Introduction
The order Galliformes is one of the most endangered avian orders both globally and in the Neotropics, while the family Cracidae is the most endangered avian family with 37% of species listed as ‘Vulnerable’ or higher (IUCN 2011). Although non-threatened species may be of lesser conservation concern, some populations of these species are at risk or have been locally extirpated. Understanding how threats that affect many species, such as habitat loss, affect the more common cracid species is not only important for the conservation of those species but is also potentially an important tool for inferring how these factors affect species of greater conservation concern (Brooks and Strahl, Reference Brooks and Strahl2000).
The Dusky-legged Guan Penelope obscura is the southernmost species of the family Cracidae, principally inhabiting riparian forests in southern Brazil, Paraguay and Argentina that reaches its southern distributional limit in the delta of the Paraná River, Argentina. Its global status is listed as ‘Least Concern’ (IUCN 2011) but at the local scale, the species has been greatly reduced in numbers or extirpated in many areas due to habitat loss and degradation and uncontrolled hunting. Based on these facts, at national level it has been considered as ‘Vulnerable’ (Lopez Lanús et al. Reference López Lanús, Grillo, Di Giacomo, Coconier and Banchs2008). In the lower delta of the Paraná River, riparian forest, the primary habitat of Dusky-legged Guan, has been largely converted or modified since the mid-19th century so that the landscape is now a mosaic of secondary forest, plantation forest, deforested land and occupied and unoccupied residences. Although Dusky-legged Guan appears to exhibit varying preferences among habitat types these preferences have not been quantified.
We surveyed Dusky-legged Guan in the lower delta of the Paraná River, Argentina during winter and summer, using occupancy modelling to estimate habitat preferences and seasonal effects on occupancy. Surveys were conducted by boat and we illustrate that this is an effective method for surveying guan species associated with riparian habitats. Moreover, our results indicate that although Dusky-legged Guans utilise a diversity of anthropogenic habitats, they exhibit a preference for habitats most similar to mature riparian forest, which has important implications for the protection of these habitats and the species in the region.
Study area
The study was conducted in the Paraná River Delta Biosphere Reserve (34°15′00′S, 58°58′33′W), located in the lower delta of the Paraná River at the southern end of the Rio de la Plata basin. The islands of the lower delta are generally saucer-shaped with natural levees around their perimeters which were originally covered with native riparian forest while lowland areas are occupied by freshwater marshes and flooded forests of Erythrina crista-galli (Kandus et al. Reference Kandus, Quintana and Bó2006). Riparian forest in the region has been highly modified so that forested areas are now dominated by poplars Populus spp. and willows Salix spp., interspersed with remnants of native forest, secondary forests dominated by exotic vegetation, and occupied or abandoned homesteads.
Methods
We surveyed 543.9 km of nine waterways (Largo, Cruz del Sauce, Inatonta, Carpincho, Dominguito, Herrera, Pantanoso, Arroyo Las Cubiertas streams and Barquita river) from a motorboat moving at approximately 20 km/hr using two observers. Each waterway was surveyed five times during January (austral summer) and July (austral winter) 2009 between 08h00 and 11h00 and 16h00-19h00 in January and 08h00-11h00 to 15h00-18h00 in June. We characterised both sides of each waterway by habitat type (secondary forest, mature plantation forest, young plantation forest, deforested area, and occupied residence) with each length of a specific habitat considered a sampling site, resulting in 158 sites. Mean segment length varied from 154 m (SE = 17.5 m) for young plantation forest to 518 m (SE = 90.7 m) for secondary forest (Figure 1). Since Dusky-legged Guans are confined to riparian forest in our study area, this was the most efficient method for sampling a large area of potentially suitable habitat.
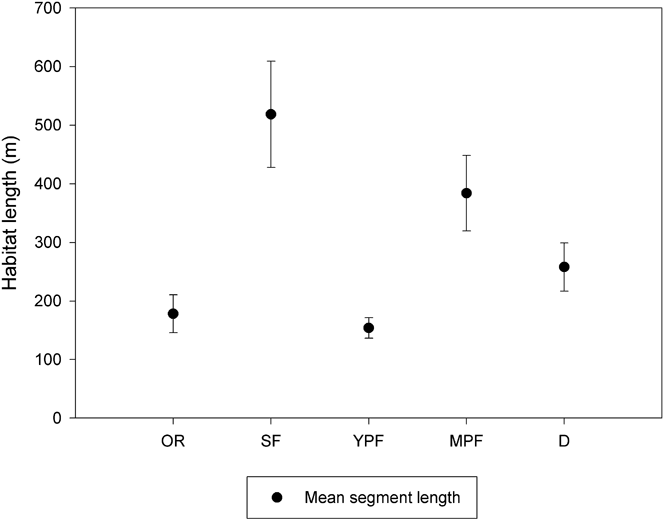
Figure 1. Mean length and 95% confidence limits of riparian habitat surveyed. OR = occupied residence, SF = secondary forest, YPF = young plantation forest, MPF = mature plantation forest, D = deforested area.
