Genetic and environmental factors influence postnatal growth and development in different ways and numerous growth factors have been shown to be responsible for the coordination of these effectsReference Barker, Hales, Fall, Osmond, Phipps and Clark1, Reference Woods, Camacho-Hubner, Savage and Clark2. The growth hormone–insulin-like growth factor (IGF)-1 axis is one of the important role players in these processesReference Boguszewski, Jansson, Rosberg and Albertsson-Wikland3. IGF-1 mediates most of the physiological actions of growth hormone and these two are major promoters of longitudinal growth. In addition to its effects on growth and cartilage metabolism IGF-1 has insulin-like and mitogenic activity on extra-skeletal structuresReference Leger, Noel, Limal and Czernichow4.
IGF-binding proteins (IGFBP) regulate the bioavailability of IGF-1. IGFBP-1 is the principal regulator of circulating IGF-1 levels and its levels rise and fall in response to hepatic portal blood insulin as such forming a link between dietary ingestion, glucose metabolism and the IGF axisReference Yasunaga, Furukawa, Katsumata, Horikawa, Tanaka, Tanae and Hibi5. IGFBP-3 is also important; it binds approximately 90 % of the IGF-1 present in serum, is thought to be an important determinant of IGF-1 bioactivity and a marker for insulin resistance. Even though primarily dependent on growth hormone, serum concentrations of IGF-1 and IGFBP-3 are also under long-term regulation by nutritional statusReference Yasunaga, Furukawa, Katsumata, Horikawa, Tanaka, Tanae and Hibi5, Reference Idohou-Dossou, Wade, Guiro, Sarr, Diaham, Cisse, Beau, Chappuis, Hoffman and Lemonnier6. Both the dietary protein levels and energy from carbohydrates are important in the regulation of IGF-1 levels.
The IGF system is extremely sensitive to metabolic alterations, and changes in IGF and IGFBP are believed to play a role in the processes that link growth and nutritionReference Thissen, Ketelslegers and Underwood7, since early postnatal growth is dependent on infant nutritional intake, especially protein and energy intakeReference Low, Tam, Kwan, Tsang and Karlberg8. In an early study on South African children Pimstone et al. Reference Pimstone, Barbezat, Hansen and Murray9 suggested that protein intake rather than total energy intake was involved in stimulating growth hormone secretion in malnutrition. The idea is that in case of protein shortage, growth hormone will break down the fat deposits in order for the individual to survive. Thus, the present study aimed to examine the concentrations of IGF-1, IGFBP-1 and IGFBP-3 in stunted and normal children from a population with high levels of stunting observed at an early age (35 % at 1 year)Reference Mamabolo, Alberts, Mbenyane, Steyn, Nthangeni, Delemarre-Van De Waal and Levitt10, and whether the high levels of stunting are nutrition related by assessing these two markers of nutrition.
Leptin, a product of the ob geneReference Brunner, Nick, Cumin, Chiesi, Baum, Whitebread, Stricker-Krongrad and Levens11, has been found to be essential in the regulation of food intake and energy metabolism, and as such body weightReference Ceddia, Koistinen, Zierath and Sweeney12. The underlying mechanisms are done by the adipose tissue mass through hypothalamic effects on satiety and energy expenditureReference Mantzoros, Moschos, Avramopoulos, Kaklamani, Liolios, Doulgerakis, Griveas, Katsilambros and Flier13. It has been shown that leptin correlates with the individual's body weight, fat mass and BMI but like the IGF axis it is affected by extremes in energy intake such as fasting and overfeedingReference Ceddia, Koistinen, Zierath and Sweeney12, Reference Kilic, Taskin, Ustundag and Aygun14. Leptin levels have been found to be low in many forms of malnutrition, including intra-uterine growth retardation and malnourishmentReference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15, Reference Isik, Kalyoncu and Okten16. Thus leptin levels were analysed in this group of children.
