15 results
Vanadoallanite-(La): a new epidote-supergroup mineral from Ise, Mie Prefecture, Japan
-
- Journal:
- Mineralogical Magazine / Volume 77 / Issue 6 / August 2013
- Published online by Cambridge University Press:
- 05 July 2018, pp. 2739-2752
-
- Article
- Export citation
Ardennite, tiragalloite and medaite: structural control of (As5+,V5+,Si4+)O4 tetrahedra in silicates
-
- Journal:
- Mineralogical Magazine / Volume 74 / Issue 1 / February 2010
- Published online by Cambridge University Press:
- 05 July 2018, pp. 55-71
-
- Article
- Export citation
Palenzonaite, berzeliite, and manganberzeliite: (As5+, V5+, Si4+)O4 tetrahedra in garnet structures
-
- Journal:
- Mineralogical Magazine / Volume 76 / Issue 5 / October 2012
- Published online by Cambridge University Press:
- 05 July 2018, pp. 1081-1097
-
- Article
- Export citation
A temperature-dependent structure study of gem-quality hibonite from Myanmar
-
- Journal:
- Mineralogical Magazine / Volume 74 / Issue 5 / October 2010
- Published online by Cambridge University Press:
- 05 July 2018, pp. 871-885
-
- Article
- Export citation
Inter-Relations between the Arctic Sea Ice and the General Circulation of the Atmosphere (Abstract)
-
- Journal:
- Annals of Glaciology / Volume 9 / 1987
- Published online by Cambridge University Press:
- 20 January 2017, p. 252
-
- Article
-
- You have access
- Export citation
A New Installation for Studying the Sidereal Anisotropy of Cosmic Rays
-
- Journal:
- Publications of the Astronomical Society of Australia / Volume 4 / Issue 4 / 1982
- Published online by Cambridge University Press:
- 25 April 2016, pp. 456-458
-
- Article
- Export citation
Optical and Near-Infrared Observations of Classical Nova V1723 Aql
-
- Journal:
- Proceedings of the International Astronomical Union / Volume 7 / Issue S281 / July 2011
- Published online by Cambridge University Press:
- 17 January 2013, pp. 121-123
- Print publication:
- July 2011
-
- Article
-
- You have access
- Export citation
HI-selected Galaxies As a Probe of Quasar Absorption Systems
-
- Journal:
- Proceedings of the International Astronomical Union / Volume 5 / Issue S262 / August 2009
- Published online by Cambridge University Press:
- 13 April 2010, pp. 402-403
- Print publication:
- August 2009
-
- Article
-
- You have access
- Export citation
Optimization of a Method for Imaging Drosophila Embryos with a Variable Pressure Scanning Electron Microscope
-
- Journal:
- Microscopy and Microanalysis / Volume 11 / Issue S02 / August 2005
- Published online by Cambridge University Press:
- 01 August 2005, pp. 420-421
- Print publication:
- August 2005
-
- Article
-
- You have access
- Export citation
Chemical enrichment of ICM in a hierarchical galaxy formation model including SNe Ia
-
- Journal:
- Proceedings of the International Astronomical Union / Volume 2004 / Issue IAUC195 / March 2004
- Published online by Cambridge University Press:
- 06 October 2004, pp. 290-292
- Print publication:
- March 2004
-
- Article
-
- You have access
- Export citation
Deep Near-Infrared Surveys and Young Brown Dwarf Populations in Star-Forming Regions
-
- Journal:
- Symposium - International Astronomical Union / Volume 211 / 2003
- Published online by Cambridge University Press:
- 26 May 2016, pp. 87-90
- Print publication:
- 2003
-
- Article
-
- You have access
- Export citation
The oxygen deficiency effect of VO2 thin films prepared by laser ablation
-
- Journal:
- Journal of Materials Research / Volume 12 / Issue 2 / February 1997
- Published online by Cambridge University Press:
- 31 January 2011, pp. 416-422
- Print publication:
- February 1997
-
- Article
- Export citation
GaAs / AlGaAs SQW Optical Switch on Si
-
- Journal:
- MRS Online Proceedings Library Archive / Volume 281 / 1992
- Published online by Cambridge University Press:
- 25 February 2011, 363
- Print publication:
- 1992
-
- Article
- Export citation
The Kamiokande Solar Neutrino Experiment
-
- Journal:
- International Astronomical Union Colloquium / Volume 121 / 1990
- Published online by Cambridge University Press:
- 12 April 2016, pp. 179-186
- Print publication:
- 1990
-
- Article
-
- You have access
- Export citation
A Study of Recrystallization Texture Formation in Cold Rolled Iron Sheets with X-Ray Diffraction Techniques
-
- Journal:
- Advances in X-ray Analysis / Volume 14 / 1970
- Published online by Cambridge University Press:
- 06 March 2019, pp. 214-230
- Print publication:
- 1970
-
- Article
- Export citation


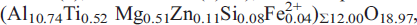 was investigated between temperatures of 100 K and 923 K by single-crystal X-ray diffraction methods. Structure refinements have been performed at 100, 296, 473 and 923 K. In hibonite from Myanmar, Ti substitutes for Al mainly at the octahedral Al4 site and, to a lesser degree, at the trigonal bipyramidal site, Al2. The Al4 octahedra build face-sharing dimers. If Ti
was investigated between temperatures of 100 K and 923 K by single-crystal X-ray diffraction methods. Structure refinements have been performed at 100, 296, 473 and 923 K. In hibonite from Myanmar, Ti substitutes for Al mainly at the octahedral Al4 site and, to a lesser degree, at the trigonal bipyramidal site, Al2. The Al4 octahedra build face-sharing dimers. If Ti