimptsch, Brand-Miller and Franz 11 ) also found an inverse association between DIL and HDL-cholesterol and a positive association between DIL and TAG, particularly among obese individuals. In a prospective study by Joslowski et al. ( Reference Joslowski, Goletzke and Cheng 21 ), DIL was associated with body fat mass, while no relation to BMI was observed.
Elderly subjects might be more at the risk for insulin resistance due to their body compositions and metabolic profiles,( Reference Guarner and Rubio-Ruiz 3 ) calling for the examination of the association between the DIL and cardiovascular risk factors in this population. Moreover, men are at higher risk of CVD compared with women( Reference Weidner 22 ). Research shows that CVD develops approximately 7–10 years earlier in men compared with women( Reference Maas and Appelman 23 ). Due to the limited studies on DIL and its association with cardiovascular biomarkers, our aim was to examine the association between DIL and cardiovascular risk factors in elderly men.
Methods
Study population
To date, little attention has been paid to men, especially the elderly men, so we prioritised this group in our study. In this cross-sectional study, we used clustered random sampling to select men referred to ten health centres in southern Tehran, Iran (March to August 2017). To calculate the number of men to be sampled from each health centre, the total population served by each centre was represented proportionally in the calculated sample size (n 313). We included men over the age of 60 years who were not already adhering to specific diets. Men were excluded if they had any malignant disease such as cancer. Moreover, we excluded subjects from our analyses with under- and overreported total energy intake (<3347 and >17573kJ/d)( Reference Saraf-Bank, Haghighatdoost and Esmaillzadeh 24 ). High-sensitive C-reactive protein (hs-CRP) was considered the main dependent variable for calculating the sample size( Reference Fard, Karimi and Baghaei 25 ). For the sample size calculation, we defined α=0·05, d=4 % and the effect size=1·5. Finally, based on hs-CRP values, we determined that 313 individuals were needed. However, to compensate for potential exclusion of participants due to under- and overreporting of total energy intake, 365 subjects were selected for inclusion. After exclusion of participants who under- and overreported the total energy intake (n 8), 357 remained in the analysis. Therefore, under- and overreported total energy intake was the only reason for exclusion. Written informed consent was obtained from all participants. Ethical approval for this protocol was given by the National Institute for Medical Research Development (grant and ethics number: 965430).
Dietary assessment
Participants’ usual dietary intake was obtained using a 168-item semi-quantitative FFQ through face-to-face interviews with a trained nutritionist. The validity and reliability of this questionnaire has been previously reported to be adequate( Reference Mirmiran, Esfahani and Mehrabi 26 ). Participants were asked to report on average frequencies of their food consumption on a daily, weekly or monthly basis. The portion size of each food was translated from household measures into grams. An adapted version of NUTRITIONIST IV modified for Iranian foods (version 7.0; N-Squared Computing) was used to calculate mean energy and nutrient intakes( Reference Fard, Karimi and Baghaei 25 , Reference Azadbakht, Kimiagar and Mehrabi 27 , Reference Esmaillzadeh and Azadbakht 28 ).