Methods and subjects
Study area
The study was undertaken in villages situated in the central region of the Limpopo Province. The villages are predominately semi-rural with poor infrastructure, lack of electricity, improper sanitation, poor roads and poorly equipped schools. The villages rely mainly on subsistence farming for a livingReference Alberts, Burger and Tollman17.
Subjects
Participants were children born to third trimester pregnant women who were recruited while attending prenatal clinics at nine randomly selected local clinics out of fourteen which are situated around the referral hospital, Mankweng Hospital. Further details on the subjects have been described in detail earlierReference Mamabolo, Alberts, Mbenyane, Steyn, Nthangeni, Delemarre-Van De Waal and Levitt10, Reference Mamabolo, Alberts, Steyn, Delemarre-van de Waal and Levitt18.
Study design
After birth the children were visited over a 3-year period. The children went for routine postnatal care at the clinics at approximately 1, 3, 6, 9, 12 and 36 months. Of the children, 156 (seventy boys and eighty-six girls) returned for the 1-year visit and leptin and growth factors were analysed in 116 samples. Of the 162 (seventy-one boys and ninety-one girls) children who came for the third year visit, growth factors were analysed in 145. The difference in numbers can be accounted for by migrations within the studied villages as well as temporary out-migrations. In addition, the lack of proper addresses, formal streets and roads in these villages complicated the process of tracing the mothers and their children. Procedures for the study were explained to mothers or caregivers who then gave informed consent before commencement of data collection.
Anthropometric measurements
At both visits, ages 1 and 3 years, anthropometric measurements were taken from the children. Weight was taken with a baby scale (TANITA Baby Scale, model 1380; Tanita Corporation, Tokyo, Japan), with the baby naked, to the nearest 0·1 kg. The baby scale was calibrated using a beam balance. Height or length was measured with a non-stretchable tape measure mounted on a board to the nearest 0·5 cm (at 1 year this was done with the baby lying down while at 3 years it was done with the child standing on a flat surface against the wall).
Blood collection and analysis
Blood samples were collected after an overnight fast for the determination of leptin and growth factors. IGF-1, IGFBP-1 and IGFBP-3 (Diagnostic Systems Laboratories Inc., Webster, TX, USA) were measured by RIA on the Cobra II Gamma Counter. Inter-assay CV were 8, 6 and 2 % for IGF-1, IGFBP-1 and IGFBP-3, respectively. Corresponding intra-assay CV were 7, 6 and 4 %, respectively. Leptin was determined on the Cobra II Gamma Counter (LINCO Research Inc., St Charles, MO, USA). The inter-assay and intra-assay CV were 10 and 7 %, respectively. Insulin levels were determined on the Access® Immunoassay System (Beckman Coulter Inc., Fullerton, CA, USA) and glucose on the Dimension SMS autoanalyzer (duPont, Wilmington, DE, USA). Blood analysis for the 1-year samples was done in 2001 and for the 3-years samples in 2003.
Insulin resistance and β-cell function were calculated by using the homeostasis model assessment (HOMA) as derived by Matthews et al. Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner19, and later corrected and computerised, then updated into a HOMA 2.2 calculatorReference Levy, Matthews and Hermans20.
Statistical analysis
Statistical analyses were performed using SPSS version 11.0 software (SPSS Inc., Chicago, IL, USA). Skewed data were log-transformed before analysis. Statistical tests done included descriptive statistics, Pearson's correlations, Student's t test and one-way ANOVA for comparison of continuous data. Statistical significance was set at the level of P < 0·05. To determine the best combination of predictors of stunting at 3 years, backward multiple regression analyses were performed, using significant variables in simple regression analysis as well as their interactions.
Ethical approval
Ethical approval and permission to undertake the study were obtained from the University of the Limpopo (Turfloop Campus) Ethics Committee and the Limpopo Province Department of Health and Welfare's Research Committee.
