Cognitive impairment in BD
Bipolar disorder is a common, complex and costly illness with a poorly defined aetiology and, despite effective treatments,Reference Goodwin1 sufferers continue to experience long-term disability and reduced quality of life.Reference Rosa, Reinares, Michalak, Bonnin, Sole and Franco2 The cause of disability in bipolar disorder is multifactorial and extends beyond the effect of acute manic or depressive episodes.Reference Sanchez-Moreno, Martinez-Aran, Tabarés-Seisdedos, Torrent, Vieta and Ayuso-Mateos3 A critical contributing factor to disability in bipolar disorder is cognitive impairment, which frequently persists even during periods of euthymic mood.Reference Tsapekos, Strawbridge, Mantingh, Cella, Wykes and Young4,Reference Cullen, Ward, Graham, Deary, Pell and Smith5 These deleterious effects of cognitive dysfunction on quality of life are widespread, ranging from everyday psychosocial functioning (including occupational, household and social function),Reference Cullen, Ward, Graham, Deary, Pell and Smith5 core illness outcomes (e.g. number and severity of affective episodes, including hospital admission rate)Reference Cullen, Ward, Graham, Deary, Pell and Smith5 and suicidal ideation.Reference Luo, Zhu, Lu, Zong and Lin6
The prevalence of clinically significant cognitive impairment in euthymic bipolar disorder is estimated to be between 30 and 57%, and is found across cognitive domains of memory, attention, processing speed and executive functioning;Reference Szmulewicz, Samamé, Martino and Strejilevich7 however, within these rates, there is significant heterogeneity as to the severity and domain specificity of impairments experienced.Reference Tsapekos, Strawbridge, Mantingh, Cella, Wykes and Young4 Although some current treatments may have some protective effects on cognition (e.g. lithium, lurasidone), intervention at present does not specifically target cognitive impairment in those with bipolar disorder, despite some emerging evidence of potential cognitive interventions.Reference Strawbridge, Tsapekos, Hodsoll, Mantingh, Yalin and McCrone8–Reference Tsapekos, Strawbridge, Cella, Wykes and Young10 However, our understanding of the physiological mechanisms underlying cognitive impairment and bipolar disorder is lacking, which hinders progress in treatment options.
Neurobiological mechanisms of cognitive impairment
Recent evidence suggests that cognitive difficulties in people with depression,Reference Krogh, Benros, Jørgensen, Vesterager, Elfving and Nordentoft11 schizophrenia,Reference Ribeiro-Santos, Lucio Teixeira and Salgado12 Parkinson's diseaseReference Lindqvist, Hall, Surova, Nielsen, Janelidze and Brundin13 and Alzheimer's diseaseReference Whittington, Planel and Terrando14 may be linked to inflammation. As with these other illnesses, bipolar disorder is understood to have an inflammatory component, with systematic reviews reporting elevated pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α), soluble tumour necrosis factor receptor type 1 (sTNF-R1) and soluble inlerleukin-2 receptor (sIL-2R) levels in patients with manic bipolar disorder compared with controls; elevated sTNF-R1 levels in manic compared with euthymic bipolar disorder;Reference Munkholm, Vinberg and Vedel Kessing15 and elevated C-reactive protein (CRP)Reference Fernandes, Steiner, Molendijk, Dodd, Nardin and Gonçalves16 and interleukin-6 (IL-6)Reference Brietzke, Stertz, Fernandes, Kauer-Sant'Anna, Mascarenhas and Escosteguy Vargas17 levels in depression compared with euthymia. Despite this, altered immune responses have also been shown to persist even when patients are euthymic.Reference Fernandes, Steiner, Molendijk, Dodd, Nardin and Gonçalves16,Reference Brietzke, Stertz, Fernandes, Kauer-Sant'Anna, Mascarenhas and Escosteguy Vargas17
The relationship between chronic inflammation and cognitive impairment is not fully understood, but several theories for other psychiatric illnesses have postulated that chronic inflammation results in hippocampal volume loss related to hypothalamic-pituitary-adrenal (HPA) axis dysregulation as a mechanism for poor verbal recognition memory in patients with depression.Reference Shah, Ebmeier, Glabus and Goodwin18 Microglial activation has been proposed as a potential mechanism of cognitive impairment in bipolar disorder,Reference Stertz, Magalhães and Kapczinski19 and immune-modulatory drugs have shown some promising initial results in improving cognition in schizophrenia.Reference Levkovitz, Mendlovich, Riwkes, Braw, Levkovitch-Verbin and Gal20 The relationship between inflammation and Alzheimer's disease has been more widely studied than associations in mood and psychotic disorders; in models of dementia, a neuroinflammatory state has been demonstrated to activate microglia and release cytokines,Reference Ferreira, Clarke, Bomfim and De Felice21 ultimately leading to neuronal loss and exacerbating Aβ and neurofibrillary pathologies.Reference Garwood, Pooler, Atherton, Hanger and Noble22,Reference Kitazawa, Oddo, Yamasaki, Green and LaFerla23 Neurogenesis is also likely to be intimately involved with these relationships, with growth factor markers being clearly associated with neurocognitive and inflammatory functions,Reference Ekdahl, Claasen, Bonde, Kokaia and Lindvall24 and linked, alongside inflammation, with severity in mood disorders.Reference Pisoni, Strawbridge, Hodsoll, Powell, Breen and Hatch25
Biological links between affect and cognition
With increasing evidence that inflammation plays a critical role in the development of cognitive impairment across a range of psychiatric conditions, it is imperative this is considered more closely in bipolar disorder, given the burden of illness faced by patients with bipolar disorder who experience cognitive impairment. Evidence to date implicates some of the commonly assessed inflammatory markers in cognitive dysfunction, although this pertains to a limited set of biomarkers and the relationship with affective symptoms is unclear.Reference Rosenblat, Brietzke, Mansur, Maruschak, Lee and McIntyre26–Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken29 Because of the difficulties disentangling specific cognitive impairment from impairments arising as a result of affective symptoms, it is important that cognitive impairment that would require treatment (i.e. be persistent beyond affective episodes) is assessed in fully euthymic states. Better understanding of the underlying pathophysiology may assist in developing/repurposing new treatment compounds or advancing the optimisation of existing treatment options in the future.
