NAFLD is a metabolic disease typified by steatosis, vacuolisation and inflammatory infiltration of hepatocytes, etc(Reference Wesolowski, Kasmi and Jonscher1). In severe cases, NAFLD may be accompanied by liver fibrosis, hepatocyte necrosis or apoptosis and other pathological changes(Reference Younossi, Koenig and Abdelatif2). The morbidity rate of NAFLD is increasing over the years, especially in western developed countries, where the average morbidity rate reaches 20–30 %; thus, preventing and treating NAFLD is of great significance(Reference Younossi, Anstee and Marietti3). However, there is no specific drug for NAFLD, and life interventions (i.e. diet, exercise, etc.) with the ultimate target of weight loss are still the first-line treatment in clinical practice at present(Reference Younossi, Koenig and Abdelatif2,Reference Martel, Esposti and Bouchet4) . Tea, one of the most consumed beverages globally, has been proven to have strong antioxidant and apoptosis-inducing effect on inflammatory cells with its various bioactive components; it has also been proven to have remarkable effects on reducing body fat and improving NAFLD(Reference Yang and Wang5–Reference Guo, Ge and Lu9). However, previous studies were mostly experimental and small clinical studies and were limited to one type of tea, lacking studies comparing the relationship between different types of tea and NAFLD(Reference Guo, Ge and Lu9,Reference Tang, Xu and Zhang10) .
Hitherto, the association between tea consumption and NAFLD, remains controversial. Hypothesis of this study is that tea has a protective impact on NAFLD. Mendelian randomisation (MR) is a novel approach of simulating randomised controlled trials using SNP as instrumental variables (IV), which effectively eliminates potential confounders or reverse causality that may introduce research bias(Reference Sekula, Del Greco and Pattaro11). The author first used MR to reveal a causal association between tea and NAFLD at genetic level and then used data from National Health and Nutrition Examination Survey (NHANES) for clinical validation, innovatively combining the two to enhance the credibility of this study. This study will provide novel insights and targets for the prevention and drug development of NAFLD from a novel genetic perspective.
Materials and methods
Study overview
Extraction of tea and non-alcoholic fatty liver disease SNP from summarised Genome Wide Association Study data
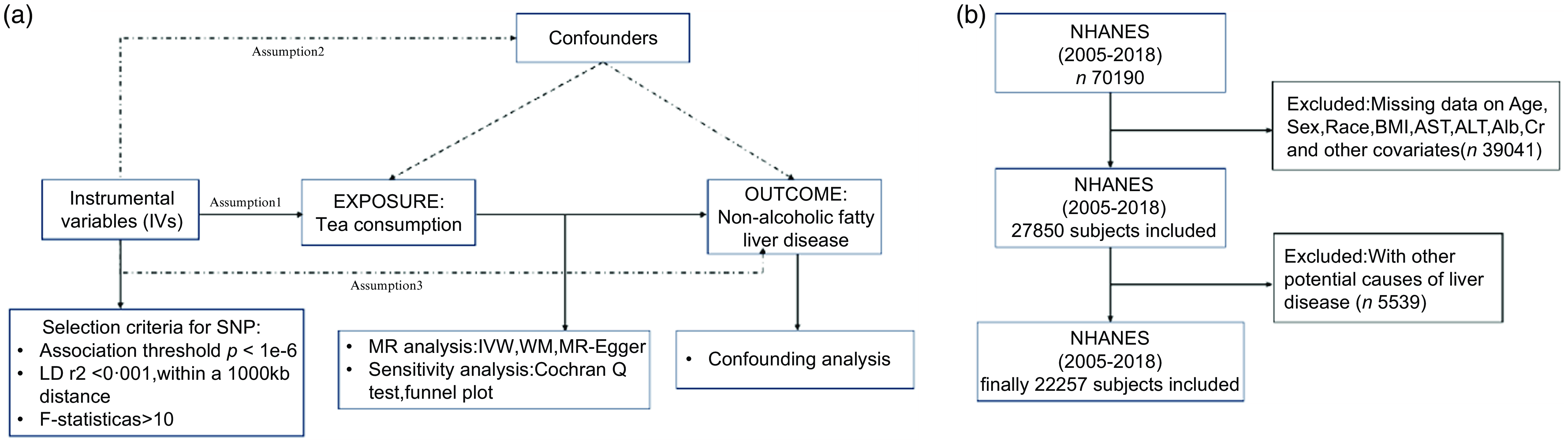
A convincing MR design should be compliant with following three basic hypotheses: Hypothesis 1, IV are strongly correlated to exposure; Hypothesis 2, genetic variation is irrelevant to confounding factors; Hypothesis 3, IV only influence outcomes through exposure. The outline of this study and three hypotheses are shown in Fig. 1(a).

Fig. 1. (a) Overview of the current Mendelian randomisation (MR) study. (b) Flow chart of participants’ selection.
Study Population in NHANES
NHANES is launched by USA National Center for Health Statistics on a two-year-cycle, consists of demographic information, diet and dietary supplement information, a variety of blood drawing indicators and test reports (e.g. colour ultrasound, etc.). All detailed information display on official websites and are available in http://www.cdc.gov/nchs/nhanes.htm. National Center for Health Statistics Research Ethics Review Committee approved the protocols of research. All individuals enrolled in the programme signed an informed consent form. All data collected for this study were coded, with personal identifiers removed, before being available to the public. Our study included a total of 7 cycles of surveys from 2005 to 2018, as shown in Fig. 1(b).The exclusion criteria included the following: (1) Under the age of 20; (2) pregnant; (3) abnormal hepatitis-related antigen or antibody tests, hepatocellular carcinoma/autoimmune hepatitis; (4) excessive alcohol consumption, with excessive alcohol consumption defined as alcohol intake of > 20 g/d for male or > 10 g/d for female; (5) abnormal energy intake, with total energy intake < 800 kcal/d, or > 4200 kcal/d; (6) participants who drank more than one type of tea; (7) participants who lack covariates such as age, sex and race.
Genome wide association studies (GWAS) data for non-alcoholic fatty liver disease
To make the genetic characteristics of the two samples similar, the database of European cohorts was selected for both exposure and outcome. Genetic information on NAFLD is accessible at the following website: https://gwas.mrcieu.ac.uk. Specifically, Professor Ghodsian conducted a meta-analysis involving cohorts from four databases (including 1 106 diseased and 8571 healthy individuals from eMERGE; 651 diseased and 176 248 healthy individuals from FinnGen; 2, 558 diseased and 395, 241 healthy individuals from UK Biobank; 4119 diseased and 190 120 healthy individuals from Estonian Biobank), resulting in a large-scale Genome Wide Association Study involving 8434 cases and 770 180 controls(Reference Ghodsian, Abner and Emdin12).
