Introduction
Infectious bovine rhinotracheitis (IBR), caused by Bovine alphaherpesvirus 1 (BoHV-1), is a disease of cattle that is responsible for significant economic losses worldwide. BoHV-1 is a member of the genus Varicellovirus in the subfamily Alphaherpesvirinae, which belongs to the Herpesviridae family. The virus is associated with major clinical syndromes, namely IBR, infectious pustular vulvovaginitis (IPV), and infectious pustular balanoposthitis (IPB). The virus also causes other clinical syndromes such as abortion, infertility, conjunctivitis, enteritis, and encephalitis (Nandi et al., Reference Nandi, Kumar, Manohar and Chauhan2009). The main sources of the virus are nasal exudates and cough droplets, genital secretions, semen, fetal fluids, and tissues. These materials can be transmitted by direct contact with infected animals or by indirect contact with infected material and personnel. BoHV-1 can become latent following a primary infection with a field isolate or vaccination with an attenuated strain. The virus is usually detectable in the sensory ganglia of the trigeminal nerve in IBR, and in the sacral spinal ganglia in IPV/IPB (Wentink et al., Reference Wentink, Van Oirschot and Verhoeff1993). Latency may also occur in tonsillar lymphoid cells and peripheral blood lymphocytes (Ackerman and Wyler, Reference Ackermann and Wyler1984). Viral reactivation may occur owing to stressful stimuli associated with delivery, transport or animal mixing, insufficient herd management, co-infections, superinfection, treatments with corticosteroids, or parturition (Winkler et al., Reference Winkler, Doster and Jones2000). As a result, the reactivated virus may be re-excreted, and an increased neutralizing antibody titer may be observed. Latently infected animals should always be considered as a potential source of infection (Bitsch, Reference Bitsch1973), though some types of vaccines can considerably reduce the amount of virus excreted following reactivation (Mars et al., Reference Mars, De Jong, Franken and Van Oirschot2001). The commercially available vaccines at present can be divided into two main categories: (i) traditional vaccines and (ii) marker vaccines. The first category, which was the only category of vaccines available until the 1990s, usually prevents severe clinical signs of disease and reduces the amounts of viral particles shed after infection; however, their use could not restrict infection spread in some herds or regions. Moreover, their use interferes with routine serological diagnosis and epidemiological surveys essential for control programs (Van Oirschot et al., Reference Van Oirschot, Kaashoek and Rijsewijk1996). In the 1990s, a new vaccine category was developed by deleting one of the non-essential viral glycoproteins, mainly glycoprotein E (gE) of BoHV-1, which was made commercially available. This vaccine allows distinction of traditionally vaccinated cattle and infected animals (gE-positive) from those vaccinated with the gE-deleted marker (gE-negative) by using a suitable serological diagnostic test (Van Oirschot et al., Reference Van Oirschot, Kaashoek and Rijsewijk1996). Therefore, gE-deleted marker vaccines can serve as a valuable tool for disease control strategies. Vaccination against BoHV-1 has been employed since the first appearance of the related disease. Indeed, BoHV-1 was recognized as the causative agent of IBR in cattle in the 1950s, in the USA (Madin et al., Reference Madin, Mckercher and York1956), and the first attenuated live vaccine was produced around the same time (Kendrick et al., Reference Kendrick, York and McKercher1956). Soon after its spread in the USA, IBR was reported in many European countries (Moretti et al., Reference Moretti, Orfei, Mondino and Persechino1964; Straub, Reference Straub1975). Despite the low mortality levels, BoHV-1 infection caused considerable economic losses everywhere the virus was introduced and spread. According to former EU regulations, IBR was listed in annex E-II of Council Directive 64/432/EEC (European Commission, 1964). Thus, every member state could receive approval for a national IBR control program for its entire territory or a part of it, and for additional guarantees for bovids destined for its territory (European Commission, 1964, article 9), and every member state could be declared as officially IBR-free in the entire territory or in a part of it (European Commission, 1964, article 10). Furthermore, the IBR-free status was one of the required animal-health conditions for intra-community trade and importation of cattle semen (European Commission, 1988) and embryos (European Commission, 1989) from third countries. Nevertheless, according to previous EU regulations, IBR was not listed in annex E-I of Council Directive 64/432/EEC (European Commission, 1964); hence, IBR was not a notifiable disease in the EU. Currently, Council Directive 64/432/EEC has been repealed by Regulation 2016/429/EU (European Commission, 2016a), the so-called ‘Animal Health Law’, and its resulting Regulations as of 21 April 2021. According to the present legislation, IBR is listed in annex II of Animal Health Law (European Commission, 2018a), is inserted in categories C + D + E (European Commission, 2018b), and disease outbreaks are notifiable if they occur in IBR-free member states or in IBR-free zones of EU countries (European Commission, 2020b). Required conditions to achieve and maintain disease-free status, as well as control strategy and surveillance organization, are reported into a new regulation (European Commission, 2020c). Sanitary measures applied to material germinal for IBR are present in a new law in force (European Commission, 2020d), while rules regarding animal health requirements for movements within the Union are codified in a new act (European Commission, 2020e). IBR can be subjected to an optional eradication program in the Member States (European Commission, 2020b). Furthermore, according to the former EU law, different European countries implemented IBR control schemes in their territories, and a few of them obtained IBR-free status (European Commission, 2017b) followed by restraint of cattle trade (European Commission, 2004b). Currently, a new regulation regards the approval of the disease-free and non-vaccination status of certain Member States or zones or compartments thereof with regard to certain listed diseases and the approval of eradication programs for those listed diseases, such as IBR (European Commission, 2021). Finally, detailed standards for the international control of IBR/IPV are contained in the World Organisation for Animal Health Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (OIE, 2018). The requirements that a country, zone, or herd should satisfy to qualify as free from IBR/IPV and to maintain its status as free are reported; recommendations are also delineated for the importation of cattle destined for herds free from IBR/IPV or intended for herds not qualified as free from the disease, as well as recommendations for the importation of fresh or frozen semen and of oocytes or embryos. The aim of this review is to provide an overview of IBR control and eradication programs in European countries. A twofold data source was required in this study. Literature references were retrieved by querying PubMed (National Library of Medicine) and Scopus (Elsevier) databases, accessed until 12 July 2021; particularly, the following keywords ‘IBR eradication’, ‘IBR control’, and ‘IBR surveillance’ were searched in all available papers and possible duplicates removed. Legislative references were obtained by querying EUR-Lex, the online gateway to EU legal documents, for most European references; for some cases, such as Switzerland and Ireland, respective official online application portals were queried.
