No CrossRef data available.
Article contents
Empiric antibiotic selection for community-acquired pneumonia in US hospitals, 2013–2020
Published online by Cambridge University Press: 29 September 2023
Abstract
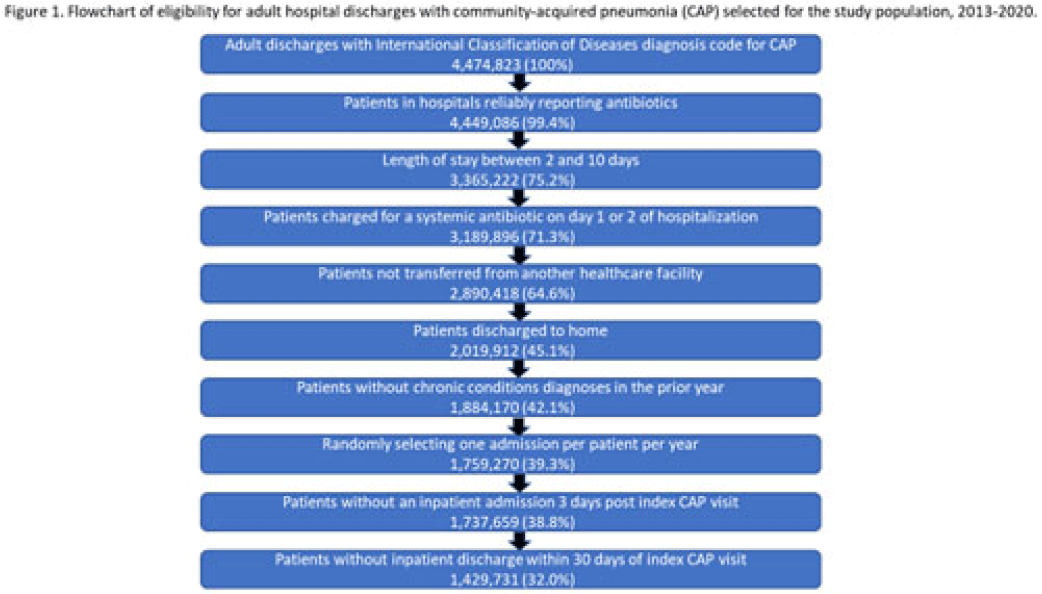
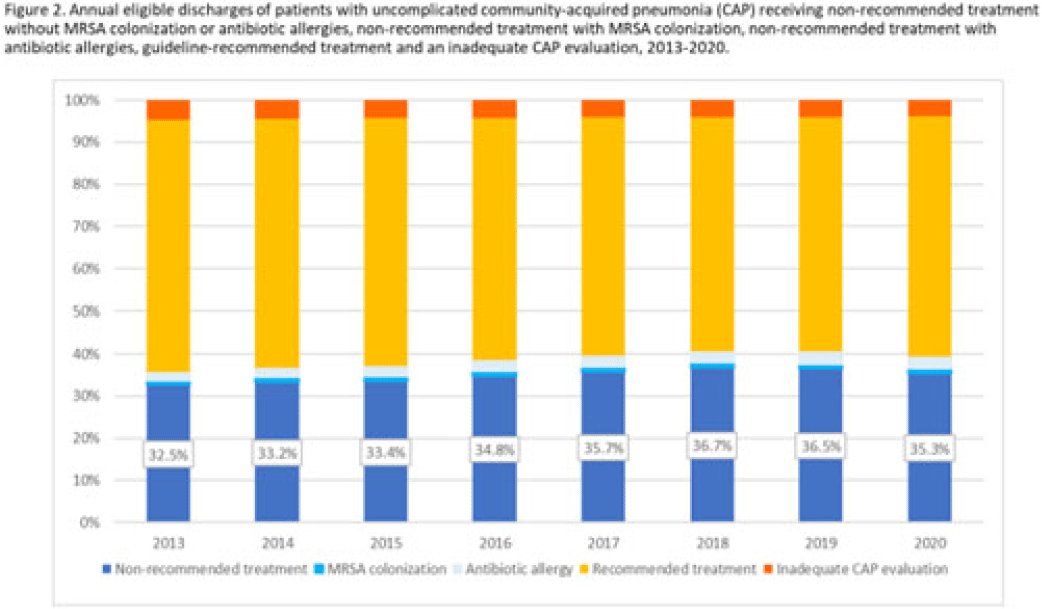
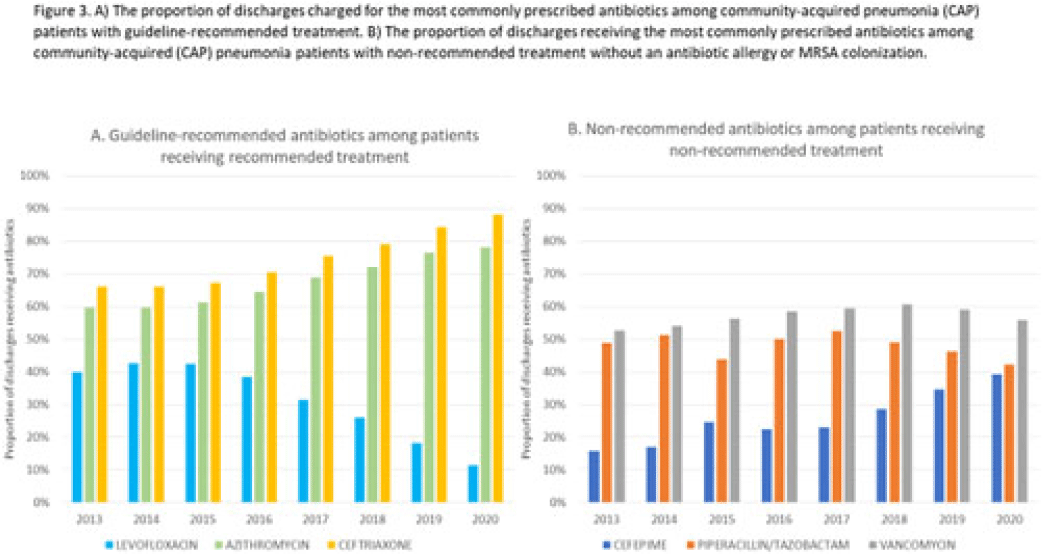
Background: Community-acquired pneumonia (CAP) is a common indication for antibiotic prescribing in hospitalized patients. Professional societies’ clinical guidelines recommend specific antibiotics for empiric treatment of CAP based on clinical factors. Manual assessments of appropriateness are time-consuming and are often conducted on a smaller scale. We evaluated empiric antibiotic selection among a large cohort of adults hospitalized with CAP using electronic health records. Methods: In this study, we used the PINC-AI healthcare database to define a cohort of adults hospitalized with CAP from 2013 to 2020. CAP was identified by International Classification of Diseases (ICD) diagnosis codes. Exclusions were applied to identify uncomplicated CAP (Fig. 1). Treatment was only evaluated if a chest radiograph or computerized tomography (CT) scan was charged during the first 2 days of hospitalization, otherwise it was considered an inadequate CAP evaluation. Administrative billing data were used to identify antibiotics charged within the first 2 days of hospitalization. Empiric guideline-recommended treatment was determined based on 2019 CAP guidelines and more recent studies. Patients who received nonrecommended treatment were evaluated for antibiotic allergies in the current hospitalization or methicillin-resistant Staphylococcus aureus (MRSA) colonization or infection in the year prior or on admission using International Classification of Disease, Tenth Revision (ICD-10) diagnosis codes. Results: We identified 4.47 million adult hospitalizations with CAP from 2013 to 2020; 32% (1.43 million) were included in this analysis (Fig. 1). Among discharges with adequate CAP evaluation (1.37 million), 59.7% received recommended antibiotics in the first 2 days of hospitalization, ranging from 62.6% in 2013 to 57.5% in 2019. Overall, 34.8% of our study population received a nonrecommended antibiotic without documentation of an antibiotic allergy or MRSA colonization (2013: 32.5%; 2018: 36.7%) (Fig. 2). Most patients in our study population received >1 antibiotic (92.3%) in the first 2 days of hospitalization. The most common antibiotics among patients receiving recommended treatment were ceftriaxone (74.2% of patients receiving recommended treatment), azithromycin (67.2%), and levofloxacin (31.8%) (Fig. 3a). The most common nonrecommended antibiotics were vancomycin (57.2% of patients receiving nonrecommended treatment), piperacillin-tazobactam (48.1%), and cefepime (25.7%) (Fig. 3b). From 2013 to 2020, cefepime charges consistently increased among CAP patients treated with nonrecommended antibiotics, whereas levofloxacin charges consistently decreased among CAP patients treated with only recommended antibiotics. Conclusions: Approximately one-third of patients with uncomplicated CAP received nonrecommended empiric antibiotics, and from 2013 to 2020 that proportion increased by 9%. Additional strategies are needed to help identify opportunities to optimize antibiotic selection among patients with CAP.
Disclosures: None
- Type
- Antibiotic Stewardship
- Information
- Antimicrobial Stewardship & Healthcare Epidemiology , Volume 3 , Issue S2: SHEA Spring 2023 Abstracts , June 2023 , pp. s26 - s27
- Creative Commons
- This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Society for Healthcare Epidemiology of America