GWAS data for tea and selection of instrumental variables
Access to genetic data associated with tea was acquired through the following website: https://www.ebi.ac.uk/gwas/, totalling 434 171 cases with a SNP count of 5 733 790(Reference Pirastu, McDonnell and Grzeszkowiak13).To guarantee the validity of IV utilised in this MR research, author took the following steps to screen eligible instrumental SNP (Fig. 1(a)). First, given the limited amount of SNP that qualified for whole-genome significance of P < 5 × 10–8, authors obtained 3, 660 SNP associated with tea from the Genome Wide Association Study summary statistics for tea using P < 1 × 10–6 as the screening criterion(Reference Li, Niu and Guo14–Reference Sun, Liang and Xia16). Then, PLINK was utilised to conduct a linkage disequilibrium test (r2 < 0·001) to guarantee independency of the chosen IV. And the F-statistic of SNP (F > 10) was calculated to avoid bias resulting from the use of weak genetic instruments(Reference Brion, Shakhbazov and Visscher17). After above screening, 58 SNP were finally acquired as IV for this study (Fig. 2). Detailed information of above 58 IV was shown in Additional file 1: online Supplementary Table 1). We calculate F-statistic according to following equation:

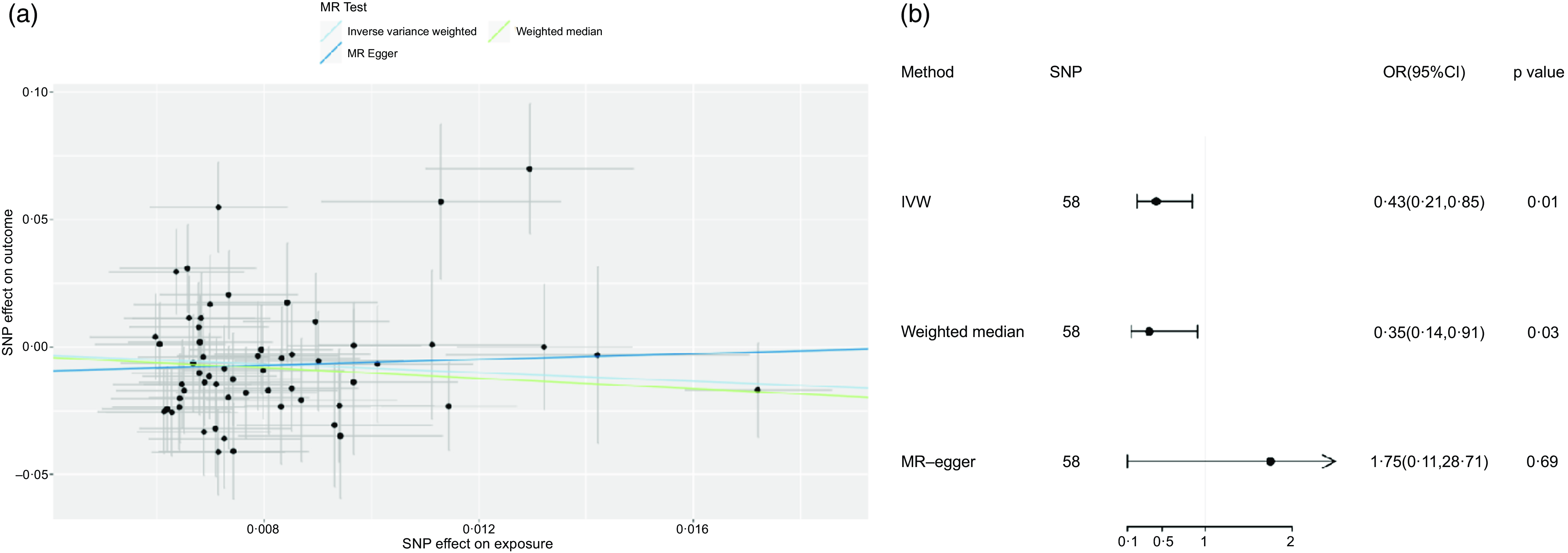
Fig. 2. (a) Scatter plot and (b) forest plot of Mendelian randomisation analyses for the associations of tea with risk of NAFLD. IVW, inverse-variance weighted method; MR, Mendelian randomisation; SNP, single nucleotide polymorphism.
R2 represents the extent to IV can explain the exposure.
(EAF: effect allele frequency)(Reference Pierce and Burgess18).
Assessment of tea consumption in National Health and Nutrition Examination Survey
NHANES collected all food information consumed by participants 24 h before the survey. All participants underwent two 24-hour dietary review surveys. Since the 2nd survey was collected by telephone after an interval of 3–10 d with low accuracy, all dietary data in this study were derived from the first 24-hour dietary survey. Food types were refined based on codes came from the USA. Department of Agriculture food as well as nutrition database for dietary studies, with different codes representing different sorts of tea consumed. Green tea and black tea were directly extracted from all beverages. After eliminating black and green teas, the remaining teas are categorised into other types of teas (e.g. herbal teas, corn teas, etc.). According to the Measuring Guides for the Dietary Recall Interview, a typical cup of tea was 8 oz (226·7 g).
Diagnostic criteria of non-alcoholic fatty liver disease
Fatty liver index, which consisted of TAG, glutamyltransferase, BMI and waist circumference, was utilised for an index to diagnose NAFLD in this study. If fatty liver index >= 60, the participant was considered to have NAFLD, and if <60, non-NAFLD(Reference Bedogni, Bellentani and Miglioli19).
Non-invasive evaluation indicators of liver fibrosis degree
Fibrosis-4 (FIB-4), which consisted of age, aspartate aminotransferase (AST), alanine transaminase (ALT) and platelet, was utilised for an index to evaluate the degree of liver fibrosis(Reference Pinzani, Vizzutti and Arena20).
FIB-4 = (age × AST)/ (platelet × ALT1/2), the higher the index value, the more severe the liver fibrosis.