Control and eradication strategies in the EU
The first IBR control programs were implemented beginning in the 1970s to 1980s. Since the EU allowed IBR-free member states to request import conditions for cattle, semen, and embryos (Noordegraaf et al., Reference Noordegraaf, Jalvingh, De Jong, Franken and Dijkhuizen2000), more efforts were undertaken in the late 1990s to achieve IBR eradication in European territory (Beer et al., Reference Beer, König, Schielke and Trapp2003). To date, a variety of IBR control programs have been performed or are in progress in the EU, whose features depend on epidemiological and economic issues. In conditions of low BoHV-1 seroprevalence, culling of seropositive animals without vaccination (test and slaughter strategy) has been the most successful method for eradication. In addition, it is advisable to create an IBR-free breeding stock by gradually removing all seropositive cattle from a conventional breeding lot and replacing them with seronegative progeny (Ackermann and Engels, Reference Ackermann and Engels2006). Scandinavian countries (Denmark, Finland, Norway, and Sweden), as well as Austria, Switzerland, and some Italian regions, have successfully achieved IBR eradication by following this strategy. Vaccination using gE-deleted marker vaccines, followed by seropositive animal removal, is an appropriate tool in conditions with medium/high BoHV-1 seroprevalence. This approach is the so-called DIVA strategy (Differentiating Infected from Vaccinated Animals). Because the total seroprevalence of gE-positive animals can reach up to 5%, the remaining seropositive animals can be removed (Vonk Noordegraaf et al., Reference Vonk Noordegraaf, Buijtels, Dijkhuizen, Franken, Stegeman and Verhoeff1998). Some countries, such as Belgium and the Netherlands, have adopted the vaccination strategy to control the disease. Once the eradication target is achieved, it is essential to reinforce this goal by implementing a subsequent surveillance program. The purpose of surveillance is to ensure early detection of infection in IBR-free herds, so that infection spread to other certified herds can be prevented (Graat et al., Reference Graat, De Jong, Frankena and Franken2001). In dairy cattle, enzyme-linked immunosorbent assay (ELISA) tests of bulk-tank milk for antibodies provide a useful and low-cost method for determining BoHV-1 seropositive status (Nylin et al., Reference Nylin, Stroger and Ronsholt2000).
Austria
In 1987, a voluntary IBR eradication program involving breeding cattle was implemented in the Styria region; this program used ‘test and removal’ procedures for serologically positive animals (Kofer et al., Reference Kofer, Wagner and Deutz1999). In 1990, IBR control became compulsory for all cattle herds in the country, involving all animals >2 years of age, without distinction between animals for slaughter, breeding, or production; therefore, the legal provisions prohibited vaccination and required reactors to be slaughtered (Republic of Austria, 1989). In 1994, when Austria did not yet belong to the European Economic Community (EEC), the European Free Trade Association (EFTA) Surveillance Authority approved the national program for the eradication of IBR, which had been applied since 1990; simultaneously, it laid down additional guarantees for cattle intended for breeding and production and destined for Austria, in order to protect the progress already made and to ensure that the program was successfully concluded (European Free Trade Association ‘EFTA’ Surveillance Authority, 1994d). In 1995, when Austria joined the EEC, the eradication program was approved by the European Commission from 1995 to 1997 (European Commission, 1995a), and Austria was granted additional guarantees (European Commission, 1995c). During the period from 1995 to 1997, a large number of IBR outbreaks were recorded; epidemiological investigations showed that most were caused either by illegal cattle imports or by legally imported cattle provided with relevant certificates. Therefore, the biennial rhythm of controls was changed to an annual rhythm, beginning from 1997 (Kofer et al., Reference Kofer, Wagner and Deutz1999). In the same year, the ongoing eradication program was approved for a further period of 3 years (European Commission, 1997). In 1998, the entire territory of Styria and some other provinces were considered as IBR-free and received additional guarantees to protect their territory (European Commission, 1998b). In 1999, IBR-free status was obtained by the entire country (European Commission, 1999). The sampling plan for IBR was changed in 2007, when bulk milk testing was established at the national level, and in 2013, when different disease control plans were harmonized (Roch and Conrady, Reference Roch and Conrady2021). Over the years 2000–2010, in Austria, single positive reactions have been detected (Raaperi et al., Reference Raaperi, Orro and Viltrop2014). The last outbreak notification was in 2015 during an export examination and caused increased animal control; IBR-positive tested cattle were detected and removed (Roch and Conrady, Reference Roch and Conrady2021). The additional guarantees remained unaffected. Currently, these cases do not invalidate IBR-free status of the whole country, as reported in the latest regulatory action (European Commission, 2021).