During each survey, the detection or non-detection of Dusky-legged Guans was recorded for each site and these data were used to construct detection histories for each of the sampling periods. Since naive estimates of occupancy from raw counts, uncorrected for incomplete detection, are potentially biased, we used occupancy modelling (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006) to model habitat occupancy (ψ) and detection probabilities (p). We used the program PRESENCE 3.1 (Hines Reference Hines2006) to test 22 models developed a priori which included habitat type and length of habitat as co-variables. Covariates were z transformed so that the mean was equal to zero. All models included the effect of sampling period on occupancy since the data from both seasons were analysed together and we utilised categorical variables to represent sampling periods so that the assumption of population closure was met, which is akin to a robust design model (Pollock Reference Pollock1982) although we did not estimate colonisation or extinction parameters.
Models were ranked using Akaike’s Information Criterion (AIC) adjusted for small sample size and overdispersion using quasi-AIC (QAICc) and models averaged for those models with a weight of at least 10% of the highest ranked model within the candidate set (Burnham and Anderson Reference Burnham and Anderson2002). Differences in estimates of occupancy and detection probability were determined using 95% confidence intervals of the beta coefficients from the composite model were utilised to determine the magnitude of the effect of covariates. Where the 95% CIs did not include zero, the effect of the covariate was considered to be strong.
Results
We detected Dusky-legged Guans at 40 sites during January 2009 and 25 sites during June 2009 (observed ψ = 0.25 and 0.16 respectively) (Table 1). Of the 22 models tested, eight had weights with at least 10% of the weight of the highest ranked model, which also accounted for 94% of all model weights (Table 2). Model fit was good with ĉ = 1.08 for the global model.
Table 1. Observed (Ψ (obs)) and estimated occupancy (Ψ (estimated)) and estimated detection (p (estimated)) probabilities by season with their respective 95% confidence intervals (95% CI) from the composite models.

Table 2. Models with model weights (w) within 10% of the top ranked model which were used for model averaging. Ψ= probability of occupancy, QAICc = Quasi-Akaike’s Information Criteria corrected for small sample size, and -2*LogL = -2 times the Log likelihood. All models include the effect of survey season on Ψ.
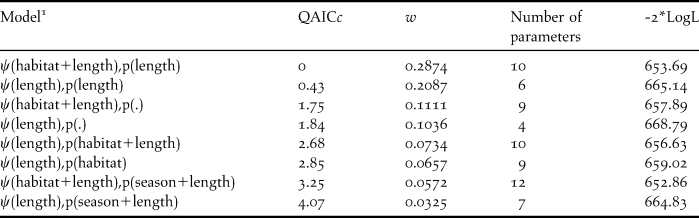
1 Occupancy and detection were modeled as constant (.) or as a function of habitat type (habitat), season (summer or winter) and length of habitat segment (length) which constituted each site.
The composite model from model averaging estimated a mean ψ across all sites for the winter sampling period of 0.26 with a 95% confidence interval of 0.11–0.40, while for the summer sampling period a mean ψ = 0.36 with a 95% confidence interval of 0.20–0.52 (Table 1). These estimates are 61% and 69% greater than the observed occupancy for the winter and summer sampling periods, respectively (Table 1). Mean detection probability was nearly equal during both sampling periods and highly variable (summer p = 0.21, 95% CI = 0.07–0.36; winter p = 0.22, 95% CI = 0.07–0.37; Table 1).
Estimated detectability was nearly constant among habitat types and season (Figure 2a). During winter and summer, detection was highest in secondary forest (winter p = 0.23, summer p = 0.24), followed by deforested land (winter p = 0.22, summer p = 0.23), mature plantation forest (winter p = 0.22, summer p = 0.22), young plantation forest (winter p = 0.19, summer p = 0.19), and occupied residence (winter p = 0.18, summer p = 0.18). Estimates of occupancy varied among habitat types and season but not significantly (Figure 2b). Occupancy estimates during both winter and summer were highest in secondary forest (winter ψ = 0.39, summer ψ = 0.51), followed by mature plantation forest (winter ψ = 0.3, summer ψ = 0.42), deforested land (winter ψ = 0.19, summer ψ = 0.29), young plantation forest (winter ψ = 0.14, summer ψ = 0.23), and occupied residence (winter ψ = 0.12, summer ψ = 0.19).