Results
Tables 1 and 2 give the biochemical and anthropometric measurements of the children at 1 and 3 years, respectively. Of the children whose growth factors were analysed at 1 year, 34 % were stunted (defined as having height-for-age Z score below − 2 sd of the National Center for Health Statistics and WHO reference guidelines), while at 3 years 46 % were stunted.
Table 1 Anthropometric and biochemical values of the children at 1 year (Mean values and standard deviations)
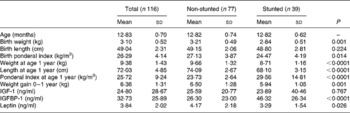
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor-binding protein.
Table 2 Anthropometric and biochemical values of the children at 3 years (Mean values and standard deviations).
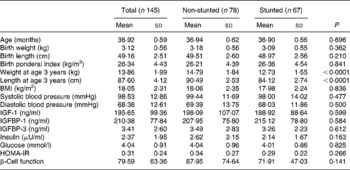
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor-binding protein; HOMA-IR, homeostasis model assessment of insulin resistance.
At 1 year, with respect to birth anthropometry, the two groups differed significantly in their birth weights and ponderal indices (PI); in all instances the stunted children had lower measurements (Table 1). Significantly lower weight gain from birth to 1 year was noted in the stunted group compared with the non-stunted group. The stunted children also had lower leptin levels (P = 0·026) but higher IGFBP-1 levels (P < 0·0001). Though there were higher values for both body weight and length at 1 year (P < 0·0001) in the non-stunted children, their PI were significantly lower (P < 0·0001) than those of the stunted children. At 3 years significant differences were observed between current body weight (P < 0·0001) and length (P < 0·0001) (Table 2).
Sex differences between the parameters at 1 year were only seen in the leptin levels wherein, even though not significant, girls had higher values than boys (4·12 (sd 2·08) and 3·53 (sd 1·92) ng/ml, respectively; P = 0·066), while at 3 years there were none.
Correlations
At 1 year IGFBP-1 correlated negatively with the following: 1-year body weight (r − 2·56; P = 0·006), length (r − 0·563; P < 0·0001), PI (r 0·345; P = 0·042) and weight gain (r − 0·202; P = 0·035). At 3 years glucose correlated positively with current weight (r 0·192; P = 0·021); BMI (r 0·252; P = 0·002) and birth length (r 0·1876; P = 0·032). Insulin correlated negatively with IGFBP-1 (r − 0·186; P = 0·025), while IGFBP-3 correlated positively with IGF-1 (r 0·181; P = 0·038).
Correlations in stunted v. non-stunted children
At 1 year, in the stunted group IGFBP-1 correlated positively with current PI and negatively with current body length. However, in the non-stunted children IGFBP-1 correlated positively with birth PI and negatively with length at 1 year. Significant negative correlations were also observed between leptin levels and both birth weight and birth PI (Table 3).
Table 3 Correlations in the stunted and non-stunted children at 1 year

IGF, insulin-like growth factor; IGFBP, insulin-like growth factor-binding protein; PI, ponderal index.
At 3 years, in the stunted group insulin correlated positively with IGFBP-3 and negatively with IGFBP-1, birth length and birth weight. In the non-stunted group of children glucose correlated positively with weight at 3 years, BMI at 3 years and birth length. Insulin correlated positively with IGF-1 and negatively with IGFBP-1. IGF-1, on the other hand, correlated positively with both IGFBP-3 and weight at 3 years (Table 4).
Table 4 Correlations in the stunted and non-stunted children at 3 years
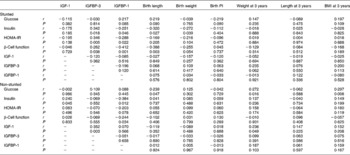
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor-binding protein; PI, ponderal index; HOMA-IR, homeostasis model assessment of insulin resistance.