Belgium
From 1991 to 1994, a voluntary program for IBR control was set up covering 70 herds in the Wallon region, where rapid culling of seropositive animals was unfeasible because of the high prevalence rate (60%). Therefore, disease control was attempted with repeated vaccinations and the use of severe biosecurity measures. This was the first experience for Belgium on a regional basis (Wergifosse et al., Reference Wergifosse, Lemaire, Pastoret and Thiry1997). Subsequently, the European Commission granted approval to Belgium for an eradication program covering the whole of its territory, and additional guarantees were granted in 2014 (European Commission, 2014).
Czech Republic
The Czech Republic has an IBR control program in place since 2005 (Nettleton and Russel, Reference Nettleton and Russel2017). A mandatory eradication program for all cattle keepers on the whole territory started in 2006, was approved in 2008 (European Commission, 2008) and finished in 2016, when most holdings had no IBR-positive animals. Eradication measures continued to be applied in the country, until all bovine animals were kept on IBR-free holdings. In 2020, Czech Republic submitted to the European Commission supporting documentation in order for the whole of its territory to be considered IBR-free, and obtained the current status for the disease; its government requested and obtained approval to apply additional guarantees in accordance with article 10 of Directive 64/432/EEC (European Commission, 2020a).
Denmark
In Denmark, BoHV-1 first appeared in the early 1970s, probably with infected cattle imports, and quickly spread, at first among bulls at some artificial insemination (AI) centers, and then among a greater number of herds (Bitsch, Reference Bitsch1978). A systematic eradication program began in 1984, when the seroprevalence among dairy herds was about 9%. It was based on serological testing of all cattle in the herd and slaughter of seropositive animals, while vaccination was not considered acceptable. Therefore, only seronegative cattle from negative herds could be transferred between herds. Throughout the eradication plan and during the surveillance phase, bulk-tank milk was used to monitor the IBR status of dairy herds; milk samples were tested at 3 or 4 months, while monthly testing of bulk-tank milk was conducted from October to May in the Jutland region, because herds in this area had a higher risk of BoHV-1 infection. In addition, blood samples were obtained randomly at slaughterhouses. As a part of the eradication plan, a central database that contained the IBR status for each tested herd, other data such as herd size and age of animals at sampling, and diagnostic results at testing was established (Nylin et al., Reference Nylin, Stroger and Ronsholt2000). At the beginning of 1991, a few seropositive herds were still present, but no acute outbreaks were recorded in these herds. The surveillance plan was initiated from March 1991, and Denmark obtained IBR-free status from the European Commission in 1992 (European Commission, 1993). Nevertheless, during 1991–1995, viral reintroduction occurred, and new seropositive herds were recorded in the Jutland region close to the German border and in other areas as well. Two different routes of viral introduction seemed to be possible: an accidental contact with infected German cattle near the German-Danish border, probably via aerosol, or an unauthorized import of infected cattle. As a part of follow-up investigations for the viral reintroduction, a special survey was carried out by testing individual serum samples from 10% of cattle in each herd. All data for suspected or infected herds in 1991–1995 were collected in the IBR database and used to develop an adequate national surveillance plan again. Field viral strains isolated from these outbreaks were subjected to restriction fragment analysis. The results demonstrated the presence of different types of BoHV-1 (Nylin et al., Reference Nylin, Madsen and Rensholt1998). The last IBR outbreak reported in Denmark was in 2005, near their German border (Raaperi et al., Reference Raaperi, Orro and Viltrop2014). Nevertheless, Denmark's status as an IBR-free country has been kept to date (European Commission, 2021).
Estonia
In Estonia, no systematic control programs have been applied against BoHV-1, except in bulls used for semen collection at AI centers. All animals introduced to the AI center must be isolated in their herd of origin, tested and confirmed to be negative for BoHV-1 antibodies 30 days before movement; therefore, bulls used for semen collection are tested serologically once a year. Nevertheless, in this country, some studies were carried out to detect the efficacy of vaccination programs in lowering seroprevalence within dairy herds and to follow the dynamics of infection in non-vaccinated herds (Raaperi et al., Reference Raaperi, Aleksejev, Orro and Viltrop2012).
