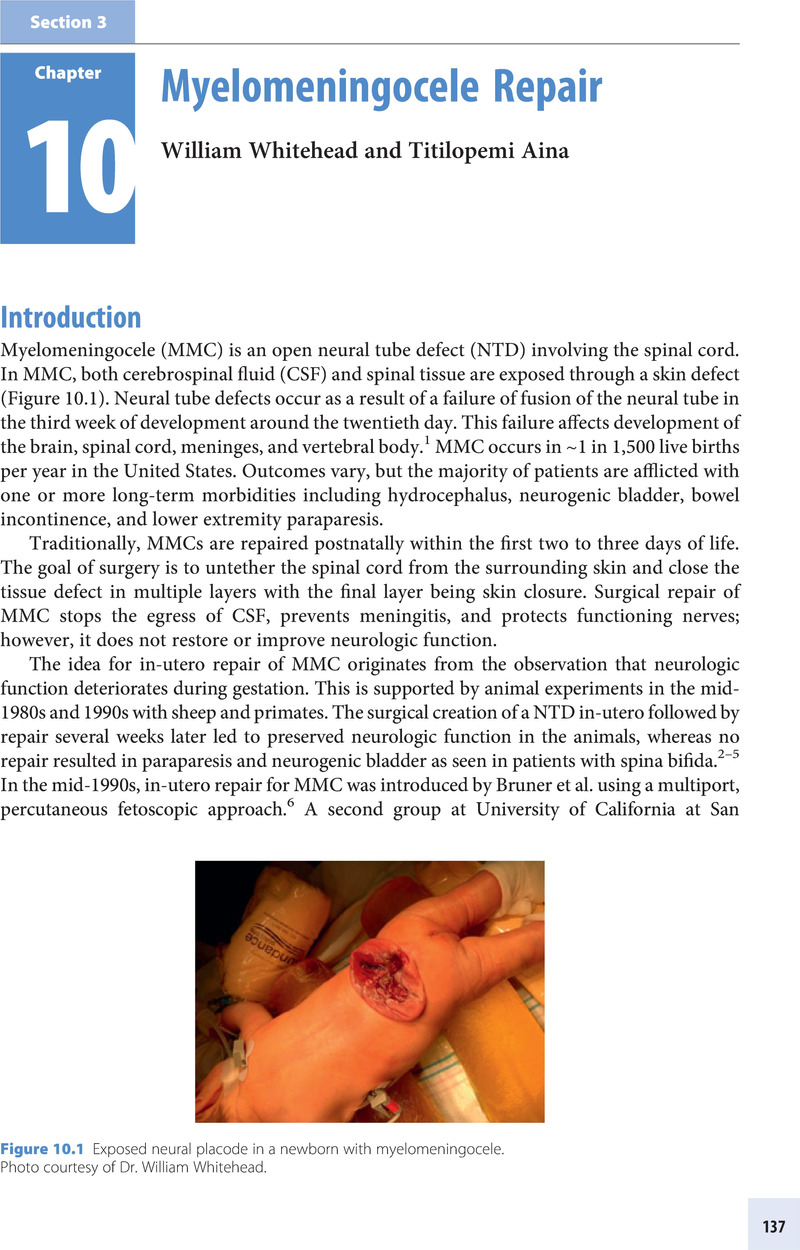
Section 3
Published online by Cambridge University Press: 19 November 2021
Summary
- Type
- Chapter
- Information
- Anesthesia for Maternal-Fetal SurgeryConcepts and Clinical Practice, pp. 137 - 206Publisher: Cambridge University PressPrint publication year: 2021