Calculation of dietary insulin load
The insulin index of each food was extracted for analysis (based on methods outlined in Kirstine Bell’s thesis)( 29 ). Insulin index was defined as the AUC representing insulin (during 120 min) in response to intake of approximately a 1000 kJ portion of the test food, which then was divided by the area below the curve after consumption of an isoenergetic reference food( Reference Holt, Miller and Petocz 17 ). The average value of DIL for each study participant during the previous year was computed using FFQ data. In this fashion, the insulin index value of each food item was multiplied by its energy content and also by the frequency of consumption. Finally, all food item values were summed. The formula used was as follows:
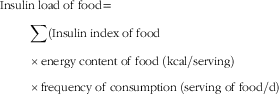 $$\eqalignno{ &{\rm Insulin}\;{\rm load}\;{\rm of}\;{\rm food {\equals}} \mathop \cr & \quad \hskip15pt {\sum}{{\rm (Insulin}\;{\rm index}\;{\rm of}\;{\rm food}} \cr & \quad \hskip15pt {{\times} {\rm energy}\;{\rm content}\;{\rm of}\;{\rm food}\;({\rm kcal}/{\rm serving})} \cr & \quad \hskip15pt {\times}{\rm frequency}\;{\rm of}\;{\rm consumption}\;({\rm serving}\;{\rm of}\;{\rm food/d}) $$
$$\eqalignno{ &{\rm Insulin}\;{\rm load}\;{\rm of}\;{\rm food {\equals}} \mathop \cr & \quad \hskip15pt {\sum}{{\rm (Insulin}\;{\rm index}\;{\rm of}\;{\rm food}} \cr & \quad \hskip15pt {{\times} {\rm energy}\;{\rm content}\;{\rm of}\;{\rm food}\;({\rm kcal}/{\rm serving})} \cr & \quad \hskip15pt {\times}{\rm frequency}\;{\rm of}\;{\rm consumption}\;({\rm serving}\;{\rm of}\;{\rm food/d}) $$
Biochemical assessment
For each subject, a single venous blood sample was taken after 12 h of fasting. Serum concentrations for fasting blood sugar (FBS), lipid profiles including total cholesterol, LDL-cholesterol, HDL-cholesterol and TAG were quantified using commercial enzymatic reagents (Pars Azmoon). Insulin serum levels were measured using the ELISA method (ELISA; Diagnostic Biochem Canada, Inc.). hs-CRP concentrations were assessed using an ultrasensitive latex-enhanced immunoturbidimetric assay (Randox Laboratory Ltd). Serum levels of inflammatory biomarkers were determined using the ELISA method (Boster Biological Technology for IL-6 and TNF-α). We used the Clauss clotting method that involves recording the rate of fibrinogen conversion to fibrin in the presence of thrombin. Insulin resistance and insulin sensitivity were assessed using the homoeostasis model assessment for insulin resistance (HOMA-IR)( Reference Matthews, Hosker and Rudenski 30 ) and the quantitative insulin-sensitivity check index (QUICKI)( Reference Katz, Nambi and Mather 31 ), respectively.
Anthropometric assessment
Anthropometric indices (body weight, height and waist circumference) were measured by a trained nutritionist. Body weight was measured using calibrated digital scales (SECA 813; Seca) after participants had removed their shoes and any heavy clothes. Body weight was reported within 100 g of precision. Height was measured using a tape metre (with measurement precision of 0·5 cm), while participants were standing against a wall and their shoulders were in a normal position. Waist circumference was measured at the narrowest point between the inferior rib and iliac crest over light clothing without applying pressure to the body. It was recorded to the nearest 0·5 cm. BMI was calculated as body weight in kg divided by height in m2.
Assessment of other variables
Blood pressure was measured twice while participants were in a seated position for 10 min. Participants waited at least 30 s between the first and second measurements. The average of the two readings was used as the final blood pressure. Socio-economic status (SES) was assessed using a questionnaire that has been validated and is reliable in the Iranian population and that was developed for measuring SES and its association with health outcomes( Reference Garmaroudi and Moradi 32 ). A total standardised score for all participants was computed (using factor analysis and a summary index), then its compliance with a normal summary index was also examined using a Kappa test. This questionnaire consists of questions about educational level, participant job, car or house ownership, having modern appliances, number of family members and trips inside or outside the country during the last year. The reported correlation of these parameters with the total score was 0·87. In the current study, participant SES was described for each category of DIL based on the calculated total scores.