Finland
In Finland, serological testing for IBR started in 1965, and seropositive samples were found for the first time in 1970–1971, from bulls at an AI station. BoHV-1 may have been introduced in 1968 by an infected AI bull imported from Denmark, which infected other AI station mates. It is quite possible that infected semen from seropositive AI bulls was used between 1968 and 1979, and spread BoHV-1 infection to a number of herds where the virus persisted for over a decade (Nuotio et al., Reference Nuotio, Neuvonen and Hyytiainen2007). In Finland, IBR is a notifiable disease; vaccine use was not considered feasible in the IBR control policy, so a decisive ‘test and slaughter’ strategy was adopted successfully. Therefore, systematic serological testing of AI bulls began in 1978. The rate of testing increased between 1986 and 1989. Since 1991, each dairy herd was tested annually, and since 1993, random samples of beef animal sera were also tested. The infection resurfaced in 1990 and was identified because of clinical suspicion, bulk-tank milk surveillance and epidemiological investigations. Five herds were involved and subjected to all provided control measures, including stamping out. The infection was eradicated in 1994, and additional guarantees for cattle destined for Finland were approved by the EFTA Surveillance Authority first, and then by the EEC (European Free Trade Association ‘EFTA’ Surveillance Authority, 1994a; European Commission, 1994). Therefore, the surveillance scheme included annual serological tests in bulk-tank milk samples and in beef animals at slaughterhouses (Nuotio et al., Reference Nuotio, Neuvonen and Hyytiainen2007).
France
In France, a voluntary eradication program based on the ‘test and removal’ strategy without vaccination was started in 1996. In 2006, a mandatory control plan based on serological testing together with vaccination and/or positive animal removal was implemented. As an increasing number of certified BoHV1-free herds were observed at the national level for 10 years, official authorities approved a compulsory program since 2016 in order to speed up the eradication plan (Valas et al., Reference Valas, Bremaud, Stourm, Croise, Meneteau, Ngwa-Mbot and Tabouret2019). In the period 2016–2020, the goals to reduce herd prevalence and to increase herd number with IBR-free qualification were achieved. In 2020, France submitted to the European Commission supporting documentation and obtained approval of its national program for IBR eradication, covering all French metropolitan departments, except for Corsica; its government requested and obtained approval to apply additional guarantees in accordance with article 9 of Directive 64/432/EEC (European Commission, 2020a).
Germany
In Germany, BoHV-1 infection control was mandated by law in 1997 (Nettleton and Russel, Reference Nettleton and Russel2017). The control program was approved by the European Commission in 2004 (European Commission, 2004a). In the same year, the approved German IBR control plan and additional guarantees for intra-Union cattle trade relating to IBR were put together in the same Decision (European Commission, 2004b). In 2007, Germany requested and obtained the right to declare a part of its territory free of BoHV-1 infection and to apply additional guarantees for the administrative units of Regierungsbezirke Oberpfalz and Oberfranken in the federal state of Bavaria (European Commission, 2007). In 2010, two other administrative units in the federal state of Bavaria were declared free of BoHV-1 infection (European Commission, 2010). In 2011, the three remaining administrative regions in the federal state of Bavaria were considered free of BoHV-1 infection and obtained the right to be covered by additional guarantees (European Commission, 2011). In addition to the Bavaria region, a series of other regions were declared officially IBR-free: the Federal State of Thuringia in 2014 (European Commission, 2014); the Federal States of Saxony, Saxony-Anhalt, Brandenburg, Berlin, and Mecklenburg-Western Pomerania (European Commission, 2015a); the Federal State of Baden-Württemberg (European Commission, 2015b); the Federal States of Bremen, Hesse, and Lower Saxony (European Commission, 2015c) in 2015; the Federal States of Rhineland-Palatinate, Saarland, and some administrative units in the Federal State of North Rhine-Westphalia in 2016 (European Commission, 2016b); and the Federal States of Hamburg and Schleswig-Holstein in 2017 (European Commission, 2017a). Finally, in the same year, when the last administrative unit, the Federal State of North Rhine-Westphalia, was considered IBR-free, the entire German territory was recognized free of BoHV-1 infection and applied for additional IBR guarantees (European Commission, 2017b).
Hungary
For Hungary, only two reference studies are available, covering the years 1990–2000. According to Tanyi and Varga (Reference Tanyi and Varga1992), in the 1990s, no national data for IBR infection prevalence were available, and the accessible records only provided preliminary data from some old studies. From 1983 to 1988, a survey was conducted by testing 160 farms. Of these, 28 farms (17.5%) were seronegative for BoHV-1; in 105 farms (65.5%), a certain proportion of seropositive cattle without clinical signs of disease was reported; in 27 farms (16.9%), most of the cattle were seropositive and IBR-induced abortions occurred as well. In a survey conducted in two AI stations in 1991, 49.3 and 48.9% of the bulls, respectively, were positive. Progressively, certain attention was paid to the IBR question, and a national eradication plan began in 2002. As part of this program, farmers had to screen their herds for seroprevalence and, subsequently, submit a vaccination program to Veterinary Services for approval, in order to eradicate infection by using gE-deleted marker vaccines (Makoschey et al., Reference Makoschey, Zehle, Bussacchini, Valla, Pálfi and Földi2007).