Covariates
Participants’ age, sex, race and prior disease history were collected by questionnaires. Diabetes, hypertension, heart failure, lung diseases, tumours are all diagnosed by doctors. Height, weight and waist circumference were collected by uniformly skilled health technicians using uniform measuring instruments at a mobile examination centre, dividing BMI into 4 groups: normal group (18·5 <= BMI < 25), overweight group (25 <= BMI < 30), obese group (BMI >= 30) and underweight group (BMI < 18·5). Laboratory parameters included TAG, glutamyltransferase, fasting glucose, HbA1c, creatinine (Cr) and albumin (Alb) that were obtained by blood drawing at the mobile examination centre by professionally medical technicians following standard operating procedures.
Statistical analysis
Mendelian randomisation
The two-sample MR package of version 4.3.0 of R software was used to conduct statistical analyses, and values were regarded as having statistical significance at P < 0·05. Inverse-variance weighted (IVW) was used as the principle method to assess if a causal association exists between tea and NAFLD, preceded with sensitivity analyses to valuate the bias of the MR hypothesis involving weighted median, MR-egger method and so on(Reference Bowden, Davey Smith and Haycock21,Reference Burgess and Thompson22) . Outlier tests were performed by MR-PRESSO. Once outliers were found, we eliminated them and replicated the MR. The existence of heterogeneity or horizontal pleiotropy in the outcomes of this study depends on outcomes of Cochran Q-test and the significance of the intercept of MR-egger(Reference Verbanck, Chen and Neale23). Leave-one-out analysis in order to assess if observed causality is strongly influenced by a single SNP. Finally, a search was performed at PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk) examining whether the IV utilised in present study were relevant to additional NAFLD risk factors, primarily including hypertension(Reference Xie, Huang and Liu24), basal metabolism, BMI, smoke(Reference Yuan, Chen and Li25) and so on. After excluding, SNP associated with the above risk factors re-examine the causal effects with the aim of ascertaining if they are still significant.
National Health and Nutrition Examination Survey
R software version 4.3.0 was used to analyse the statistics, and P < 0·05 was taken to be statistically difference. To avoid oversampling and reduce the rate of non-response, the weights in this analysis were adjusted with reference to the published NHANES article. Continuous and classified variables were characterised by weighted means and weighted percentages, separately(Reference Cai, Zhang and Chen26,Reference Tao, Zhang and Zuo27) .When comparing intergroup difference, weighed chi-square tests were utilised to classified variables and weighted linear regression models were utilised to the continuous variables. To investigate the connection between tea consumption and NAFLD, the author groups different types of tea separately according to the quartile of daily tea intake (Green tea: Q1 (0, 255) g, Q2 (255, 402·5) g, Q3 (402·5, 720) g, Q4 (> 720) g; Black tea:Q1 (0, 257·5) g, Q2 (257·5, 435) g, Q3 (435, 754·8) g, Q4 (> 754·8) g; Other tea: Q1 (0, 240) g, Q2 (240·0, 355·2) g, Q3 (355·2, 518·0) g, Q4 (> 518) g. With the lowest tea consumption group (Q1) as reference, crude model and models correcting for confounders were constructed by univariate and multivariate logistic regression, respectively. Subgroup analyses were performed based on age, sex, BMI, CHD, lung disease, diabetes, hypertension and tumour. Finally, the effect of tea consumption on hepatic fibrosis levels was also analysed by multivariate linear regression.