Statistical analysis
The Kolmogorov–Smirnov test and histogram curves were used to examine whether variables had normal distributions. Parameters with normal distributions were presented as means and standard deviations. We categorised participant characteristics, dietary intake, anthropometric indices and biochemical parameters based on the median DIL scores. Basic participant characteristics were provided for the total population and each category of DIL. To investigate the differences in characteristics between categories of DIL, χ 2 (qualitative variables) and independent t tests (quantitative variables) were used. Dietary intakes within categories of DIL were compared using ANCOVA to adjust for daily energy intake. The levels of anthropometric measures, and biochemical parameters within categories of DIL were compared using independent t tests in crude models and ANCOVA in adjusted models. To assess the association between DIL and cardiometabolic risk factors, binary logistic crude and adjusted regression models were used. In the adjusted models, we controlled for a wide range of confounders (model 1: energy intake, marital status, SES and smoking; model 2: energy intake, marital status, SES, smoking, disease status, anti-diabetic drugs, thyroid drugs and heart disease drugs). The low category of DIL was considered the reference group and high and low categories were compared to predict the risk of CVD. Glycaemic control parameters and lipid profiles were considered primary outcomes, while inflammatory biomarkers were considered secondary outcomes. All statistical analyses were performed using SPSS software (version 18; SPSS Inc.). P<0·05 was considered statistically significant.
Results
The mean age of participants (n 357) was 64·96 years. General participant characteristics in the two DIL categories are represented in Table 1. A larger percentage of participants in the high category were married (P=0·003), had lower education levels (P=0·009), were non-smokers (P=0·0001), had no disease (P=0·02), did not use anti-diabetic drugs (P=0·02), thyroid drugs (P=0·001) or drugs for heart disease (P=0·0001).
Table 1 General participant characteristics and median dietary insulin loads (DIL) (Numbers and percentages; mean values and standard deviations)
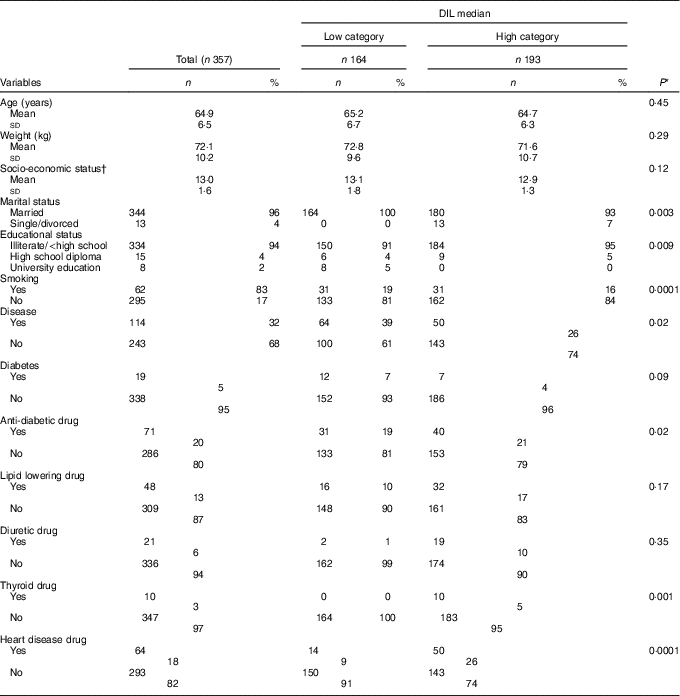
* Calculated using χ 2 tests and t tests for qualitative and quantitative variables, respectively.
† Socio-economic status; minimum: 10, maximum: 18.
Participant dietary intakes in each DIL category are represented in Table 2. Participants in the high DIL category had higher consumption of energy (P=0·0001), carbohydrates (P=0·0001), fruits (P=0·002), vegetables (P=0·006), meats (P=0·04) and grains (P=0·0001), compared with those in the low category. However, participants who were in the high category of DIL had lower fat (P=0·02) and oil consumption (P=0·0001).