To our knowledge, no studies have yet compared a comprehensive panel of proteomic inflammatory markers and growth factor proteins with clinically relevant cognitive measures in bipolar disorder; this might facilitate a broader consideration of related biological networks as cognitive correlates in clinical practice. Besides, most studies of inflammation have only adjusted for limited clinical or demographic factors in these analyses, which limits the translational utility of findings.
Objectives
This study takes a comprehensive exploration of inflammatory predictors of cognitive impairment in adults with bipolar disorder not currently experiencing an episode of depression or (hypo)mania. We examine several demographic and clinical factors alongside a wide range of inflammatory and growth factor protein markers, defining cognitive performance according to international recommendations.Reference Miskowiak, Burdick, Martinez Aran, Bonnin, Bowie and Carvalho30
Previous evidence has suggested that elevated pro-inflammatory cytokines and/or CRP partially explain severity of cognitive impairment, but several other constructs, not always adjusted for, are known to influence inflammation and be associated with bipolar disorder. As the majority of protein markers examined in this study have not previously been assessed in association with cognitive function in individuals with bipolar disorder, this is considered an exploratory study, the results of which can be used to guide future hypothesis-driven studies.
Method
Design
This study is a secondary analysis of cross-sectional (baseline) data from the Cognitive Remediation in Bipolar (CRiB) study.Reference Strawbridge, Tsapekos, Hodsoll, Mantingh, Yalin and McCrone8 Methodological details of the CRiB study have been described previously.Reference Strawbridge, Tsapekos, Hodsoll, Mantingh, Yalin and McCrone8,Reference Strawbridge, Fish, Halari, Hodsoll, Reeder and Macritchie31 The CRiB study investigated a sample of 60 participants; the present analysis focuses on a subsample of 44 individuals who provided blood for biomarker analysis (N = 44), which was an optional assessment in the CRiB study.
Participants
Individuals were included in the study if they had a diagnosis of bipolar disorder (type 1 or type 2), had been in a euthymic affective state for at least 1 month, were aged 18–65 years, fluent in English and did not have a current substance use or personality disorder, or an impairing organic neurological disorder. Participants already had a formal bipolar disorder diagnosis, which was validated with the MINI-International Neuropsychiatric Interview (MINI).Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller32 The MINI was also used to ensure an absence of substance use disorders. To qualify as euthymic, participants needed to meet the Newcastle Euthymia Protocol criteria, scoring ≤7 on the Hamilton Rating Scale for Depression (HRSD)Reference Hamilton33 and Young Mania Rating Scale (YMRS)Reference Young, Biggs, Ziegler and Meyer34 at two time points 1 week apart, covering the month before inclusion.
Procedure
The study had received prior approval from the UK's Health Research Authority and London City Road & Hampstead NHS Research Ethics Committee (identifier 15/LO/1557; trial registration ISRCTN-32290525). Participants were recruited via community advertisement and primary and secondary care healthcare services, and all provided written informed consent before taking part. The data examined in this study was then provided in a single session, before participants were randomised to receive a cognitive remediation intervention or continue treatment as usual.
Measures
Clinical, psychosocial and demographic
Continuous variables assessed were age, body mass index, number of medications currently taken, health-related quality of life (as measured by the EuroQol-5D questionnaireReference Dolan, Culyer and Newhouse35), number of lifetime affective episodes, psychosocial functioning (measured by the Functioning Assessment Short Test (FAST)Reference Rosa, Sánchez-Moreno, Martínez-Aran, Salamero, Torrent and Reinares36), subsyndromal symptoms of depression (measured by the HRSDReference Hamilton33) and mania (measured by the YMRSReference Young, Biggs, Ziegler and Meyer34), anxiety symptoms (measured by the Hamilton Rating Scale for Anxiety (HRSA)Reference Hamilton37) and history of childhood trauma (measured by the Childhood Trauma Questionnaire (CTQ)Reference Bernstein, Fink, Handelsman, Foote, Lovejoy and Wenzel38). Binary variables assessed were gender (all participants identifying as male or female based on free-text self-report), type of bipolar disorder (type 1 or type 2), current physical illness (yes/no), alcohol use (nil/low or medium/high, as per thresholds on the MINI interviewReference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller32) and smoking (yes/no). These ‘non-biological’ factors were selected a priori according to their understood associations with cognition and/or inflammation, as well as availability from the primary study, and all were considered in analyses as described in the statistical analysis section below.