Ireland
BoHV-1 was first isolated in Ireland in 1971 from a conjunctivitis case; many other cases were reported in the following years, and this number began increasing in the late 1980s. However, BoHV-1 seroprevalence was reported to be low, <20%, even in the periods with a high IBR incidence. Due to legislative requirements (Minister for Agriculture and Food, 2002), all IBR vaccines allowed in Ireland since 2004 were DIVA vaccines (Simon, Reference Simon2004). However, some recent studies showed a markedly increased seroprevalence in both dairy and beef herds and a widespread distribution of infection in Ireland. For instance, in the 2010s, herd seroprevalence rate was found to be between 28 and 42%, with significant regional differences (Graham, Reference Graham2013). This increase in the incidence and severity of IBR outbreaks has prompted some local authorities to consider IBR more than a sporadic disease. For instance, Animal Health Ireland (AHI) and Animal Health and Welfare NI (AHWNI), two not-profit partnerships for important non-regulated diseases involving farmers, processors, service providers, and the government, and acting in Ireland and Northern Ireland, respectively, have identified IBR management as a priority in local farms (Graham, Reference Graham2013). Nevertheless, as of 2015, a coordinated approach to IBR control did not exist in Ireland (Sayers et al., Reference Sayers, Byrne, O'Doherty and Arkins2015). Currently, a new discussion just started about IBR infection, in order to define structure and implementation of a potential national eradication plan. A new and innovative spatially explicit, individual-based, regional cattle disease model was proposed. It could be used as an effective tool for decision-makers to facilitate the assessment of IBR eradication strategies in the country (Brock et al., Reference Brock, Lange, Tratalos, More, Guelbenzu-Gonzalo, Graham and Thulke2021).
Italy
In Italy, the acute respiratory form of the disease was first reported in 1964 (Moretti et al., Reference Moretti, Orfei, Mondino and Persechino1964). Nevertheless, according to former law, IBR is not a notifiable disease (Ruffo et al., Reference Ruffo, Beghetto, La Greca and Fossati2017a) and, to date, not subjected to a national-level eradication plan for the whole cattle population. Disease control programs started at the regional and provincial levels at the beginning of the 1990s, to facilitate trading. In fact, some regions in North Italy were dependent on cattle trading and faced restrictions on season movements, imposed by the bordering already IBR-free countries. These territories include the Friuli Venezia Giulia region and Trento province on one hand, and the Valle d'Aosta region and Bolzano province on the other hand: the first group obtained approval for its eradication programs from the European Commission, and the second one was considered officially IBR-free (European Commission, 2017b, annex II). Within some years, other Italian territories developed programs to eradicate IBR from their territories by controlling the disease in breeding cattle populations and by using marker vaccines in some cases (Tamba et al., Reference Tamba, Pallante, Petrini, Feliziani, Iscaro, Arrigoni, Di Sabatino, Barberio, Cibin, Santi, Ianniello, Ruocco and Pozzato2021). Furthermore, in 2015 and 2016, the Italian Ministry of Agriculture, Food and Forestry (Ministero delle Politiche Agricole Alimentari e Forestali; MIPAAF) and Italian Ministry of Health approved two surveillance plans for controlling IBR in some beef cattle breeds, which have been recorded in the National Herd Book (Ministero delle Politiche Agricole Alimentari e Forestali, 2015, 2016). The plans are voluntary, and monetary incentives are paid if farmers achieve the annual target seroprevalence range (Maresca et al., Reference Maresca, Scoccia, Dettori, Felici, Guarcini, Petrini, Quaglia and Filippini2018).
Luxembourg
Luxembourg obtained approval for its national IBR eradication program covering the whole territory, and for additional guarantees for intra-Union cattle trade from the European Commission in 2017 (European Commission, 2017a).
Norway
IBR is a reportable disease in Norway (Paisley et al., Reference Paisley, Tharaldsen and Jarp2001). The first two IBR outbreaks were reported in the early 60s, while the latest single positive herd was found in 1993. Until 1992, importation of live cattle into Norway was prohibited. When the General Agreement on Trade and Tariff (GATT) was approved, this prohibition was discharged and Norway mandated BoHV-1 serological testing for all imported cattle to maintain its high animal-health standards. According to the World Trade Organization (WTO) and EFTA guidelines, in order to apply import restrictions based on freedom from a disease, any member state has to prove to be free from that disease itself. For this reason, Norway began serologically testing its cattle population since 1992. Since 1993, IBR has not been diagnosed clinically or serologically in Norway. In 1994, Norway was recognized as an IBR-free country by the EFTA Surveillance Authority and additional guarantees for bovines to be imported were granted (European Free Trade Association ‘EFTA’ Surveillance Authority, 1994c). Until 1996, the surveillance plan provided for annual testing of bulk-tank milk samples from all dairy herds; however, since 1996, this has been reduced to only 10% of the herds. In 2010, Norway requested the EFTA Surveillance Authority to update the previous decision in order to take account new legislative changes covering additional guarantees for intra-Union cattle trade relating to IBR (European Commission, 2004b). The new decision maintained the same guarantees previously granted to Norway and aligned them with the present European criteria (EFTA, Surveillance Authority, 2010).
