Article contents
Inverse power law potentials about polygonal prisms and in polygonal cavities
Published online by Cambridge University Press: 17 February 2009
Abstract
The effects on adsorption of the geometry of the solid may be studied through calculations based on a (distance)−ε (ε> 3) intermolecular potential. This paper establishes the result that the potential due to an infinitely long polygonal homogeneous solid prism, at position r in the plane of its right section, is – 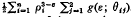 . Here ρi = ∣ r − ri ∣, where the ri are the position vectors of the n vertices of the polygon, and θij are the angles r − ri makes with the two sides of the polygon which meet at vertex ri. The g's are exact functions of θij. They are, in general, integrals of associated Legendre functions, but they are elementary for ε an even integer. A similar result holds for the potential within an infinitely long polygonal prismatic cavity. The analysis involves a systematic superposition schema and the concept of a supplementary potential with datum within the solid at infinity. The cases ε = 6 and ε = 4 are treated in detail and illustrative solutions given for the following configurations: semi-infinite laminae, deep rectangular cracks, square prisms, square prismatic cavities and regular n-gonal prismatic cavities.
. Here ρi = ∣ r − ri ∣, where the ri are the position vectors of the n vertices of the polygon, and θij are the angles r − ri makes with the two sides of the polygon which meet at vertex ri. The g's are exact functions of θij. They are, in general, integrals of associated Legendre functions, but they are elementary for ε an even integer. A similar result holds for the potential within an infinitely long polygonal prismatic cavity. The analysis involves a systematic superposition schema and the concept of a supplementary potential with datum within the solid at infinity. The cases ε = 6 and ε = 4 are treated in detail and illustrative solutions given for the following configurations: semi-infinite laminae, deep rectangular cracks, square prisms, square prismatic cavities and regular n-gonal prismatic cavities.
- Type
- Research Article
- Information
- Copyright
- Copyright © Australian Mathematical Society 1977
References
REFERENCES
- 2
- Cited by