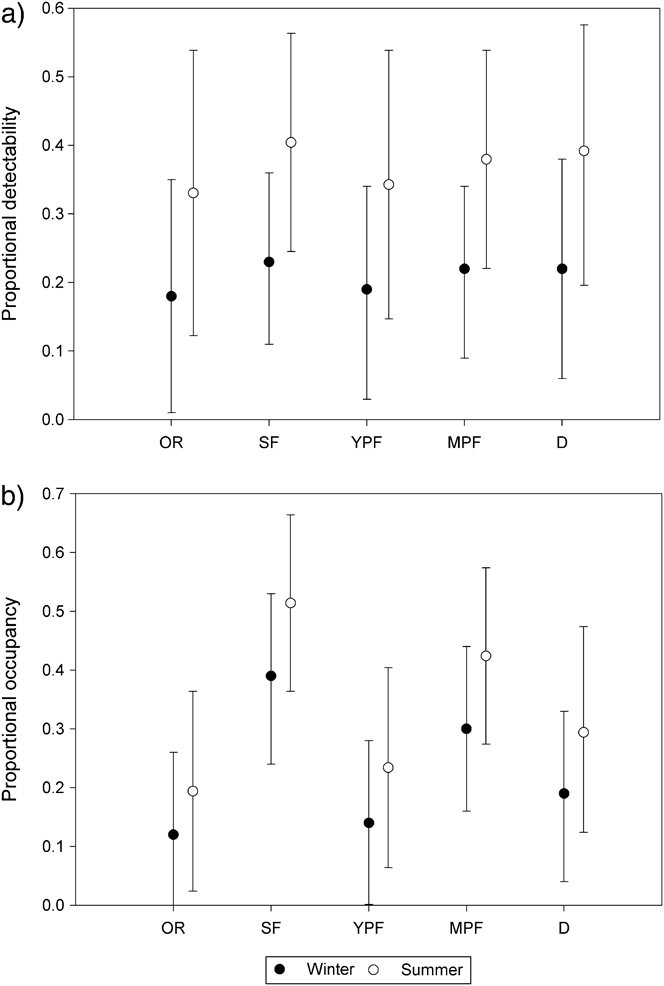
Figure 2. Estimated detection probability (a) and occupancy (b) by habitat type and season from the composite occupancy model. Error bars represent 95% confidence limits. OR = occupied residence, SF = secondary forest, YPF = young plantation forest, MPF = mature plantation forest, D = deforested area.
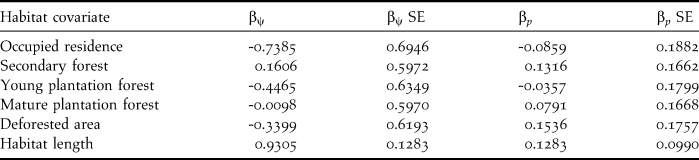
Beta coefficients and their 95% confidence intervals from the composite model indicated a strong positive effect of length of riparian habitat segments on occupancy of Dusky-legged Guans, the 95% CI interval did not span zero. Occupied residences appeared to have a weak to moderate negative effect since zero was included in the lower limit of the confidence interval (Table 3). Additionally, there was a moderate positive effect of habitat length on detection probability since the lower limit of the 95% confidence interval included zero (Table 3).
Table 3. Beta coefficients and their standard errors for the habitat covariates used in the analysis from the composite occupancy (βψ) and detection (βp,) models.
Discussion
Mean estimated occupancy was higher during summer than winter although highly variable and not significantly different. If higher occupancy is indicative of greater abundance (MacKenzie and Nichols Reference MacKenzie and Nichols2004) then the difference between the two seasons is likely due to increased population from post-breeding recruitment. Although estimates of occupancy were highly variable, guans exhibited a preference for secondary forest followed by mature plantation forest. Given the lack of mature native forest within our study area, this suggests that secondary forest and mature plantation forest are preferred since they have a well developed understorey and offer resources and shelter most similar to what would be provided by mature native forest (Malzof et al. Reference Malzof, Villar, Saccone, Casaburi, Bilinsky and Quintana2006). For example, secondary forests provide important fruit resources throughout the year (S. Malzof unpubl. data), such as Ligustrum lucidum and L. sinense, whose fruits are staple food items for guans (Merler et al. Reference Merler, Diuk Wasser and Quintana2001).