Table 2 Energy-adjusted dietary intakes and medians of dietary insulin load (DIL) (Mean values and standard deviations)
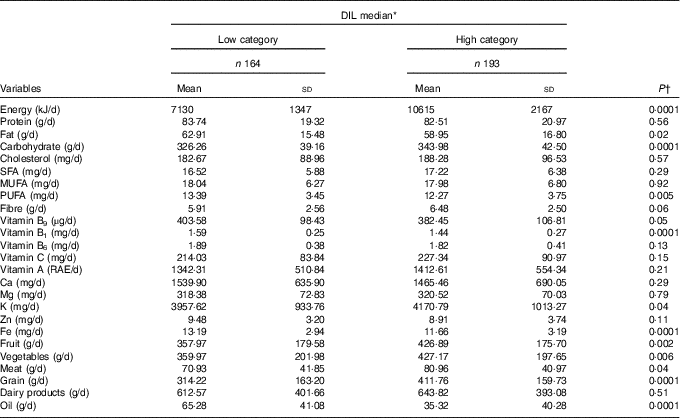
RAE, retinol activity equivalents.
* All the variables, except energy, were adjusted for energy intake.
† Calculated using multivariate ANCOVA.
Participants’ anthropometric measurements and biochemical markers are displayed in Table 3. With regard to blood pressure, participants in the high DIL category had higher systolic blood pressure (P=0·004), insulin (P=0·0001), HOMA-IR (P=0·005) and hs-CRP (P=0·04) levels compared with participants in the low category. However, the differences between anthropometric measurements, glycaemic parameters, lipid profiles, liver enzymes and inflammatory biomarkers did not significantly differ between elderly men classified in the low and high DIL categories.
Table 3 Medians of dietary insulin load (DIL) by anthropometric indices, biochemical markers and blood pressure (Mean values and standard deviations)
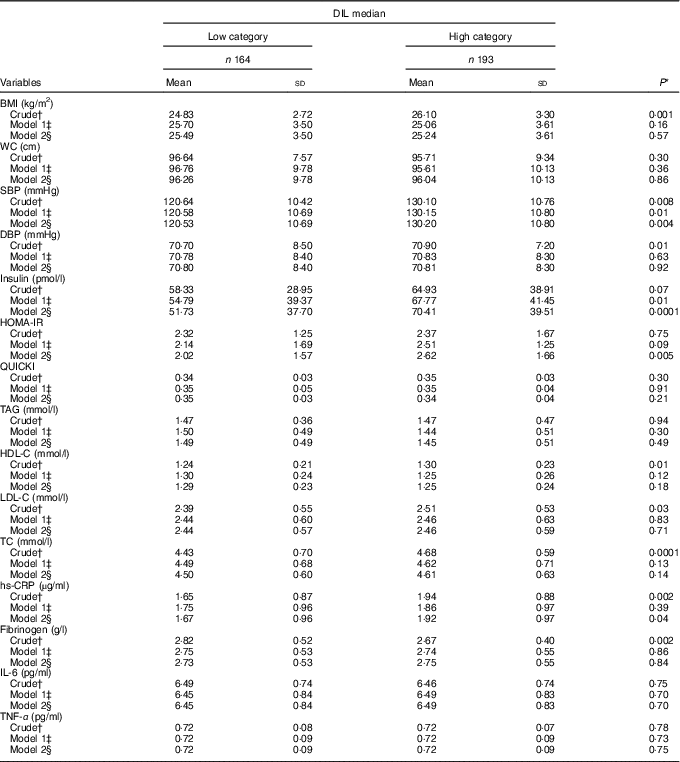
WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homoeostasis model assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol; hs-CRP, high-sensitive C-reactive protein.
* Calculated using t tests for the crude model and ANCOVA in the adjusted models.
† Crude: not adjusted for any variables.
‡ Model 1: this model was adjusted for energy intake, marital status (which includes educational level), socio-economic status and smoking.
§ Model 2: this model was adjusted for energy intake, marital status, socio-economic status (which includes educational level), smoking, disease, anti-diabetic drugs, thyroid drugs and heart disease drugs.