Result
Baseline characteristics
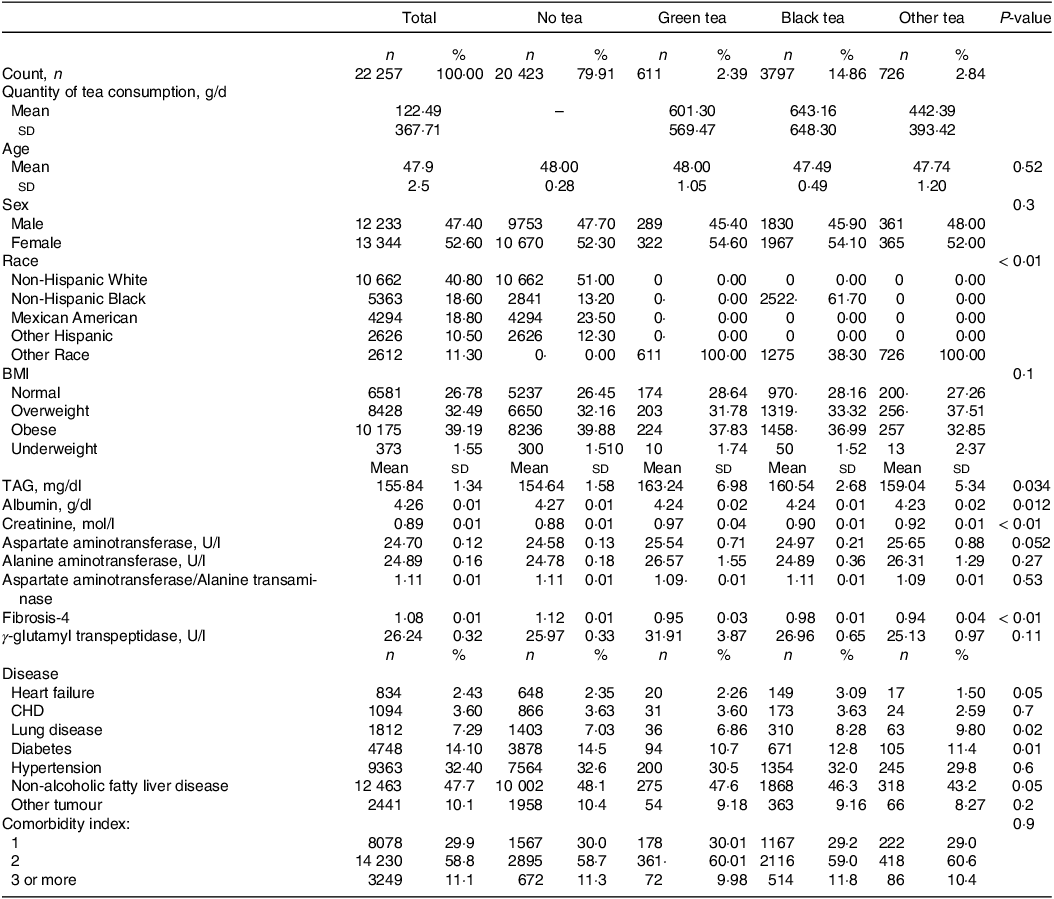
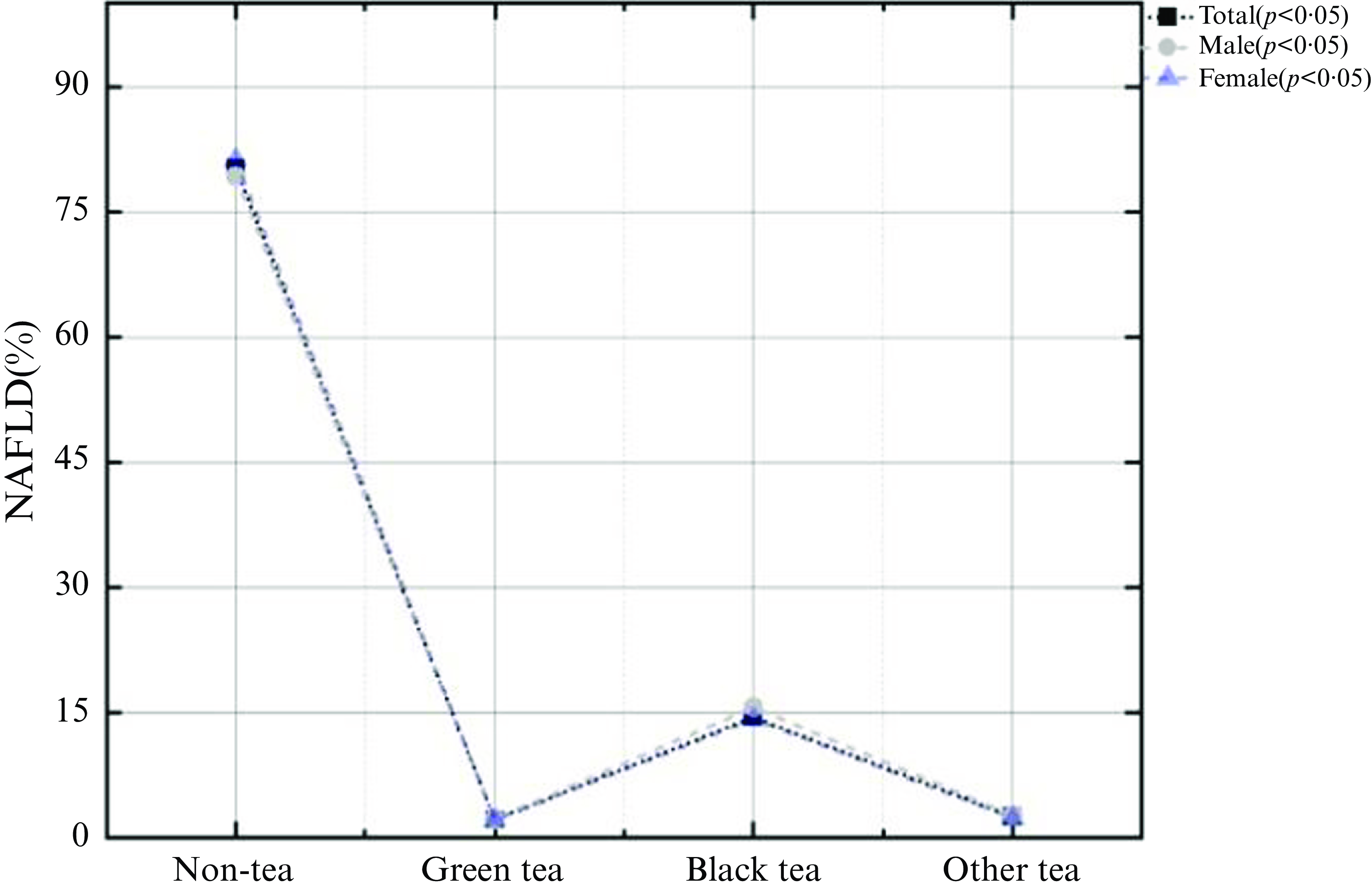
This study involved 22 557 subjects, of which 12 463 (47·7 %) were NAFLD patients. Average age of all involved participants was 47·9 ± 2·5 years and average daily tea consumption was 122·49 ± 367·71 g. There were 5134 tea drinkers in this USA population, with a total tea consumption rate of 20·09 %.Among them, the drinking rate of black tea was the highest (about 14·86 %), with an average daily tea consumption of 643·16 ± 648·30 g, followed by other teas (about 2·84 %), with an average daily tea consumption of 442·39 ± 393·42 g, and the drinking rate of green tea was the lowest (about 2·39 %), with an average daily tea consumption of 601·30 ± 569·47 g. In all three types of tea, the rate of tea consumption was higher in women than in men (Table 1). Among non-tea drinkers, there were 10 002 NAFLD patients (48·1 %), among green tea drinkers, 275 NAFLD patients (47·6 %), among black tea drinkers, 1868 NAFLD patients (46·3 %) and among other tea drinkers, 318 NAFLD patients (43·2 %) (Fig. 3).
Table 1. Baseline characteristics of study population (Numbers and percentages; Mean values and s d)


Fig. 3. Proportion of NAFLD in the different types of tea drinkers.
Causal association between tea and non-alcoholic fatty liver disease
Utilising IVW as the principle method of MR analysis, the outcomes upheld a potentially negative causal association between tea and NAFLD (OR: 0·43, 95 % CI: 0·21, 0·85, P = 0·01). It means that for every s d increase in tea consumption, the risk of developing NAFLD was reduced by 57 %.Weighted median method was used for sensitivity analysis, and the correlation and direction were consistent with those obtained by IVW (OR: 0·35, 95 % CI: 0·14, 0·91; P = 0·03). Nevertheless, nothing significant was observed between tea consumption and NAFLD by utilising MR-egger (OR: 1·75, 95 % CI: 0·11, 28·70, P = 0·69) (Fig. 2). The difference between IVW and MR-egger analysis lies in that MR-egger takes intercept term’s presence in the regression into account, while P = 0·31 of the MR-Egger intercept in this paper is of no significance. In addition, weighted median method has the advantage of retaining higher estimation precision in comparison with MR-egger analysis. And the results of weighted median method and IVW method in this study were consistent; therefore, the author concluded that there exists a negative causal relationship between tea and NAFLD. Heterogeneity test indicated no significant heterogeneity (Q-value (df) = 56, P = 0·08). P-value of MR-egger intercept was 0·31, implicating non-existence of horizontal pleiotropy. Followed by leave-one-out analysis, after removing any SNP sequentially, the remaining SNP was on the left side of the invalid line, indicating that a single SNP had a limited contribution to the outcomes. Finally, through PubMed retrieval, a total of 6 SNP were found to have secondary phenotypes, and after excluding SNP with secondary phenotypes, MR analysis was re-conducted, the results were basically consistent (IVW method was adopted, OR: 0·46, 95 % CI: 0·21, 0·97, P = 0·04), as shown in Additional file 1: online Supplementary Table 2).