Independent predictors of stunting at 3 years: multivariate analyses
In order to select the best combination of independent predictors of stunting at 3 years, we entered all the variables that were significantly related to height-for-age Z score in simple regression analysis into backward multiple regression analyses (Table 5). The model revealed that both 1-year IGFBP-1 levels and height-for-age Z scores were independent predictors of stunting at 3 years (multiple r 0·653; P < 0·0001).
Table 5 Backward multiple regression analyses for stunting as assessed by height-for-age Z scores (HAZ)*
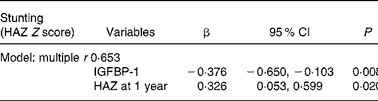
IGFBP, insulin-like growth factor-binding protein.
* Model: HAZ at 1 year, ponderal index at 1 year, IGFBP-1 at 1 year, insulin-like growth factor-1 at 1 year, BMI at 3 years, homeostasis model assessment, β-cell function and insulin-like growth factor-1 at 3 years, as well as their interactions, were used as predictors of stunting, as they were related to stunting in simple regression analysis.
Correlations within the various nutritional states
The study further examined the children based on their respective malnutrition states at 3 years (i.e. normal; stunted; overweight; stunted and overweight)Reference Mamabolo, Alberts, Steyn, Delemarre-van de Waal and Levitt18. There were no differences in biochemical parameters and birth anthropometry amongst these groups.
In children who were normal (n 41), glucose correlated positively with BMI at 3 years (r 0·266; P = 0·020) and insulin negatively with IGFBP-1 (r − 0·242; P = 0·038). IGF-1 correlated positively with both weight at 3 years (r 0·394; P = 0·021) and BMI at 3 years (r 0·423; P = 0·013).
In the 3-year-old stunted group of children (n 45) glucose correlated positively with current body weight (r 0·278; P = 0·010), BMI (r 0·346; P = 0·001) and birth length (r 0·309; P = 0·007). Insulin correlated positively with IGFBP-3 (r 0·324; P = 0·032) and negatively with both IGFBP-1 (r − 0·300; P = 0·048) and birth weight (r − 0·325; P = 0·043), and IGFBP-3 showed a positive correlation with current body length (r 0·349; P = 0·020).
In the overweight children (n 38) glucose correlated positively with BMI at 3 years (r 0·410; P = 0·010), birth weight (r 0·416; P = 0·018) and birth length (r 0·476; P = 0·006). IGFBP-3 correlated positively with IGF-1 (r 0·405; P = 0·014) and negatively with both birth weight (r − 0·405; P = 0·021) and birth PI (r − 0·370; P = 0·037). In the stunted and overweight group of children (n 21) IGF-1 correlated negatively with birth PI (r − 0·512; P = 0·036) and IGFBP-1 correlated negatively with BMI at 3 years (r − 0·440; P = 0·040).
Discussion
The mechanisms by which growth factors affect postnatal growth have not yet been clearly elucidated. Though it is known that nutrition plays a role in these mechanisms, other factors have been demonstrated to affect growth via the growth factors; these include inflammatory diseases, renal diseases and diabetesReference Kilic, Taskin, Ustundag and Aygun14. It is also known that in underprivileged populations such as this community with a generally low socio-economic statusReference Alberts, Burger and Tollman17 the major cause of poor growth is inadequate food intakeReference Wan Nazaimoon, Rahmah, Osman, Khalid and Livesey21.
Palacio et al. Reference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15 have revealed that IGF-1 levels are decreased in malnourished children, a finding not observed in the present study. However, the stunted children had increased IGFBP-1 at 1 year, which was also an independent predictor of stunting at 3 years. This increase in IGFBP-1 has been postulated to be an adaptive mechanism in an attempt by the body to inhibit the synthesis and activity of IGF-1 in modulating growth, such that the available nutrients can be channeled to other essential metabolic processes such as brain developmentReference Kilic, Taskin, Ustundag and Aygun14, Reference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15. But surprisingly these differences disappeared by 3 years of age in the studied children.