Cognitive
The CRiB study included measurement of a neuropsychological battery producing numerous cognitive variables, as described previously.Reference Strawbridge, Tsapekos, Hodsoll, Mantingh, Yalin and McCrone8,Reference Strawbridge, Fish, Halari, Hodsoll, Reeder and Macritchie31 To reduce the (already extensive) number of comparisons undertaken, two measures of cognition were computed as informed by the International Society of Bipolar Disorders Cognitive Taskforce recommendations.Reference Miskowiak, Burdick, Martinez Aran, Bonnin, Bowie and Carvalho30 These essentially measure global cognitive performance as a continuous measure, and cognitive impairment as a classified (dichotomous) construct.Reference Tsapekos, Strawbridge, Mantingh, Cella, Wykes and Young39 The continuous measure of ‘global’ cognitive performance (higher scores indicating less impairment) is calculated from eight cognitive tests across four domains: processing speed (using the Digit Symbol Substitution Test and symbol search (Wechsler Adult Intelligence Scale)Reference Wechsler40), working memory (using the digit span (Wechsler Adult Intelligence Scale)Reference Wechsler40), verbal learning and memory (from the verbal paired associates tests I and II (Wechsler Memory Scale)Reference Wechsler41), and executive functioning (from the Hotel test,Reference Manly, Hawkins, Evans, Woldt and Robertson42 matrix reasoning (Wechsler Abbreviated Scale of Intelligence)Reference Wechsler43 and verbal fluency F-A-S testReference Delis, Kaplan and Kramer44). For each test, the raw score was transformed into standardised normative scores (correcting for age and education) as per test manuals, and the composite global score was then calculated by averaging each participant's z-scores across individual cognitive tests.Reference Tsapekos, Strawbridge, Mantingh, Cella, Wykes and Young4 The binary summary variable representing clinically significant cognitive impairmentReference Miskowiak, Burdick, Martinez Aran, Bonnin, Bowie and Carvalho30 categorises participants scoring ≥1 s.d. below published norms on two or more of the aforementioned cognitive tests as impaired and others as unimpaired.
Biomarker
For each participant, 5 mL of blood was drawn. Plasma was obtained after centrifugation and samples were stored at −80 until thawing for assay. Plasma samples were assayed using a high-sensitivity Meso Scale Discovery (MSD) V-Plex kit (Meso Scale Diagnostics, Maryland, USA) as previously.Reference Strawbridge, Marwood, King, Young, Pariante and Colasanti45 This kit was selected because of its ability to assess an extensive range of inflammatory and trophic proteins understood to be relevant to affective disorders and with a high detection sensitivity.Reference Pisoni, Strawbridge, Hodsoll, Powell, Breen and Hatch25,Reference Strawbridge, Marwood, King, Young, Pariante and Colasanti45 The panel of protein markers comprised brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), CRP, eotaxin, eotaxin-3, fms-like tyrosine kinase (Flt-1), intracellular adhesion module (ICAM-1), interferon-γ (IFN-γ), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-15 (IL-15), interleukin-16 (IL-16), interleukin-17 (IL-17), interleukin-1α (IL-1α), IL-6, interleukin-7 (IL-7), interleukin-8 (IL-8), interferon-γ-induced protein-10 (IP-10), macrophage chemoattractant protein-1 (MCP-1), macrophage chemoattractant protein-4 (MCP-4), macrophage inflammatory protein-1α (Mip-1α), macrophage inflammatory protein-1β (Mip-1β), placental growth factor (PlGF), serum amyloid-A (SAA), thymus and activation-regulated chemokine (TARC), angiopoietin-1 receptor (Tie-2), TNF-α, tumour necrosis factor-β (TNF-β), vascular cell adhesion molecule-1 (VCAM-1), vascular endothelial growth factor (VEGF), vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor D (VEGF-D). Plasma was assayed with the MSD array, according to manufacturer's manual, with seven-point standard curves run in duplicate to calculate the levels of each protein with each sample, and a no-template control included to control for background fluorescence. The standard curves demonstrated very high concentration-fluorescence correlations (r 2 > 0.99), indicating reliability. Protein levels are expressed as picograms per millilitre, unless otherwise stated.
Statistical analyses
Protein levels were transformed (log base 10) and normality of distribution assessed (via skewness and kurtosis values, visual inspection of stem and leaf diagrams and box plots in addition to the Kolmogorov–Smirnov test). Because of issues surrounding the removal of outliers, particularly in small samples, bootstrapping of 1000 samples was employed on all statistical tests instead, because in these cases outliers hold less weight without removing potentially valid data altogether, and this also has the benefit of dealing with slightly non-normally distributed variables.Reference Davison and Hinkley46 Initially, univariate indications of proteins associated with the two cognitive variables was assessed with Spearman's correlation (for global cognitive performance) and t-tests (for cognitive impairment). Any protein associated with either cognitive variable at P < 0.1 (‘potentially indicative’) was decided a priori to be considered further as putative predictors of cognitive function in respective multivariable models. Before multivariable models, the respective univariate tests also compared the above-mentioned ‘non-biological factors’ selected as putative confounders with the cognitive outcomes, and with the indicated protein markers; any associated at P < 0.01 were also included as covariates in these multiple regression analyses. Multiple regressions thus aimed to explore all potentially indicative markers (i.e. biological and non-biological factors associated with cognition at P < 0.1 in univariate tests, as independent variables) of each cognitive outcome (dependent variable). Both linear regressions (predicting global cognitive performance) and logistic regressions (predicting impairment status) were conducted. Note that the terms ‘predicting’ or ‘predictors’ here refer to cross-sectional statistical associations, rather than longitudinal prediction. Model assumptions for collinearity were checked (Hosmer–Lemeshow test in logistic regressions, and Durbin–Watson in linear regressions) and a P-value of <0.05 was considered nominally significant.