Slovakia
For Slovakia, only one reference study is available (Mandelik et al., Reference Mandelik, Bires, Ozsvari, Hodnik and Vilcek2021). According to the authors, a voluntary IBR control program was implemented in 1996 but, in the subsequent years, only a small rate of national herds enrolled in this plan. Since 2006, the program became compulsory for all cattle farms in the country. First, serological tests were used to identify infected animals. Then, according to prevalence rate, eradication was based on culling (herd seroprevalence <15%), or on use of gE-deleted marker vaccines in combination with culling (herd seroprevalence >15%). When appropriate, especially in very small farms, all animals were slaughtered, with agreement form the farmers. Depending upon the selected method, seropositive cattle were gradually replaced by animals originating from officially IBR-free herds, in order to limit economic losses. After the replacement of all infected animals, a monitoring program started, to maintain IBR-free herd status. Further measures were implemented, such as strict control on animal movement and farmer education, to improve disease awareness. According to official data, 60.2% of herds were IBR-free in the country in 2020.
Spain
For this country, very poor information is available regarding IBR control plans. According to Villaamil et al. (Reference Villaamil, Arnaiz, Allepuz, Molins, Lazaro, Benavides, Moya, Fabrega, Yus and Dieguez2020), in Galicia (north-west of the country), an official voluntary IBR control plan has been in place since 2004 and now involves a large proportion of the cattle population. The program is based on a serological survey of herds and progressive seroprevalence reduction, by means of replacement control and not by culling animals. Furthermore, additional measures are required, such as the mandatory control for IBR in all purchased animals.
Sweden
In 1994, the EFTA Surveillance Authority granted approval to the Swedish national IBR eradication program until 1997, along with additional guarantees for cattle intended for breeding and production and destined for Sweden, to protect the progress already made and to ensure that the program was successfully concluded (European Free Trade Association ‘EFTA’ Surveillance Authority, 1994b). In 1995, an official eradication program was approved (European Commission, 1995b). At the same time, the European Commission granted additional guarantees for cattle destined for Sweden, in order to protect territory under the IBR eradication program (European Commission, 1995c). In 1998, Sweden submitted supporting documentation to the Commission and obtained the right to be considered as IBR-free in the whole country (European Commission, 1998a).
Switzerland
The first IBR outbreak in Switzerland was reported in 1978, when data about infection prevalence and its distribution were not available and national laws concerning IBR were yet to be established (Ackermann et al., Reference Ackermann, Muller, Bruckner and Kihm1990). Retrospective studies indicate that, in 1978, the proportion of positive farms varied from 1 to 15% regionally. The disease was made notifiable and some measures were provided, such as trading bans for farms with confirmed or suspected IBR cases, the performance of serological tests before movement since 1980 and only allowing movement of seropositive cattle to slaughterhouses. Serological surveys were performed nationwide in order to determine the prevalence and incidence of disease, and since 1983 national law was modified to eradicate IBR. In particular, trade restrictions were extended to all cattle present in a positive farm, seropositive cattle had to be slaughtered, and the ban was lifted from the farm only if two consecutive serological tests within 6 months indicated freedom from BoHV-1 in all cattle. All farms had to be tested for antibodies to BoHV-1 in serum samples once per year or in pooled milk samples twice per year, and IBR vaccines were never licensed. In a first step, all these measures concerned dairy farms only, because fattening animals originated from IBR-free sources and were transported directly to the slaughterhouse. Nevertheless, a serological survey in 1985 indicated that fattening cattle were the last infection reservoir. For this reason, fattening farms were also subjected to restrictive measures related to farm biosecurity management as well as accurate serological tests. At the same time, new laws regulated cattle and semen importation as well to prevent IBR introduction from other countries. Finally, Switzerland eradicated IBR in 1988, 10 years from the first outbreak, by slaughtering about 50,000 seropositive cattle and without using vaccines. Since then, the Swiss Confederation has proven its IBR-free status annually by serological testing of serum and bulk-milk samples from randomly selected farms. Sample size had to be sufficient to exclude an effective prevalence rate higher than 0.2% (Swiss Confederation, 2018). Since 1994, different IBR outbreaks were reported in the country, probably because of viral reintroduction via imported infected cattle. As a result, in 2012, the surveillance program was modified by increasing the number of tested dairy farms number and by testing some other farms associated with IBR risk factors (seasonal cattle movement, high importation or trade rates, nearness to national borders). At present, the Swiss surveillance program provides for annual serological testing in randomly selected farms, high IBR-risk farms, and bulls aged >24 months. Vaccine use is not permitted, and seropositive cattle are considered virus carriers without further virological tests. Particularly, the national law in force in case of outbreaks provides for immediate notification, loss of IBR-free status, farm attachment, abortion-cause analysis, immediate serological tests, and annual random tests (Swiss Confederation, 1995). The European acknowledgement is regulated by a trading agreement between the Swiss Confederation and European Commission since June 1999 (Swiss Confederation, 1999). As a result, additional guarantees were valid for cattle destined for Switzerland according to former European regulations (European Commission, 2004b).