IGFBP-1, on the other hand, has been shown to inhibit the effect of IGF-1-stimulated somatic linear growth and differentiation, weight gain and tissue growthReference Yasunaga, Furukawa, Katsumata, Horikawa, Tanaka, Tanae and Hibi5, Reference Garrone, Radetti, Sidoti, Bozzola, Minuto and Barreca22. And similarly IGFBP-1 can counteract the insulin-like hypoglycaemic activity of IGF-1Reference Katz, Deleon, Zhao and Jawad23, a phenomenon confirmed by the observed negative association between the two parameters at 3 years in the present study. In view of all these findings, the observed increase in IGFBP-1 during nutritional deprivation may be a protective mechanism serving to mobilise energy in order to survive and not for linear growth.
Unlike what has been previously reported about leptin levelsReference Hassink, Sheslow, De Lancey, Opentanova, Considine and Caro24, in the present study there was no relationship between leptin levels at 1 year and body weight. But as previously reportedReference Hassink, Sheslow, De Lancey, Opentanova, Considine and Caro24, Reference Caprio, Tamborlane, Silver, Robinson, Leibel, McCarthy, Grozman, Belous, Maggs and Sherwin25, there were sex differences in the leptin levels, with girls having higher levels than boys. This lack of association between leptin and body weight is similar to what was reported by Jaquet et al. Reference Jaquet, Leger, Tabone, Czernichow and Levy-Marchal26, who attributed this discrepancy to the fact that body weight does not truly reflect body fat mass at this early stage of life.
Several researchers have found an association between IGF-1 and leptin, and have suggested that IGF-1 might be involved in controlling leptin secretion in malnourished individualsReference Kratzsch, Lammert, Bottner, Seidel, Mueller, Thiery, Hebebrand and Kiess27. However, in the present study no relationship between the two factors was found. The pattern of increased IGFBP-1 and reduced leptin levels observed in the stunted children at 1 year is similar to previous studies on malnourished adults and childrenReference Kilic, Taskin, Ustundag and Aygun14, Reference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15. Indeed, Palacio et al. Reference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15 suggested that leptin might have a direct effect on the liver and other sites of IGF synthesis or reduce growth hormone resistanceReference Carro, Scenaris, Considine, Casanueva and Dieguez28, Reference Clement, Vaisse and Lahlou29.
Hall et al. Reference Hall, Yamasaki, Kucera, Waltner-Law, O'Brien and Granner30 reported that IGFBP-1 transcription is suppressed by insulin in a manner similar to the regulation of the rate-limiting gluconeogenesis enzyme phosphoenolpyruvate carboxykinase. With this observation, they suggested that serum IGFBP-1 has the potential to reflect hepatic gluconeogenesis and insulin sensitivity.
From previous reportsReference Mueller, Gregoire and Stanhope31, Reference Couillard, Lamarche, Mauriege, Cantin, Dagenais, Moorjani, Lupien and Despres32, it is possible that both circulating levels and long-term maintenance of insulin and glucose levels in these children have an effect on the observed pattern of stunting accompanied by impaired growth factor levels. It is thus becoming increasingly apparent that diet is an important regulator of the IGF system. Severe energy or protein restriction has been shown to lower IGF concentrations considerablyReference Thissen, Ketelslegers and Underwood7. Several studies have suggested that even within the normal IGF reference range, nutrient intakes are associated with variations in IGF concentrations. The most consistent associations have been positive associations with dairy products, protein and mineral intakes, including Zn, K, Ca, P and MgReference Holmes, Pollak, Willett and Hankinson33, Reference Giovannucci, Pollak, Liu, Platz, Majeed, Rimm and Willett34. In children, milk has been shown to stimulate linear growth via IGF-1, supporting the notion of the importance of nutrient intakeReference Hoppe, Udam, Lauritzen, Molgaard, Juul and Michaelsen35. However, it is still unclear whether total protein or protein high in essential amino acids is the most important factor in this regard.