Results
Sample characteristics
Forty-four participants were assessed (based on protein marker availability in addition to the study eligibility criteria). Participants who were not currently well on the day of the baseline assessment rescheduled their appointment, and therefore, to our knowledge, none had a cold or current infection. Twenty-one participants were grouped as cognitively impaired (48%) and 23 participants were grouped as unimpaired. The mean composite cognitive performance scores were z = −0.675 (s.d. 0.426) for the impaired group and z = 0.261 (s.d. 0.350) for the unimpaired group (overall mean z = −0.186, s.d. = 0.609 across the sample). Supplementary Tables 1 and 2 available at https://doi.org/10.1192/bjo.2021.966 contain descriptive data related to inflammation and cognition respectively. Clinical and demographic characteristics of the sample are presented in Table 1. None of the non-biological variables had any missing values. Four proteins (IFN-γ, IL-10, TARC and TNF-β) had 1–19% levels undetected by the assay, and these missing values were imputed with half the lower limit of detection.Reference Croghan and Egeghy47 Eight proteins had ≥20% levels undetected by the assay (granulocyte-macrophage colony-stimulating factor, interleukin -2p70, -13, -1β, -2, -4 and -5, and interferon-α) and were excluded from analyses (see Supplementary Table 1); these rates of non-detection align with previous reports in similar samples.Reference Pisoni, Strawbridge, Hodsoll, Powell, Breen and Hatch25,Reference Strawbridge, Marwood, King, Young, Pariante and Colasanti45 A total of 32 proteins were analysed.
Table 1 Participant characteristics

BMI, body mass index; HRSD, Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale; FAST, Functioning Assessment Short Test; HRSA, Hamilton Rating Scale for Anxiety; CTQ, Childhood Trauma Questionnaire.
a. Data missing for two participants. No other missing data.
b. Originally smoking coded as current/past/never, but previous smokers did not differ from those who had never smoked in any cognitive or inflammatory measure and the two were pooled.
c. The only significant association with cognition was that participants with cognitive impairment were more frequently smokers (χ 2 = 4.91, P = 0.027).
d. Alcohol use coded according to the MINI interview. Cognitive impaired/unimpaired groups were compared with participant characteristics, using t-tests (continuous) and chi-squared tests (binary). Composite cognitive performance were compared with participant characteristics, using Spearman's correlation (continuous) and t-test (binary).
Univariate associations between proteins and cognition
Table 2 displays the univariate associations between biomarker and cognitive variables. Twenty-three proteins were not associated at P < 0.1 with cognitive summary measures; nine proteins were indicated as potentially associated with at least one of the cognitive variables, summarised below and in Fig. 1.

Fig. 1 Summary of univariate associations between cognitive and protein markers. BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; IL-6, interleukin-6; IL-7, interleukin-7; IL-16, interleukin-16; Mip-1β, macrophage inflammatory protein-1β; PlGF, placental growth factor; TNF-β, tumour necrosis factor-β; VEGF-C, vascular endothelial growth factor C.
Table 2 Univariate associations between proteins and cognitive function
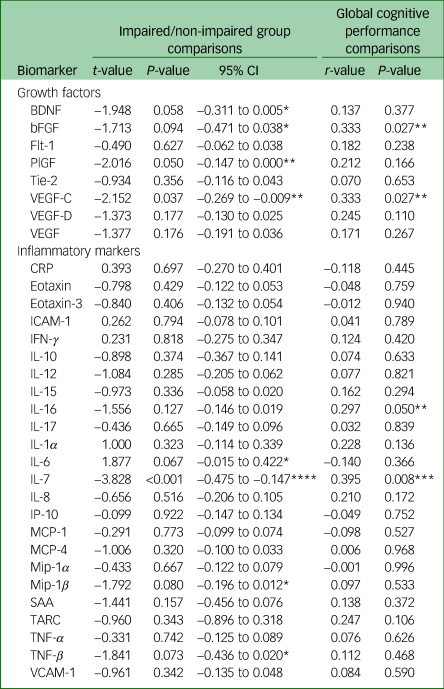
Positive t-values for the impairment group indicates higher protein levels in participants with cognitive impairment (negative t-values indicate lower protein levels in participants with cognitive impairment). A positive correlation between protein and cognitive performance indicates higher protein levels in those with better cognitive performance. BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; Flt-1, fms-like tyrosine kinase; PlGF, placental growth factor; Tie-2, angiopoietin-1 receptor; VEGF-C, vascular endothelial growth factor C; VEGF-D, vascular endothelial growth factor D; VEGF, vascular endothelial growth factor; CRP, C-reactive protein; ICAM-1, intracellular adhesion module; IFN-γ, interferon-γ; IL-10, interleukin-10; IL-12, interleukin-12; IL-15, interleukin-15; IL-16, interleukin-16; IL-17, interleukin-17; IL-1α, interleukin-1α; IL-6, interleukin-6; IL-7, interleukin-7; IL-8, interleukin-8; IP-10, interferon-γ-induced protein-10; MCP-1, macrophage chemoattractant protein-1; MCP-4, macrophage chemoattractant protein-4; Mip-1α, macrophage inflammatory protein-1α; Mip-1β, macrophage inflammatory protein-1β; SAA, serum amyloid-A; TARC, thymus and activation-regulated chemokine; TNF-α, tumour necrosis factor-α; TNF-β, tumour necrosis factor-β; VCAM-1, vascular cell adhesion molecule-1.
*P < 0.1, **P < 0.05, ***P < 0.01, ****P < 0.001.
VEGF-C and IL-7 were significantly associated with both measures of cognition, with higher levels in the cognitively unimpaired group and a positive correlation with cognitive performance. The same pattern was observed for bFGF, although the association with the group was non-significant at P < 0.05. IL-16 was also positively correlated with continuous performance, but not to a significant extent with impairment group. PlGF was also higher in the cognitively unimpaired group, but not correlated to a significant extent with continuous performance. Three further markers were non-significantly (P < 0.1) higher in participants without cognitive impairment (BDNF, TNF-β, Mip-1β). IL-6 was the only marker found to be higher in participants with cognitive impairment, although the association did not meet the threshold for statistical significance.