OR and 95 % CI for cardiovascular risk factors by medians of DIL are provided in Table 4. In subjects who had diets with high DIL, serum levels of FBS were 7·52 times greater than those with low DIL (OR: 7·52; 95 % CI 3·38, 16·75; P=0·0001). Moreover, subjects with high DIL showed 3·03 times greater hs-CRP levels than those with low DIL (OR: 3·03; 95 % CI 1·54, 5·94; P=0·001). No associations were found between high DIL and BMI (OR: 1·43; 95 % CI 0·75, 2·75; P=0·27), serum levels of TAG (OR: 0·82; 95 % CI 0·26, 2·59; P=0·73), HDL-cholesterol (OR: 2·03; 95 % CI 0·79, 5·23; P=0·13) or fibrinogen (OR: 1·57; 95 % CI 0·80, 3·06; P=0·18).
Table 4 Crude and multivariable OR and 95 % CI in medians of dietary insulin load (DIL) (Odds ratios and 95 % confidence intervals)
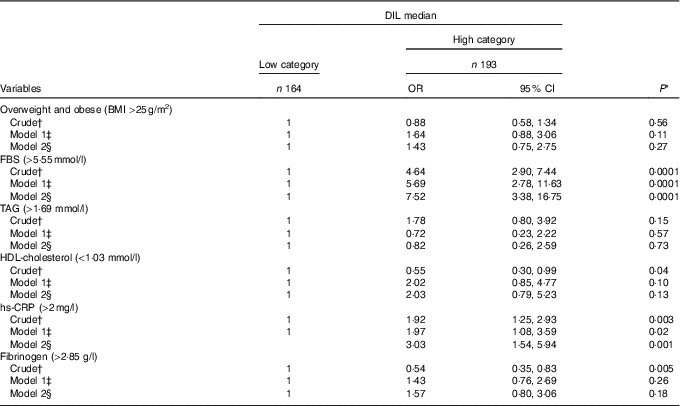
FBS, fasting blood sugar; hs-CRP, high-sensitive C-reactive protein.
* Calculated using logistic regression.
† Crude: not adjusted for any variables.
‡ Model 1: the model was adjusted for energy intake, marital status, socio-economic status (which includes educational level) and smoking.
§ Model 2: the model was adjusted for energy intake, marital status, socio-economic status (which includes educational level), smoking, disease, anti-diabetic drugs, thyroid drugs and heart disease drugs.
Discussion
In the present cross-sectional study, DIL was positively associated with serum levels of FBS and hs-CRP. However, there was no association between DIL and BMI or between DIL and lipid profiles. To the best of our knowledge, this is the first study, in which glycaemic parameters, lipid profile and also inflammatory biomarkers were investigated to provide better insight into the association between DIL and CVD risk factors in elderly men.
DIL is an indicator that adequately reflects insulin secretion of the whole diet, rather than a single nutrient( Reference Bao, de Jong and Atkinson 18 ). In the field of nutritional epidemiology, DIL is a suitable indicator to examine the link between insulin exposure and the development of metabolic diseases( Reference Nimptsch, Brand-Miller and Franz 11 , Reference Bao, Nimptsch and Meyerhardt 19 ). Apart from carbohydrates, dietary protein and fat can affect insulin secretion( Reference Collier, Greenberg and Wolever 33 – Reference Gannon, Nuttall and Westphal 35 ). Therefore, macronutrients might act synergistically to increase insulin secretion and reduce blood glucose levels( Reference Collier, Greenberg and Wolever 33 – Reference Azadbakht and Esmaillzadeh 36 ). White bread, potato, skim milk, low-fat ice cream or yogurt, melon, fruit juice, canned fruits, jam, chocolate and jelly beans are examples of food items with high insulin index( 29 ).