Relationship between intake of different sorts of tea and non-alcoholic fatty liver disease
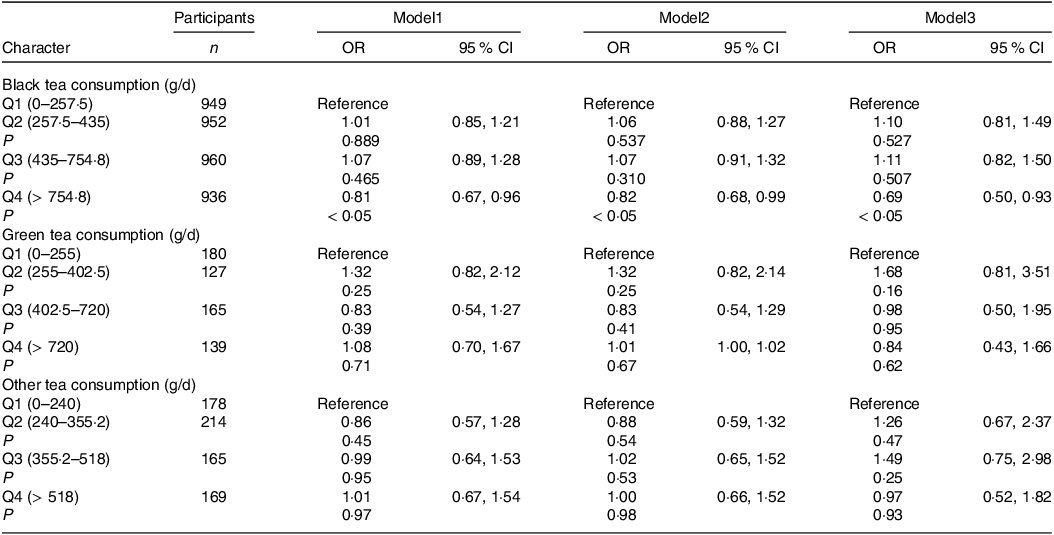
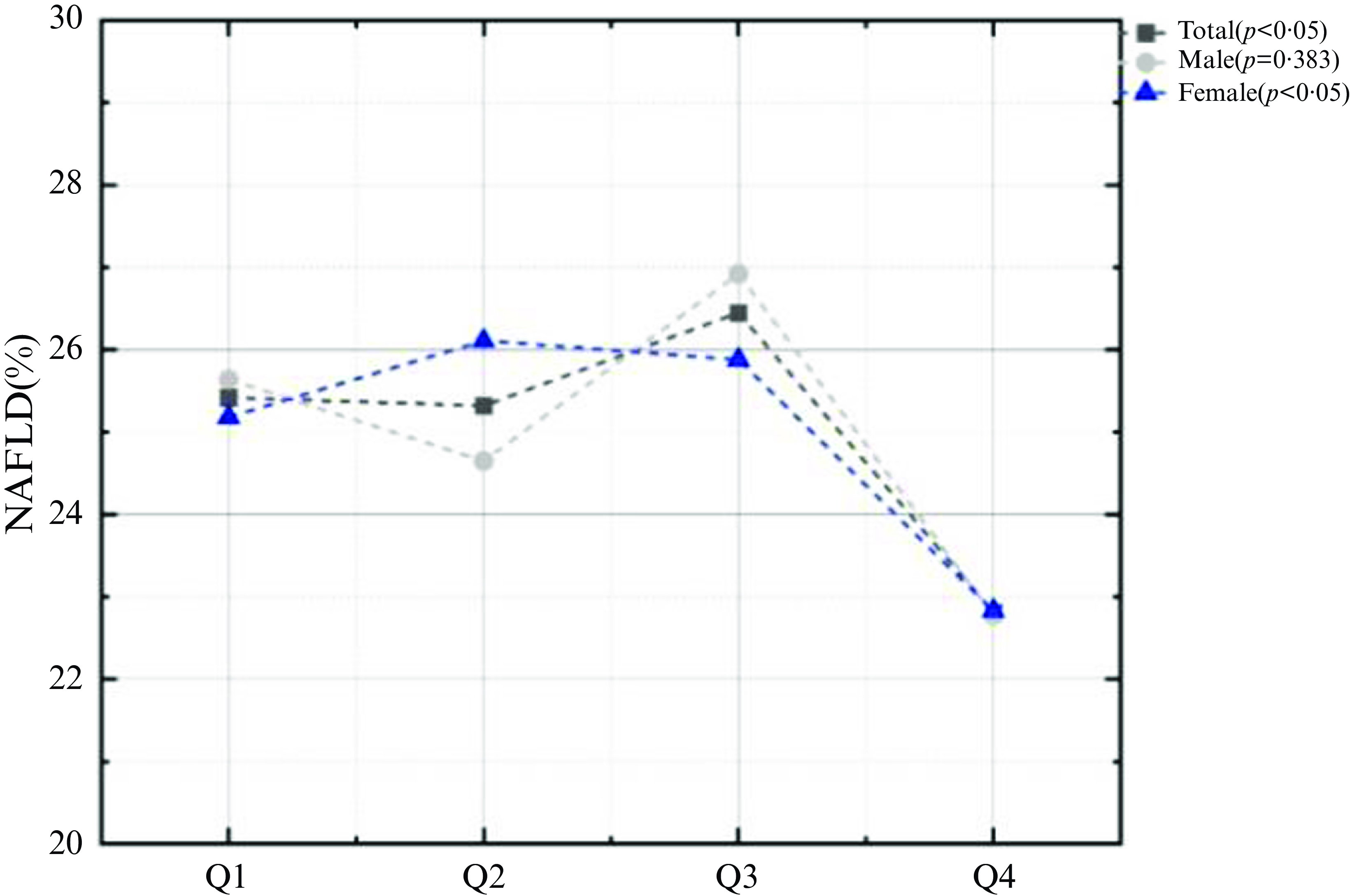
To further investigate the connection between tea consumption and NAFLD, the author grouped tea consumption by quartiles. The results showed that the highest black tea intake group (Q4) was significantly negatively associated with NAFLD in model1 which was not adjusted for any confounders (OR: 0·81, 95 % CI: 0·67, 0·96, P < 0·05) and maintained negatively correlated with NAFLD after adjustment of age, sex and race in model2 (OR: 0·82, 95 % CI: 0·68, 0·99, P < 0·05). This correlation remained steady after continuing to adjust for BMI, co-morbidity index, creatinine, albumin, FIB.4, AST to ALT (OR: 0·69, 95 % CI: 0·50, 0·93, P < 0·05) (Table 2), but neither green tea nor other tea displayed significant association with NAFLD. Also, the prevalence of NAFLD was significantly lower in the highest black tea intake group (Q4) than in the other groups (Q1: 25·42 %, Q2: 25·32 %, Q3: 26·45 %, Q4: 22·81 %, P = 0·011) (Fig. 4).