Because of the number of comparisons, the above analyses are to be interpreted as only preliminary, non-inferential indications. Before regression analyses, the nine indicated proteins were compared with non-biological variables, presented in Supplementary Tables 2 and 3. The only non-biological markers associated with cognition were the FAST measure of psychosocial functioning, which was tentatively and positively associated with global cognitive performance (r = −0.275, P = 0.071), but not impairment group; and smoking, which was more prevalent in participants grouped as cognitively impaired versus unimpaired (χ 2 = 4.91, P = 0.027), but was not associated with global performance as a continuous measure. Multivariable models included FAST in all linear regressions, and smoking in all logistic regressions.
Multivariable regression analyses
Tables 3 and 4 present the results of regression models predicting, cross-sectionally, cognitive group status (logistic; Table 3) and the continuous measure of global cognitive performance (linear; Table 4) with each of the nine indicated proteins as independent variables.
Table 3 Multivariable logistic regressions predicting cognitive impairment group

Multivariable logistic regressions did not indicate a significant concern of collinearity within any of the models (Hosmer–Lemeshow test). The P-values provided are following bootstrapping. Bold text indicates significance at P < 0.05. BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; IL-16, interleukin-16; HRQOL, health-related quality of life (EQ-5D score); CTQ, childhood trauma severity (Childhood Trauma Questionnaire); IL-6, interleukin-6; FAST, functional impairment (Functioning Assessment Short Test); IL-7, interleukin-7; Mip-1β, macrophage inflammatory protein-1β; PlGF, placental growth factor; TNF-β, tumour necrosis factor-β; VEGF-C, vascular endothelial growth factor C.
a. For underpowered models (those containing more than one independent variable per ten participants, i.e. more than four in total), regressions were re-run containing only covariates that were significant at P < 0.05 (between inflammatory and non-biological, or cognitive and non-biological). The results were similar; IL-6 differed in that health-related quality of life was no longer significantly associated with impairment group.
Table 4 Multivariable linear regressions predicting global cognitive performance

Multivariable linear regressions did not indicate a significant concern of collinearity within any of the models (Durbin–Watson value between 1 and 3.) Bold text indicates significance at P < 0.05. BDNF, brain-derived neurotrophic factor; FAST, functional impairment (Functioning Assessment Short Test); bFGF, basic fibroblast growth factor; IL-16, interleukin-16; HRQOL, health-related quality of life (EQ-5D score); CTQ, childhood trauma severity (Childhood Trauma Questionnaire); IL-6, interleukin-6; IL-7, interleukin-7; Mip-1β, macrophage inflammatory protein-1β; PlGF, placental growth factor; TNF-β, tumour necrosis factor-β; VEGF-C, vascular endothelial growth factor C.
a. For underpowered models (those containing more than one independent variable per ten participants, i.e. more than four in total), regressions were re-run only containing covariates that were significant at P < 0.05 (between inflammatory and non-biological, or cognitive and non-biological). The results were similar, with the exception of PlGF, for which the model as a whole was no longer significant.
As in the univariate associations, both VEGF-C and IL-7 were significantly lower in participants with poorer cognition in both cognitive outcomes (P < 0.01). In both logistic regressions, smoking also remained a significant predictor of impairment. IL-7 was the only significant independent predictor in the relevant linear regression, whereas VEGF-C was accompanied by FAST, which was also significant at P < 0.05. bFGF was significantly lower (P < 0.01) in those with poorer cognitive performance (with FAST also significant at P < 0.05), but the association with impairment group in logistic regression was not significant. IL-16 did not predict better cognitive performance or unimpaired group status when considered in regressions alongside health-related quality of life, childhood trauma severity, bipolar type and FAST/smoking.
Of the four proteins (PlGF, BDNF, Mip-1β and TNF-β) that indicated a univariate association with group (higher in participants without cognitive impairment) but not continuous performance, only PlGF contributed significantly (P < 0.05) to both cognitive outcomes in multivariable regression models (also containing medications, health-related quality of life, gender, smoking, age and FAST). The other three biomarkers were not significantly associated with cognitive performance. BDNF and Mip-1β also did not predict impairment group status, but TNF-β (alongside number of episodes and smoking) significantly predicted impairment status at P < 0.05.
IL-6 was the only protein indicated at P < 0.1 as higher in the impaired versus unimpaired group. In multivariable regressions (adjusting for health-related quality of life, smoking, physical illness and FAST), this cytokine was not a significant predictor of either cognitive outcome, although was the only protein indicating an effect of medium (as opposed to small) effect size (odds ratio 3.52).
Post hoc analyses
We maximised inclusiveness of eligibility criteria for this secondary analysis because of the small sample size. This meant that, a priori, participants with autoimmune illnesses or taking anti-inflammatory medications were not excluded. However, since these clearly affect inflammatory marker levels (and also may influence cognition), these factors were considered post hoc. After data were accessed, two participants were identified who had an autoimmune condition (n = 1) or were taking anti-inflammatory medications (n = 2). Sensitivity analyses were conducted, reanalysing the above comparisons with these two participants removed. Results were largely unaffected by this (differences are described in Supplementary Table 4), with the main changes being that TNF-β and IL-6 no longer indicated association with cognitive functioning. In the multivariable models, the same cytokines were significant as in the planned analyses, with one exception (TNF-β; significance reduced to P = 0.057).
An exploratory comparison employed Spearman's correlation to indicate associations between this subset of putative biomarkers with the individual cognitive domains that comprised the cognitive summary variables (see Supplementary Table 4). Most correlations between proteins and individual domains were small and the only associations where P < 0.01 were IL-7 with verbal memory (Verbal Paired Associates II) and processing speed (symbol search), and VEGF-C with symbol search. Four biomarkers (IL-6, Mip-1β, PlGF and TNF-β) were not significantly associated with any individual domain, and executive functioning was not significantly associated with any protein levels.