Associations between dietary insulin indices and metabolic features such as glycaemic status, lipid profile, inflammatory biomarkers and body composition have been addressed only in limited studies and results have been inconsistent( Reference Nimptsch, Brand-Miller and Franz 11 , Reference Mirmiran, Esfandiari and Bahadoran 16 , Reference Joslowski, Goletzke and Cheng 21 ). Moreover, documented associations between diet and disease in young adults cannot be generalised to the elderly due to the differences in the grade of systematic inflammation as well as differences in the quantity and distribution of fat mass( Reference Guarner and Rubio-Ruiz 3 ). In addition, men are at higher risk of CVD compared with women( Reference Weidner 22 ). Research suggests that CVD develops approximately 7–10 years earlier in men v. women( Reference Maas and Appelman 23 ).
In the present study, DIL was not associated with BMI. These findings are consistent with a prospective study conducted by Joslowski et al. ( Reference Joslowski, Goletzke and Cheng 21 ). This study found that high intake of dietary insulin index (DII) (45 compared with 39) or DIL (362 compared with 321) during puberty (among healthy subjects) was not associated with BMI in young adulthood( Reference Joslowski, Goletzke and Cheng 21 ). However, Chaput et al. ( Reference Chaput, Tremblay and Rimm 37 ) showed that high insulin secretion can predict weight gain in adulthood. In Chaput et al.’s study, adults with the highest level of insulin concentration and with the lowest level of dietary fat gained approximately 4·5 kg more weight after 6 years of follow-up compared with those with the lowest levels of insulin and dietary fat( Reference Chaput, Tremblay and Rimm 37 ). It has been demonstrated that high insulin secretion due to high consumption of insulinogenic foods during a long period can result in the development of fat mass( Reference Joslowski, Goletzke and Cheng 21 ) and insulin resistance( Reference Mirmiran, Esfandiari and Bahadoran 16 ). Following insulin resistance, the risk of obesity can increase( Reference McKeigue, Shah and Marmot 38 ). Moreover, high insulin concentrations can suppress lipolysis and stimulate glucose uptake, which in turn enhances lipogenesis in adipocytes( Reference Ludwig, Majzoub and Al-Zahrani 39 ).
Regarding glycaemic control, a significant positive association was observed between DIL and FBS concentrations. Although high secretion of insulin can result in lower FBS levels, it seems that prolonged consumption of foods with high insulin index causes β-cell dysfunction( Reference Nimptsch, Brand-Miller and Franz 11 ). This condition subsequently can lead to insulin resistance and increased serum glucose levels.
We found no association between DIL and HDL-cholesterol concentrations. In the study by Nimptsch et al., they observed an inverse association between DIL (≥858 compared with <648) and HDL-cholesterol. However, after stratification by BMI, DIL was no longer associated with HDL-cholesterol levels in normal (BMI <25 kg/m2) and overweight (BMI=25–29·9 kg/m2) subjects. However, an inverse association remained among obese (BMI ≥30 kg/m2) subjects( Reference Nimptsch, Brand-Miller and Franz 11 ). A reason why we failed to observe any association between DIL and HDL-cholesterol might be due to the overall low mean BMI of our participants (approximately 25·4 kg/m2).
It appears that the inverse association between DIL and HDL-cholesterol found by Nimptsch et al., especially in obese subjects, is due to the high insulin resistance in this group. A possible mechanism is that an insulinogenic diet aggravates insulin secretion, which in turn may lead to insulin resistance in the long-term, as was observed in the study by Mirmiran et al.( Reference Mirmiran, Esfandiari and Bahadoran 16 ) (DIL ≥1097 compared with <794 was associated with a 69 % increase in the risk of insulin resistance). Based on previous research, insulin resistance and disturbance of glycaemic control is associated with lower HDL-cholesterol serum levels( Reference Reaven 40 ). Moreover, studies have revealed that high carbohydrate consumption is associated with low serum levels of HDL-cholesterol( Reference Liu, Manson and Stampfer 41 , Reference Rock, Flatt and Thomson 42 ). In the present study, no association was found between DIL and serum TAG levels. However, in a study conducted by Nimptsch et al. ( Reference Nimptsch, Brand-Miller and Franz 11 ), a significant positive association between dietary insulin indices (DII: ≥46·2 compared with <38·3; DIL: ≥858 compared with <648) and TAG concentration was observed in all BMI categories, particularly in the obese.