Table 2. Associations between tea consumption and non-alcoholic fatty liver disease (OR and 95 % CI)

Model1: Crude model (without adjustment for any confounders). Model2 was corrected for age, sex and race. Model3 was corrected for variables included in Model2 and BMI, comorbidity index, albumin, creatinine fibrosis-4 index, aspartate aminotransferase, alanine transaminase.

Fig. 4. Proportion of NAFLD in the quartile of black tea gram.
Subgroup analysis
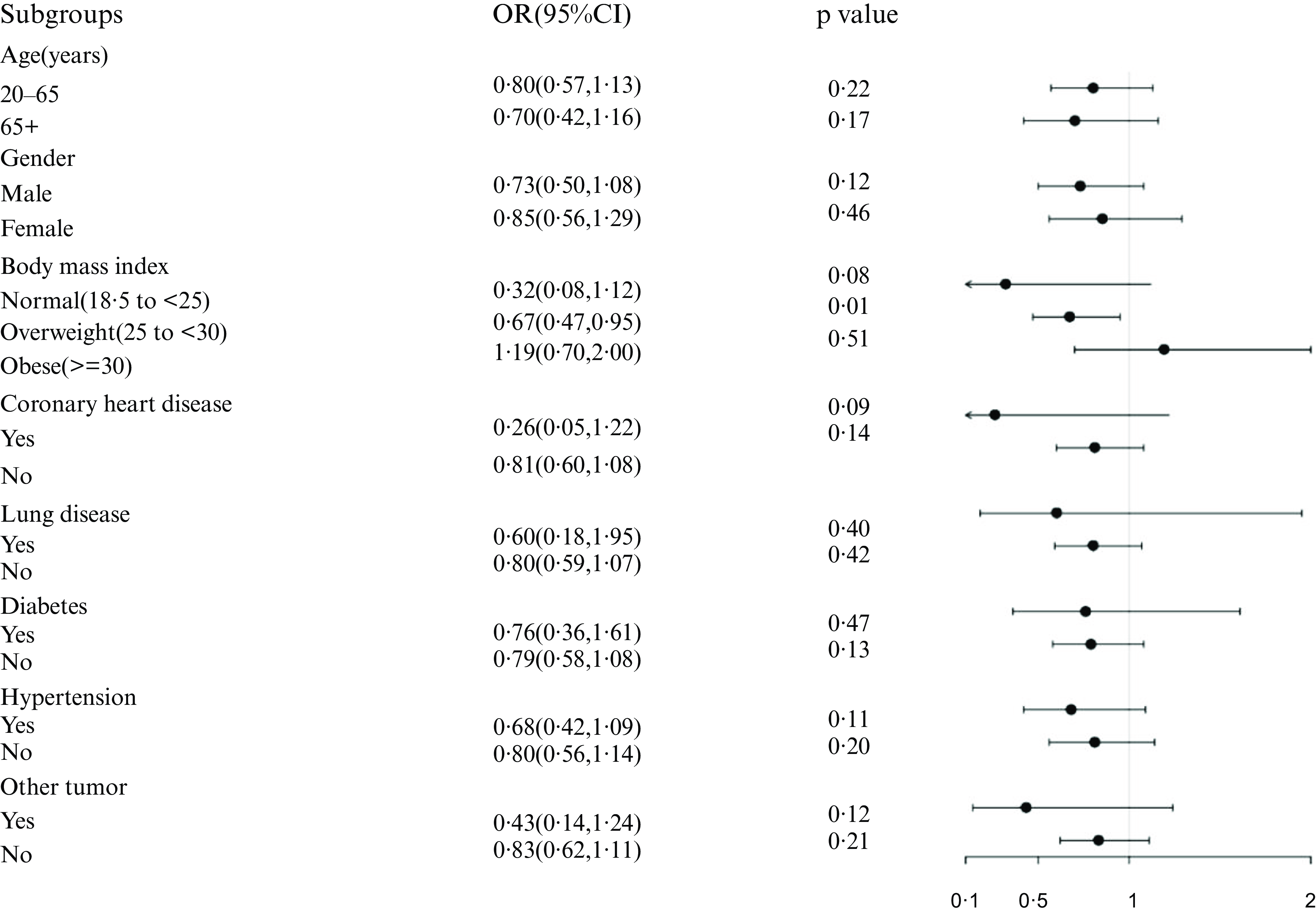
Black tea is the most consumed tea beverage in developed countries; thus, authors further investigated the correlation between black tea intake (Q4) and NAFLD (Fig. 5). Following stratification of the study populations by age, sex, BMI and disease condition, independent multifactorial logistic regression analyses were performed for each subgroup. Except for the variables used for stratification, the variables adjusted in Model3 remain in this analysis. The association remained stable among the overweight subgroup (OR: 0·67, 95 % CI: 0·47, 0·95, P < 0·05).

Fig. 5. Association between black tea (Q4) and NAFLD in different stratifications. The model adjusted for covariates such as age, gender, BMI, comorbidity index, albumin, creatinine, FIB-4, AST, ALT, but the model did not adjust for the stratification variables themselves.