Discussion
In this sample of 44 euthymic participants with bipolar disorder, 6 of 32 examined proteins were associated with composite cognitive outcomes and 4 remained significant in regression models, after adjusting for potentially relevant non-biological covariates (both when including and excluding participants with an inflammatory condition or anti-inflammatory medication).
Three of these (VEGF-C, IL-7 and PlGF) were all lower in participants with poorer cognition as measured by both outcomes examined (clinically relevant impairment and global cognitive performance). With the same direction of effect, bFGF was predictive of global performance and, to a lesser extent, TNF-β was predictive of impairment group status cross-sectionally.
Neurobiology, mood and cognition in bipolar disorder
It might be expected that neurobiological dysregulations in bipolar disorder would be confined to mood episodes, and most research has investigated pro-inflammatory states during mania or depression.Reference Barbosa, Bauer, Machado-Vieira and Teixeira48,Reference Ortiz-Domínguez, Hernández, Berlanga, Gutiérrez-Mora, Moreno and Heinze49 However, elevated inflammatoryReference Doganavsargil-Baysal, Cinemre, Aksoy, Akbas, Metin and Fettahoglu50 and attenuated trophicReference Monteleone, Serritella, Martiadis and Maj51 biomarkers have also been reported in periods of euthymia. Within-participants comparisons of these biomarkers across different affective states are scarce, but suggest more pronounced dysregulations in mood states.Reference Ortiz-Domínguez, Hernández, Berlanga, Gutiérrez-Mora, Moreno and Heinze49,Reference Kapczinski, Dal-Pizzol, Teixeira, Magalhaes, Kauer-Sant'Anna and Klamt52,Reference Tramontina, Andreazza, Kauer-Sant'anna, Stertz, Goi and Chiarani53 Thus, we may have identified stronger or more frequent biomarker associations if the study been conducted when participants were experiencing an episode. However, our main interest was in cognitive associations with these biomarkers, and focusing on individuals with euthymia allowed an investigation essentially independent of mood, which has been subject to more intensive research. It is notable that none of the nine proteins assessed were correlated with subsyndromal manic, depressive or anxious symptom severity.
To date, relatively few studies have explored the relationship between inflammation and cognition, as recently reviewed.Reference Rosenblat, Brietzke, Mansur, Maruschak, Lee and McIntyre26 A 2018 study reported that in the presence of poor performance in tasks of affective processing, verbal memory, working verbal memory and executive functioning, participants with euthymic bipolar disorder had elevated plasma pro- and anti-inflammatory cytokines compared with controls, with IL-6 being negatively correlated with global cognitive performance.Reference Barbosa, de Ferreira, Rocha, Mol, da Mata Chiaccjio Leite and Bauer27 A subsequent study reported that another prominent pro-inflammatory cytokine, TNF-α (but not IL-6 or other cytokines), was elevated in patients with poorer global cognition, processing speed and working memory.Reference Chakrabarty, Torres, Bond and Yatham28 This association has recently also been reported for CRP,Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken29 although the patients in the latter two studies were in variable affective states at the time of assessments, and the biomarker/cognitive assessments were not always collected on the same day. Previous studies tended to assess cognitive function by using domain-specific outcomes, often testing several individual cognitive tests, rather than defining cognitive impairment by using consensus-recommended definitions or computing global cognition composite scores.Reference Miskowiak, Burdick, Martinez Aran, Bonnin, Bowie and Carvalho30 Critically, they also examined a limited set of inflammatory biomarkers. In measuring a comprehensive panel of proteins, we captured a broader neurobiological impression of inflammatory and growth factor markers whose functions are understood to interact.
Candidate biomarkers of cognitive dysfunction in bipolar disorder
Our results only tentatively support an association, as identified previously,Reference Barbosa, de Ferreira, Rocha, Mol, da Mata Chiaccjio Leite and Bauer27 between elevated IL-6 and impaired cognition. Mechanistic evidence suggests that not only can this cytokine cross the blood–brain barrier, but it may be directly involved in memory consolidation.Reference Benedict, Scheller, Rose-John, Born and Marshall54 However, as supported by our analyses, it may be that this pro-inflammatory cytokine is more directly associated with well-being (e.g. quality of life or psychosocial functioning, which are putatively associated with reduced cognition) or affective symptoms. IL-6 has been found to rise particularly in mania,Reference Ortiz-Domínguez, Hernández, Berlanga, Gutiérrez-Mora, Moreno and Heinze49 and may even fluctuate in association with subclinical symptoms in samples categorised as broadly euthymic.
BDNF is well-understood to be critical in cognitive function via neural plasticity and affected by inflammatory states,Reference Stertz, Magalhães and Kapczinski19 so it is unsurprising that this neurotrophic factor has been found as attenuated in the presence of cognitive impairment in bipolar disorder.Reference Bauer, Pascoe, Wollenhaupt-Aguiar, Kapczinski and Soares55 Known to be particularly implicated in memory synthesis via neuronal action in the hippocampus, cortex and basal forebrain,Reference Yamada and Nabeshima56 it is possible that the BDNF–cognition association in our study did not reach significance because cognition was measured as a global construct, rather than domain-specific comparisons. However, this growth factor has often been strongly correlated across a variety of cognitive domains.Reference Bauer, Pascoe, Wollenhaupt-Aguiar, Kapczinski and Soares55,Reference Dias, Brissos, Frey, Andreazza, Cardoso and Kapczinski57
bFGF, another neurotrophic factor and signalling protein involved in tissue repair and angiogenesis, has been found to enhance hippocampal neurogenesis following brain injuries.Reference McDermott, Raghupathi, Fernandez, Saatman, Protter and Finklestein58 The positive association between bFGF and cognitive performance was significant only for the continuous measure and not for impairment group. To our knowledge, this is the first study assessing bFGF and cognition in bipolar disorder.