The Netherlands
In the Netherlands, IBR outbreaks were observed for the first time in 1973 and associated with severe clinical signs; BoHV-1 spread in the whole territory in the years thereafter, so the severity of clinical signs decreased as the infection became enzootic (De Wit et al., Reference De Wit, Hage, Brinkhof and Westenbrink1998). Most of the dairy cattle population became seropositive because of viral dissemination and extensive vaccination: in 1993, about 42% of dairy cows were seropositive and about 85% of herds were infected (Vonk Noordegraaf et al., Reference Vonk Noordegraaf, Buijtels, Dijkhuizen, Franken, Stegeman and Verhoeff1998). Since 1993, local authorities allowed the Dutch cattle industry to begin a voluntary eradication program. In 1996, the Dutch farmers board decided to start a national compulsory eradication program. It began in 1998 and provided vaccination with a marker vaccine for all cattle >3 months of age twice a year; therefore, herds were certified as IBR-free when all cattle >12 months of age tested seronegative in ELISA for gB or gE (De Wit et al., Reference De Wit, Hage, Brinkhof and Westenbrink1998). At the start of the eradication program, about 25% of dairy herds and about 18% of non-dairy herds were certified as IBR-free in the country. These farms were allowed to purchase cattle from other certified herds only; therefore, their status was monitored through monthly bulk-milk tests and semiannual serological tests in dairy herds and non-dairy herds, respectively (Vonk Noordegraaf et al., Reference Vonk Noordegraaf, Labrovic, Frankena, Pfeiffer and Nielen2004). Surveillance program demands were determined by means of mathematical models (Graat et al., Reference Graat, De Jong, Frankena and Franken2001). Dutch agriculture is characterized by intensive animal production, implemented since the 1980s when cattle concentrations, as well as national and extra-national cattle movements, increased considerably (Van Schaik et al., Reference Van Schaik, Dijkhuizen, Huirne, Schukken, Nielen and Hage1998). The Netherlands has a high cattle importation rate: >900,000 cattle are imported annually, and most of them (94%) are calves fattened for a few months and then sent to the slaughterhouse. Most imported cattle originate from IBR-free or nearly IBR-free countries. Nevertheless, a notable proportion of cattle are imported from countries where the disease is still endemic. When the Netherlands becomes IBR-free, cattle import flow will be the largest threat for virus reintroduction (Santman-Berends et al., Reference Santman-Berends, Mars, Waldeck, Van Duijn, Wever, Van den Broek and Van Schaik2018).
United Kingdom
IBR was first described in the UK in 1961, when a BoHV-1.2b strain was isolated, being designated as the British-type virus since then (Oxford strain; Graham, Reference Graham2013). From 1977 to the mid-1980s, a sudden rise in IBR incidence and severity was reported, associated with a high herd morbidity rate and a variable but significant mortality rate. The seropositive cattle rate increased from 5% in the early 70s to 12% in the mid-1980s. A new viral strain was possibly introduced from North America to the UK in that period because of the movement of infected cattle (Edwards, Reference Edwards1988). Subsequently, IBR outbreaks have been constantly reported. According to official sources, in the IBR surveillance data in the UK from 2010 to 2015, the disease has been diagnosed throughout the whole country, but mainly in Scotland, Western regions, and Wales (Veterinary Investigation Diagnosis Analysis, VIDA, 2015). Within this period, a decrease in the number of submitted IBR cases has been reported, confirming better infection control, although these results may have been biased by the use of different diagnostic methods (Ackermann et al., Reference Ackermann, Muller, Bruckner and Kihm1990). In 2017, UK authorities submitted supporting documentation to the Commission that let Jersey territory to be recognized as free of BoHV-1 infection and applied for additional IBR guarantees (European Commission, 2017a).
Discussion
The IBR status and history of several European countries were investigated by using data obtained from available literature and legislative sources. The first IBR outbreak in Europe was reported as a vesicular coital exanthema in Germany in the early 1900s, and its viral etiology was demonstrated in 1928. Genital signs of infection were the main form of the disease until the 1950s. At the same time, a more severe respiratory form of the disease, caused by the same virus, rapidly spread in America. This disease involving the upper respiratory tract is considered as the proven expression of IBR in cattle. IBR rapidly spread to Europe when American dairy cattle were imported to improve the milk production performance of European cattle (Muylkens et al., Reference Muylkens, Thiry, Kirten, Schynts and Thiry2007). At present, BoHV-1 is still widespread (despite several territories that are officially free), although there are significant differences in herd-level prevalence; disease incidence between and within regions is depending, of course, on epidemiological conditions, but it is important to consider the geographical relationships and cattle management practices (Nettleton and Russel, Reference Nettleton and Russel2017). To manage the disease, control schemes were