In the present study, we did not find an association between DIL and fibrinogen levels. However, there was a positive relationship between high DIL and serum levels of hs-CRP. In contrast to our study, Nimptsch et al. failed to find any association between dietary insulin indices (DII: ≥46·2 compared with <38·3; DIL: ≥858 compared with <648) and inflammatory biomarkers including IL-6 and C-reactive protein (CRP). In another study, hyperglycaemia was associated with increased levels of inflammatory biomarkers (OR for CRP: 1·33; for IL-6: 1·51 and TNF-α: 1·14)( Reference De Rekeneire, Peila and Ding 43 ). Under normal conditions, the pro-inflammatory effects of glucose are controlled by the anti-inflammatory action of insulin( Reference Esposito, Marfella and Giugliano 44 ). However, in the current study, high levels of FBS in participants with high DIL (who might have reduced insulin secretion due to older age), might be an explanation for increased levels of hs-CRP.
Our failure to find relationships with a number of biomarkers may be due to several limitations. The cross-sectional design of the study prevents us from making causal inferences. Therefore, prospective studies are needed to evaluate these associations over longer periods. Second, since our study only included men, the results are not generalisable to the both sexes. Third, in this study basal insulin secretion was assessed by taking fasting insulin samples. However, DII is based on postprandial insulin secretion. Fourth, in addition to dietary factors that affect the insulin levels, it is important to consider multiple other factors that determine insulin levels such as physical activity ( Reference Steele, Brage and Corder 45 , Reference Fedewa, Gist and Evans 46 ), anthropometric characteristics and genetic predisposition( Reference Strawbridge, Dupuis and Prokopenko 47 – Reference Wang, Wu and Li 49 ). Fifth, the insulin index values for foods were derived from a study that was conducted in young lean students whose insulin responses are relatively different from elderly and obese subjects( Reference Holt, Miller and Petocz 17 ). However, according to a validation study, the positive link between insulin index and TAG concentrations is expected to be stronger among overweight subjects( Reference Nimptsch, Brand-Miller and Franz 11 ). This suggests that the insulin index would also be applicable in heavier subjects. Sixth, using an FFQ as a retrospective dietary assessment tool might cause misclassification. Despite our best effort to control for major confounders, some additional confounders may not have been accounted for or residual confounding may remain. One such confounder might be recent changes in body weight as it has been shown to be associated with CVD risk factors( Reference Alnasir and Masuadi 50 – Reference Vasunilashorn 52 ), particularly incidence and remission of insulin resistance( Reference Chang, Sung and Yun 51 ).
The current study has several strengths. First, limited research is available on the association between insulin indices and cardiovascular risk factors. Second, not all published studies have comprehensively taken into account different cardiovascular risk factors. However, in the present study, glycaemic parameters, lipid profile and also inflammatory biomarkers were investigated to provide better insight into the association between DIL and CVD risk factors. Third, as little information is available about dietary patterns and indices such as DIL in the elderly, attention to this group is critical. Fourth, the elderly are at higher risk of insulin resistance, therefore examining the association between dietary insulin indices and cardiovascular risk factors is important.
Conclusion
In this cross-sectional study, DIL was positively associated with serum FBS and hs-CRP levels. However, no association was observed between DIL and BMI or lipid profiles. More research is needed to elucidate the association between DIL and other cardiovascular risk factors and to understand potential differences by sex.
Acknowledgements
The authors thank the subjects who participated in the study.
This study was funded by the National Institute for Medical Research Development (grant no. 965430). The National Institute for Medical Research had no role in the design, analysis or writing of this article.
H. M., N. N. and L. A. designed the study. H. M., N. N., B. L., P. J. S. and L. A. contributed to the statistical analysis, interpretation of the data and to the drafting of the manuscript. The final version of the manuscript was approved by all authors before submission.
The authors declare that there are no conflicts of interest.