Relationship between tea consumption and the degree of hepatic fibrosis
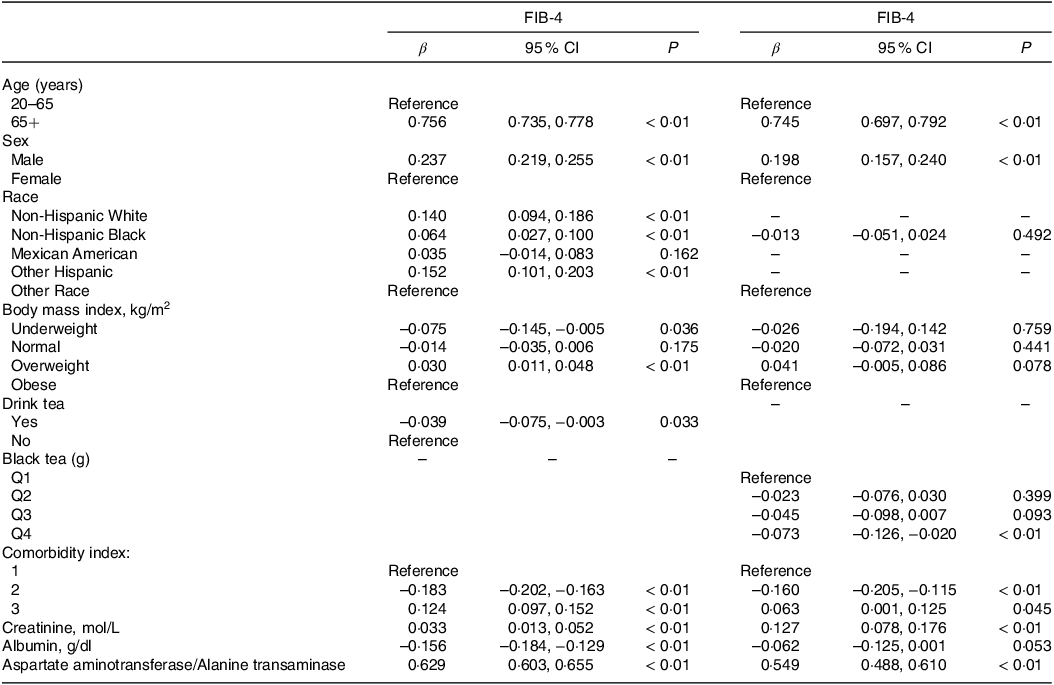
NAFLD could evolve into liver fibrosis or even cirrhosis if it continued to progress. To further explore the connection between tea consumption and disease progression, linear regression analysis was used to analyse the effect of tea consumption on FIB-4, a non-invasive index of hepatic fibrosis (Table 3). After adjusting for confounders (Model3), with the non-tea drinker as the reference, tea drinkers was significantly negatively correlated with FIB-4 (β = –0·039, 95 % CI: –0·075, −0·003, P < 0·05). Further analysis showed that consumption of black tea > 754·8 g (Q4) had the most protective effect on fibrosis degree in NAFLD (Q4: β = –0·073, 95 % CI: –0·126, −0·020, P < 0·01).
Table 3. Linear regression analysis of tea and black tea intake (classification) and degree of liver fibrosis (95 % CI)

Corrected for age, sex, race, BMI, tea consumption, comorbidity index, albumin, creatinine, aspartate aminotransferase and alanine transaminase.
Discussion
This study explored the association between tea consumption and NAFLD using genetic data from Genome Wide Association Study and clinical data from the large cross-sectional study NHANES. Our findings suggest that there exists a causal association between tea consumption and NAFLD based on genetic prediction. And when grouped according to the type of tea, it was found that consumption of black tea > 754·8 g per day reduced the risk of NAFLD by 24 % and had a protective effect against hepatic fibrosis. However, green tea and other types of tea were not significantly associated with a low prevalence of NAFLD.
Epidemiologic studies on the association between tea consumption and NAFLD are scarce, and we are aware of only 4 studies(Reference Guo, Ge and Lu9,Reference Soleimani, Ranjbar and Rezvani28–Reference Huo, Bai and Zhang30) .In a large prospective cohort study in 2022(Reference Guo, Ge and Lu9), 372, 492 participants without liver disease at baseline were followed for 12 years, with 3527 cases of NAFLD, 1643 cases of cirrhosis and 669 cases of hepatocellular carcinoma occurring during follow-up. Grouped by different dietary groups to explore NAFLD risk ratios, it was found that higher tea consumption (HR: 0·85, 95 % CI: 0·77, 0·94) was significantly associated with a lower risk of NAFLD, and similar results were observed in cirrhosis and hepatocellular carcinoma. Despite the study type and statistical approach was different, this multivariable-adjusted HR was comparable to that in our analysis of black tea (OR: 0·69, 95 % CI: 0·50, 0·93), i.e. tea consumption was a protective factor for NAFLD.A cross-sectional study conducted by Soleimani et al of 170 NAFLD participants using Fibroscan to assess hepatic fibrosis and using three 3-day dietary records during a 1-month period to assess diet showed that the risk of developing fibrosis was significantly lower in patients in the highest tertile of tea consumption (OR: 0·38; 95 % CI: 0·17, 0·71) than those in the lowest tertile, suggesting a protective role of tea on hepatic fibrosis(Reference Soleimani, Ranjbar and Rezvani28). Due to limited data, in our study we used FIB-4 rather than Fibroscan to assess hepatic fibrosis and obtained comparable results to their study, the highest quartile of black tea consumption significantly improved the hepatic fibrosis (β:–0·073, 95 % CI: –0·126, –0·020). Another cohort study enrolled 20, 051 participants without fatty liver at baseline. Using NAFLD onset as the outcome event, multivariate Cox analysis showed that tea consumption was a protective factor against NAFLD (OR: 0·86, 95 % CI: 0·78, 0·94)(Reference Huo, Bai and Zhang30).The results of these studies support our conclusions, but it is important to notice that they do not detail the tea types. There is only one cross-sectional study exploring the association between different tea types and NAFLD. Yang et al found a positive association between green tea (OR: 1·48, 95 % CI: 1·33, 1·65), oolong tea (OR: 1·50, 95 % CI: 1·33, 1·68),and black tea (OR: 1·28, 95 % CI: 1·13, 1·46) and the prevalence of NAFLD(Reference Xia, Wang and Zhang29). However, no significant association was found between tea consumption and the prevalence of NAFLD after correcting for factors that differed in baseline information and factors that were judged to be possible risk factors for NAFLD from a clinical perspective, suggesting that the confounding effect of common risk factors for NAFLD should not be underestimated. Our study took this fully into consideration when developing the regression model by including variables that did not differ in the baseline information, but were common risk factors for NAFLD. A prospective cohort study conducted in Yoshimi Town, Saitama Prefecture, recorded residents’ disease history, health status, medication history and tea consumption (categorised as </= 3, 4–9, or >/= 10 cups per day).The result showed that tea consumption reduced hepatocyte injury. Maximum tea consumption (>/= 10 cups) was significantly and negatively correlated with AST and ALT(Reference Imai and Nakachi31). However, the results of our study suggested that there was no significant difference in AST and ALT between tea drinkers and non-tea drinkers, which may be due to the fact that the distribution of the populations was not balanced in our study, with the number of tea drinkers being significantly smaller than the number of non-tea drinkers. In addition, Hoofnagle et al. found in their study that high doses of tea extracts may cause liver injury(Reference Saleh, Ali and Abe32–Reference Cho, Wang and Yeung34), so further longitudinal observational studies and randomised controlled trials are needed to evaluate the changes in liver function (such as AST and ALT) among tea drinkers. In conclusion, studies investigating the effect of tea consumption on NAFLD have inconsistent results, so in addition to observational studies using NHANES, we also used MR to provide a higher level of evidence(Reference Smith and Ebrahim35). Both the exposure and outcome SNP of this study were from European populations, potentially reducing population heterogeneity. Also, we used two models (IVW and weighted median model) to co-validate the existence of a causal relationship between tea consumption and NAFLD. All these make the results of this study more convincing.