The overall nutrient intakes of the children in the present study were lowReference Mamabolo, Steyn and Alberts36, a consistent finding in black South African preschool children over the yearsReference Steyn, Badenhorst, Nel and Jooste37, Reference Bourne, Langenhoven, Steyn, Jooste, Laubscher and Bourne38. Furthermore, the children's diet was of poor quality, comprising mainly of carbohydrate-rich foods with a low intake of dairy products and fruit and vegetablesReference Mamabolo, Steyn and Alberts36.
These findings suggest that the high prevalence of stunting in these children was a result of a combination of the following: chronic energy deficiency, poor nutrient density and poor-quality protein with respect to essential amino acids. The fact that the diet was very low in fat, and perhaps in some essential fatty acids, also needs to be considered. The importance of healthy fat intake during infancy needs to be emphasised as all these dietary factors are known to be essential for optimum growth and development to occur, and are supported by the finding that these children were introduced to supplementary feeds at a very early ageReference Mamabolo, Alberts, Mbenyane, Steyn, Nthangeni, Delemarre-Van De Waal and Levitt10. Thus the reduced IGF-1 levels in stunted children at 1 year might be the result of poor nutrition. Furthermore, it is known that IGF-1 exerts a negative feedback on the synthesis and release of growth hormoneReference Palacio, Perez-Bravo, Santos, Schlesinger and Monckeberg15.
At 3 years growth factors were mainly associated with weight measures, contrary to the situation at 1 year when the association was with length. This could probably be accounted for by the high proportion of overweight children at this ageReference Mamabolo, Alberts, Steyn, Delemarre-van de Waal and Levitt18. Thus it is speculated that the loss of association between stunting (a measure of chronic malnutrition) and the growth factors at 3 years might be due to the children having more fat stores, resulting in the observed changes in their biochemical profile in an attempt to adapt to the situation. It is also possible that the children are now exposed to a variety of food available within the household, and they may be failing to thrive due to the diet's poor quality (especially the lack of animal protein) or other factors such as poor living conditionsReference Alberts, Burger and Tollman17.
On the other hand, it is a generally accepted concept that overweightness in childhood is becoming an epidemic worldwide, though an internationally accepted definition has not yet been established. When using the reference ranges recommended by the International Task Team on ObesityReference Cole, Bellizzi, Flogal and Dietz39, there were no differences in the growth factor levels between the overweight children and the normal ones. This is a phenomenon also reported by other researchersReference Slowinska-Srzednicka, Zgliczynski, Makowska, Jeske, Brzezinska, Soszynski and Zgliczynski40, but contrary to othersReference Colletti, Teale and Marks41.
There have been previous reports of adverse outcomes being evidenced in young children who have been exposed to poor conditions in utero and early life such as low birth weight, catch-up growth, current weight, poor nutritional status and poor living conditionsReference Pulkki, Keltikangas-Jarvinen, Ravaja and Viikari42. However, this was not observed in the present study nor reported by researchersReference Wilkin, Metcalf, Murphy, Kirkby, Jeffery and Voss43, Reference Singhal, Cole, Fewtrell, Deanfield and Lucas44.
In conclusion, the study shows that the observed stunting in this group of children may be a result of chronic undernutrition resulting in long-term growth faltering which is already evident at 1 year. The early introduction of supplementary feeds and a diet of poor quality later in childhood observed in these children may be a major player in this regard. As such, this calls for public health measures aimed at promoting proper feeding practices and weaning methods.
Acknowledgements
The present study was supported by grants from the Institute for Research in Extramural Medicine (EMGO), Vrije University (Amsterdam, The Netherlands). We would like to thank Ms A. M. Makwela and Mr R. P. Mamabolo (field workers), and also the nursing staff at the nine clinics for their assistance in conducting the study. We are also grateful to Ms M. H. Mamabolo for her assistance during blood collection.