VEGF growth factors are signalling proteins involved in the growth and maintenance of both vascular and neural cells, and appear protective against cognitive impairment,Reference de Almodovar C, Lambrechts, Mazzone and Carmeliet59 particularly in the context of Alzheimer's disease.Reference Garcia, Ornellas, Martin, Patti, Mello and Frussa-Filho60 Notably, VEGF-A and VEGF-D were not associated with cognitive functioning in this study, but VEGF-C was markedly higher in participants with unimpaired cognitive performance (according to both cognitive outcomes). To our knowledge, VEGF-C has not been compared with cognitive function in individuals with bipolar disorder, but our results are supported by recent preclinical evidence of decreased cerebrospinal VEGF-C having a negative effect on cognitive task performance.Reference Valente, Begg and Filiano61
PlGF is a ligand of VEGFR and is involved in the recruitment of monocytes and macrophages, which promote vessel growth and angiogenesis.Reference Pisoni, Strawbridge, Hodsoll, Powell, Breen and Hatch25 Similar to VEGF-C above, we identified a significant and positive association between PlGF and overall cognitive performance, although this was not apparent when assessing individual cognitive domains, and we are not aware of previous studies of PlGF in people with bipolar disorder. Unlike PlGF, individual cognitive domain examinations supported a positive relationship across multiple tests of processing speed and memory with IL-7 and VEGF-C.
Similar to the above, IL-7 (which acts as a growth factor and cytokine important for B and T cell development) was positively and strongly significantly associated with cognitive function in both outcomes, in this study. Our findings accord with its understood mechanisms of action, although it has not, to our knowledge, previously been studied in association with cognition in bipolar disorder.
We are also not aware of previous comparisons between lymphotoxin (or TNF-β) and cognition in a sample of participants with euthymic bipolar disorder. TNF-β levels were lower in participants with cognitive impairment, which is slightly surprising given its functional proximity to TNF-α and sTNF receptors. which have documented involvement in both bipolar disorder and cognitive impairments, with effects in the reverse direction.Reference Munkholm, Vinberg and Vedel Kessing15,Reference Rosenblat, Brietzke, Mansur, Maruschak, Lee and McIntyre26,Reference Chakrabarty, Torres, Bond and Yatham28 Despite this, TNF-β has previously been reported as attenuated in the presence of inflammatory signals in those with severe depressive episodes,Reference Strawbridge, Hodsoll, Powell, Hotopf, Hatch and Breen62 and we highlight that the association we identified with this marker was not robust (i.e. did not persist to a significant extent after exclusion of participants whose inflammatory activity was likely influenced by a health condition or medication).
The chemokine Mip-1β (CCL4) is produced in response to pro-inflammatory cytokines, and elevated levels have been reported in people with bipolar disorder with lower cortical thickness,Reference Poletti, Leone, Hoogenboezem, Ghiglino, Vai and de Wit63 as well as severity of cognitive impairment after stroke.Reference Angelia, Andrea, Anna, George and Calin64 In this study, Mip-1β was non-significantly lower in participants with cognitive impairment (and was also positively correlated with PlGF), which warrants further examination. It is worth noting that attenuated levels of this chemokine have been reported in people with depression (who typically present with pro-inflammatory indications) compared with healthy controls.Reference Lehto, Huotari, Niskanen, Herzig, Tolmunen and Viinamäki65
When examining associations between these markers and individual cognitive domains post hoc, memory and processing speed were frequently correlated with these proteins, but a striking absence of significant relationships with executive functioning is noted, contrary to previous assertions.Reference Rosenblat, Brietzke, Mansur, Maruschak, Lee and McIntyre26
Methodological considerations
As below, we first emphasise that this exploratory study was underpowered and that numerous statistical comparisons were undertaken without adjusting for multiple testing. Therefore, clearly all nominally significant findings from the current investigation require replication in larger samples of individuals with euthymic bipolar disorder with and without cognitive impairment, and including a matched group of non-affected controls. Our study was considered an exploratory investigation of a large panel of inflammatory and trophic proteins, many of which had not been subject to examination in samples with bipolar disorder, in only 44 participants. Thus, the comparisons made were underpowered statistically, and it is possible that type 2 errors may help to explain non-significant associations in this study that contrast with established markers of impaired cognition (e.g. BDNF). Proteins identified in this study as candidate biomarkers of cognitive dysfunction need to be considered in a unified predictive model controlling for more non-biological factors, to assess the predictive value of each putative marker as well as account for their inter-associations. Our analysis tentatively suggests that modelling a binary construct (i.e. clinically significant cognitive impairment) rather than a continuous global cognitive outcome might facilitate the identification of cognitive biomarkers and subsequent neurobiological treatment targets for improving cognition in this population.
One of the methodological issues common to neurobiological and neuropsychological research is the number of data points (or markers) required to fully ascertain the complexities of constructs such as ‘inflammation’ or ‘cognitive function’. Batteries of cognitive assessment commonly contain measurements of short-term and working memory, attention and executive functions, but there is much variation is the number and focus of tasks covering the umbrella of executive function domains. Typically, a well-rounded cognitive battery might be expected to comprise five to ten outcome variables. However, inflammatory research tends to focus on a few (two to five) traditional pro-inflammatory/T1 proteins.
Despite this, there are several other relevant constructs that we were not able to assess in this study, including waist circumference (which may better reflect adipose tissue, closely related to inflammation better than body mass index), biological gender in addition to or instead of identified gender, and use of specific medications that may have particular influence on cytokines (as opposed to general medication load). Another factor to consider is the imputation of half the limit of assay detection for proteins not detected by the MSD kit; although it is usual practice to make the assumption that this represents a low protein level present in the blood, it is indeed possible that there may have been other reasons for non-detection, and we note here that some putative biomarkers could not be assessed because of a particularly high rate of non-detection (including interleukin-1 (IL-1), which may have a prominent role in affective disorders).