first introduced in Europe in the late 1970s. Depending on the seroprevalence rate, eradication schemes are based on identification and removal of seropositive animals or employment of gE-deleted marker vaccines in infected herds (Raaperi et al., Reference Raaperi, Orro and Viltrop2014). In case of low seroprevalence, at herd level, as well as at territorial level, researching positive cases and slaughtering them could be a cheaper and more effective strategy; on the other hand, if viral circulation is reported in most of the enrolled herds, vaccination strategy should be implemented. Consequently, to improve the IBR status of the territory, it is necessary to apply the proper strategy. These considerations influence both the efficacy and efficiency of the activities; the applied strategy is relevant in terms of cost/benefit ratio. The eradication process also differed in the period of measures application and in duration. Some countries began early with the eradication process, beginning from the 1980s, and the target was achieved in quite a short time. Other countries, although they implemented control measures in the 1990s, took a longer time to achieve eradication goals. In other cases, although an IBR control program has been in force for a long time, substantial results cannot be yet recorded. Finally some countries or parts of them implemented the eradication plans recently, so these plans are still in progress (Table 1). Therefore, in most cases, European countries obtained IBR-free status in their whole territory, and others in limited zones only (Table 2). Scandinavian countries, as well as Austria and Switzerland, were the first countries implementing and successfully concluding an IBR eradication program between the late 1970s and the early 1990s. The official approvals for both the eradication plan and the IBR-free status were obtained in a short period in the 1990s. Their low initial seroprevalence rate allowed them to adopt a ‘test and slaughter’ strategy. Germany, which showed an infection prevalence rate of 20%, implemented an eradication program based on the same strategy and obtained officially IBR-free status after 20 years (1997–2017). Belgium and the Netherlands started control programs for BoHV-1 infection in the early 1990s and, to date, have not received official IBR-free status; in these countries, a vaccination strategy has been employed since the initiation of the control programs. In Italy, a national eradication program became effective since 2015/2016, but involved only specific beef breeds; nevertheless, some Italian regions and provinces implemented IBR control plans since the early 1990s and 2000s, and obtained official IBR-free status for their territories, such as the Valle d'Aosta region and Bolzano province. The Czech Republic started to control IBR in 2005, and obtained official approval of its eradication plan by the European Commission some years later (2008). In Hungary, a national eradication program has been in force since 2002. Slovakia implemented a compulsory control plan in 2006, whereas Spain implemented a voluntary one only in the region of Galicia, since 2004. Luxembourg adopted an eradication program and the European Commission approved it in 2017. In France, official authorities approved a compulsory eradication program in 2016.
Table 1. Length of time of the infectious bovine rhinotracheitis (IBR) control programs in European countries
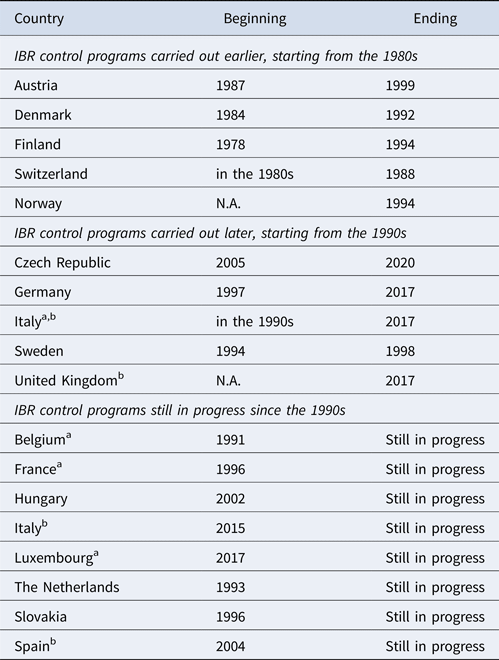
N.A., not available.
a IBR control program approved by European Commission (art. 9, Directive 432/64/EECc).
b IBR control program limited to some territories.
c Currently repealed by Regulation 2016/429/EU
Table 2. IBR free-status in the whole country or some territories only (art. 10, Directive 432/64/EECa) in European countries subjected to UE approved or not UE approved control programs
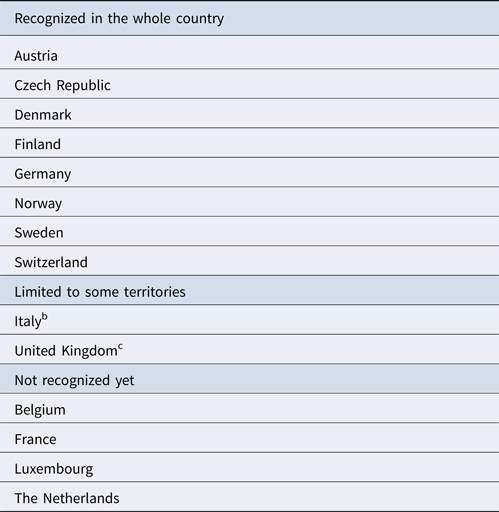
a Currently repealed by Regulation 2016/429/EU.
b Valle d'Aosta region; Bolzano province.
c Isle of Jersey.
Conclusions
All investigated countries managed IBR issues from the 1970s–1980s to date, by implementing various alternative strategies, successfully or not. Consequently, the European IBR status is diverse. This is an unfavorable condition for individual countries and the entire European territory. Therefore, as in the past, every State developed and implemented plans for disease management at the national level, the European legal and epidemiological background on IBR are not homogeneous. An opportunity to realize a harmonized approach for the management of IBR in Europe could be the new legislative tool, called the Animal Health Law (European Commission, 2016a) and, in general, the legal framework has been in force since 21 April 2021. Finally, a critical point involves data collection about IBR epidemiological surveillance in European countries. Currently, there is a lack of a European IBR informative system, and data collection on IBR management is fragmented even at a national level. If an efficient data sharing system be available, the analysis of surveillance data would support the progression of IBR control strategies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1466252321000116.
Financial support
This study was financially supported by internal funds of the Istituto Zooprofilattico Sperimentale Umbria Marche ‘Togo Rosati’, Perugia, Italy.