Tea contains a variety of bioactive compounds, green tea being abundant in catechins and theanine, and black tea being abundant in theaflavins, theabrownin and caffeine, which could improve NAFLD via their anti-inflammatory, apoptosis-inducing and antioxidant abilities(Reference Yang and Wang5–Reference Wu, Huang and Li7,Reference Nehmi-Filho, Santamarina and de Freitas36–Reference Ohishi, Goto and Monira38) . The mechanism of the protective effect of black tea on NAFLD can be explained as follows: theabrownin, a major component of black tea, was found to inhibit obesity and NAFLD in mice through serotonin-related signalling pathways and the gut-liver axis in animal studies(Reference Li, Huang and Zhou39). Theaflavin, another major component of black tea, directly binds to and inhibits the activation of plasma kinin-releasing enzyme, which further stimulates adenosine monophosphate-activated protein kinase and its downstream targets to reduce the lipid deposition of hepatocytes(Reference Zhang, An and Li40). Theaflavin also regulates lipid metabolism through the Fads1/PPARδ/Fabp4 axis(Reference Valim, Martins-Filho and Gouvea41). Caffeine in black tea may alleviate NAFLD by increasing low density lipoprotein receptor expression through direct binding to epidermal growth factor receptor (EGFR) and activating the EGFR-ERK1/2 signalling pathway(Reference Huang, Wang and Zhang42).There are also studies that regard black tea as a whole. Shen et al. found that black tea alleviated high-fat diet-induced NAFLD by promoting the expression of PPARα in liver tissue and thereby promoting fatty acid β-oxidation and VLDL synthesis(Reference Shen, Xiao and Wu43). Moreover, black tea could significantly decrease the ratio of Firmicutes to Bacteroidetes, preventing NAFLD by modulating the intestinal microbiota(Reference Li, Mao and Xiong44).
There was no association between green tea or other teas and low prevalence of NAFLD in our study. Instead, Karolczak found that green tea reduced the prevalence of NAFLD in rats fed a high-fat diet(Reference Karolczak, Seget and Bajerska45). Considering that among 22 257 participants included in our study, only 611 consumed green tea and 726 consumed other tea, which may result in poor statistical efficacy, subsequent studies in larger sample sizes are needed. Consistent with previous studies(Reference Guo, Ge and Lu9,Reference Soleimani, Ranjbar and Rezvani28) , high black tea consumption was negatively correlated with the degree of hepatic fibrosis, exerted a protective effect against NAFLD and had a therapeutic optimal effect in overweight NAFLD patients (OR: 0·67, 95 % CI: 0·47, 0·95).This may be because black tea polyphenols are more effective than green tea polyphenols in lowering body weight and anti-obesity(Reference Pan, Gao and Tu46), and weight control is an important measure in NAFLD disease management. Interestingly, in a study that included 1013 people with type 2 diabetes, it was found that drinking tea more than twice a day led to an increased risk of developing NAFLD(Reference Yang, Zhou and Zhu47). In our study, high consumption of black tea also had no therapeutic effect on subgroups such as diabetes, probably because when NAFLD is co-morbid with diabetes, accompanied by worsening of insulin sensitivity and losing of glycaemic control(Reference Forst, Botz and Berse48), which can deteriorate the condition of NAFLD further, and so the therapeutic effect of tea may not be significant. The reasons for the differences in the effects of black tea on NAFLD with different underlying diseases still need to be further explored.
To the authors’ knowledge, this study is the first to combine genetic inference from MR with clinical validation in a large NHANES cohort to elucidate the relationship between tea-drinking habit and the risk of developing NAFLD. In addition, this study is the first large cross-sectional study to focus on the association between black tea and NAFLD. However, some limitations do exist in this study. First, in MR analysis, only summary-level statistics from general population were available, so subgroup analyses based on age, sex, etc. could not be performed, and the results could not be mutually verified with the subgroup analyses of NHANES study. Second, due to limited data or insufficient number of subjects analysed, we could not further analyse the association between other types of tea (e.g. oolong tea, white tea, etc.) and NAFLD, or between tea consumption and different subtypes of NAFLD (e.g. simple fatty liver, non-alcoholic steatohepatitis, etc.). Third, the subjects of this study were adult Americans, excluding adolescents and children, which may affect the generalisation of the results.
In conclusion, our study revealed that high black tea consumption was a protective factor for NAFLD and negatively correlated with hepatic fibrosis. These findings provide new ideas for the clinical treatment of NAFLD and encourage people to reduce the prevalence of NAFLD by adopting the habit of drinking tea, and provide new insights on diet-based health interventions for NAFLD. Further prospective studies are necessary in large populations to determine the association between different types of tea and NAFLD.
Acknowledgements
The authors thank the participants and staff of 2005–2018 of NHANES for their valuable contributions.
The authors report there is no funding associated with the work featured in this article.
The authors’ responsibilities were as follows: S. L. conceived and designed the study. Q. L., P. C., Y. W. and X. G. conducted the data extraction, and F. W., J. X. and Y. Z. assisted in data analysis. S. L. wrote the manuscript. L. M. and X. D. revised the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524002277