As an exploratory study, we did not control for multiple comparisons, and (even limiting the number of regression models by initially conducting univariate analyses to focus on the potentially indicative proteins) several models were conducted, increasing the possibility of type 1 errors. The reporting of effect sizes in addition to P-values serves to aid interpretation of the effects observed. Future studies may consider these results to guide the investigation of candidate biomarkers of cognition in populations with bipolar disorder. Future work should also attempt to build on this work by assessing longitudinal relationships, as this cross-sectional study is not able to infer causality of association.
Potential mechanisms for cognitive impairment in bipolar disorder
In addition to neurogenesis, associations between reduced hippocampal volume and cognitive impairment in bipolar disorder may be mediated by inflammation or neuronal toxicity,Reference Aas, Haukvik, Djurovic, Bergmann, Athanasiu and Tesli66 although longitudinal studies are needed to ascertain temporal associations between these putatively related phenomena. Oxidative stress may also implicate mitochondrial,Reference Colasanti, Bugiardini, Amawi, Poole, Skorupinska and Skorupinska67 HPA axis,Reference Strawbridge, Young and McIntyre68 monoamineReference Rosenblat, Brietzke, Mansur, Maruschak, Lee and McIntyre26 and/or white matterReference Miyamoto, Maki, Pham, Hayakawa, Seo and Mandeville69,Reference Macritchie, Lloyd, Bastin, Vasudev, Gallagher and Eyre70 dysfunctions, all of which have been linked with bipolar disorder and cognitive difficulties. Additionally, genetic interactions may provide additional support for some of the above relationships, e.g. IL-1Reference Tsai71 or IL-6Reference Frydecka, Misiak, Pawlak-Adamska, Karabon, Tomkiewicz and Sedlaczek72 polymorphisms.
It is worth noting that both inflammatory proteins and cognitive impairments indicate dysregulations that persist into periods of recovery, but are exacerbated during acute affective episodes. However, the proteomic markers identified relate to cognitive and affective illness characteristics that manifest in individuals with bipolar disorder, and clearly inflammatory and neurotrophic systems play a role in the key features of bipolar disorder. Relevant interventional research has focused thus far on the antidepressant and antimanic effects of anti-inflammatory medications in individuals with mood disorders,Reference Husain, Strawbridge, Stokes and Young73 but the same agents have also shown pro-cognitive and neurogenic effects.Reference Jiang, Liu, Zhu, Ma, Ma and Zhou74 Despite the promise of anti-inflammatory treatments for affective episodes, the use of minocycline and celecoxib was not supported in the largest study of people with bipolar disorder in a depressive episode to date,Reference Husain, Chaudhry, Husain, Khoso, Husain and Hamirani75 although there was no apparent measurement of fundamental cognitive outcomes. In addition to translational research of pharmacological treatments that regulate inflammatory activity, it may likewise be informative to assess the effects of pharmacological and psychosocial pro-cognitive interventions on inflammatory and neurotrophic protein outcomes in populations with bipolar disorder.
In conclusion, this study has provided insight into possible biological markers of cognitive impairment in individuals with bipolar disorder currently free from affective symptoms. The proteins implicated include some established markers, such as VEGF-C and bFGF, and some novel putative targets, including IL-7 and PlGF. In addition to further investigation of putative neurobiological underpinnings of cognitive dysfunction in larger and longitudinal studies, future work should consider the implications of this work for potentially detecting and treating cognitive impairment in those with bipolar disorder, which is an emerging field that is gaining momentum.Reference Strawbridge, Tsapekos, Hodsoll, Mantingh, Yalin and McCrone8,Reference Pisoni, Strawbridge, Hodsoll, Powell, Breen and Hatch25 Anti-inflammatory treatments appear to have some antidepressant effects, and these may also be ameliorating cognitive impairments observed in bipolar depression (where cognitive difficulties are more pronounced than in euthymia).
This exciting area of investigation is still in its infancy, and as such, more clinical studies are needed to more fully understand the nature and mechanisms underlying the relationship of inflammation and growth factors with cognitive deficits in bipolar disorder. This could then guide future clinical trials involving existing or potentially novel anti-inflammatory interventions addressing cognition, rather than just mood, more directly.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2021.966
Data availability
Data availability requests should be submitted to the corresponding author.
Acknowledgements
The authors sincerely thank all participants and contributors to the CRiB study.
Author contributions
R.S. and A.H.Y. conceptualised the study and were responsible for funding acquisition. D.T. and R.S. were responsible for data curation and acquisition. R.S. and F.S. conducted the formal analysis. All authors were involved in data interpretation. R.S., D.T. and A.H.Y. were responsible for the study investigation/methodology. R.S. and R.C. wrote the original draft of the manuscript. All authors contributed to reviewing and editing the manuscript, and gave final approval for submission. The corresponding author should be contacted for any requests for anonymised data.
Funding
This project is funded through the National Institute of Health Research (NIHR) Research for Patient Benefit programme (grant number PB-PG-0614-34075) and NIHR Maudsley Biomedical Research Centre. This article presents independent research; the funder did not have a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
Declaration of interest
In the past 3 years, A.H.Y. has received honoraria for speaking from AstraZeneca, Lundbeck, Eli Lilly and Sunovion; honoraria for consulting from Allergan, Livanova, Lundbeck, Sunovion and Janssen; and research grant support from Janssen. R.S. has received an honorarium for speaking from Lundbeck. Other authors declare no conflicts of interest.











eLetters
No eLetters have been published for this article.