Young plantation forest, deforested areas, and occupied residences were least occupied, consistent with the negative effect of occupied residences and young plantation forest on occupancy exhibited by the β values of the process model. Insufficient resources and habitat structure are likely causes of lower occupancy in these habitats, although in occupied residences and young plantation forest, anthropogenic disturbance, both direct and indirect, also contributes to lower occupancy. Human activity around occupied residences is high and young plantations require considerable management during the first five years of establishment which likely presents a significant amount of human disturbance. Moreover, an increased human presence likely equates to increased hunting pressure.
As with occupancy, detection by habitat type was highly variable, although similar across habitats and seasons. Detectability by habitat type, excluding deforested areas was highly correlated with occupancy (r 2= 0.98), suggesting that abundance in these habitats affects detectability. The greater detectability in relation to occupancy in deforested areas is likely to be due to increased visibility. As with seasonal differences, if higher occupancy is related to greater abundance, then the moderate positive effect of secondary forest on detectability can be attributed to higher abundance of guans in this habitat.
Habitat length was the only factor that had a strong effect on occupancy and appeared to positively influence detection rates. Increased detectability in longer habitat sections is attributable to greater probability of detecting an individual as function of survey effort. After correcting for the effect of habitat length on detectability, the length of habitat positively affects occupancy which suggests that regardless of habitat type, larger habitat fragments have a greater probability of being occupied.
The relatively low detectability highlights the importance of accounting for incomplete detection, since on average 78–79% of individuals are not detected, depending upon the season. Additionally, although we found a strong correlation between occupancy and detection in four of the five habitat types, the greater detectability in deforested areas and the positive effect of habitat length on detectability further highlights the importance of accounting for incomplete detection when evaluating habitat preferences. If not accounted for, the higher detectability in deforested habitats relative to occupancy would erroneously place greater importance on deforested areas, while greater detectability in longer habitat segments would overestimate the importance of secondary forest and mature plantation forest, since on average these habitats represented the longest habitat segments.
Accounting for incomplete detectability is fundamental to estimating population parameters (Anderson Reference Anderson2001, Williams et al. Reference Williams, Nichols and Conroy2002), which is evident by our results. In our case, a failure to account for incomplete detectability would underestimate occupancy in general and would produce biased estimates of habitat preference. Cracids generally occur at low densities, even in pristine habitats, which often makes surveying these species difficult, but we believe that we developed a sound methodology for large-scale surveys of cracids associated with riparian forest that produces robust estimates of site occupancy. Based upon our results, the use of the common methodology for cracid surveys (Strahl and Silva Reference Strahl, Silva, Strahl, Beaujon, Brooks, Begazo, Sedaghatkish and Olmos1997) should be reconsidered and we urge researchers to adopt methods that account for incomplete detection to produce reliable and robust estimates of cracid population parameters.
The pattern in habitat occupancy that we observed is consistent with the ecology of cracids in general, preferring more mature forests and exhibiting a high level of sensitivity to anthropogenic disturbance (Strahl et al. Reference Strahl, Beaujon, Brooks, Begazo, Sedaghatkish and Olmos1997, Brooks and Strahl Reference Brooks and Strahl2000, Brooks Reference Brooks2006). Even though the Dusky-legged Guan utilised a diversity of habitats, including highly modified habitats, it still illustrated the relative avoidance of such habitats, preferring those most analogous to mature riverine forest. A similar response was observed in the congeneric Penelope perspicax which preferred forest patches over exotic tree plantations (Ríos et al. Reference Ríos, Kattan, Londoño and Muñoz2008). Therefore, the abundance and distribution of Dusky-legged Guan in the lower delta of the Paraná River is likely to be highly dependent upon the availability of native forest remnants and secondary forest.
Although the Dusky-legged Guan is still relatively common in the lower delta of the Paraná River, its distribution is dependent upon the availability of preferred habitat. In our study area, the habitats with the highest estimated occupancy, secondary forest and mature plantation forest, represented 43% and 30% of the habitat area respectively and in part explains the relative commonness of guans in the area. The maintenance of these habitats in the landscape is critical for the conservation of Dusky-legged Guan in the lower delta of the Paraná River and suggests that management to increase habitat quality, particularly mature plantation forest, may have particular positive effects on guan populations.
Acknowledgements
We thank Carlos Zoppi and family for logistic support, BEX (Birders Exchange) and Idea Wild for equipment support and Interisleña for providing us free passes to motorboats.









