Introduction
The Ordovician culminated in one of the major Phanerozoic mass extinctions, ranked roughly fourth in severity, equivalent to the Cretaceous-Paleogene boundary mass extinction (Alroy, Reference Alroy2008, Reference Alroy2010a, Reference Alroyb). Mass extinctions due to multiple glaciations in Gondwana severely affected the tropical coral-sponge reef ecosystem in the Late Ordovician (Copper, Reference Copper2002, Reference Copper2011; Webby, Reference Webby2002), and its concomitant tropical shelly faunas, in which athyride brachiopods played a significant role. Several extinction events mark the Ordovician-Silurian (O-S) boundary section on Anticosti, as evident within the Hirnantian Ellis Bay Formation (Copper et al., Reference Copper, Jin and Desrochers2013). The Hirnantian carbonate-dominated succession, ~80 m thick, was deposited over some two million years and marked the arrival of a rich and diverse suite of early spire-bearers (atrypides, hindellides, but no spiriferides), not seen in the Katian Vaureal Formation below. These all suffered losses at the end of the Hirnantian. The general recovery of brachiopod shelly faunas is recorded in the lower Silurian for Anticosti (Copper and Jin, Reference Copper and Jin2012, Reference Copper and Jin2014, Reference Copper and Jin2015). The earliest shelly community of the Becscie Formation (Rhuddanian) was characterized by low diversity and small shells such as Koigia, described herein (Fig. 1). The upper Becscie Formation was marked by the appearance of the large-shelled pentameride Virgiana community, which became ubiquitous in Laurentia during the late Rhuddanian (Jin et al., Reference Jin, Long and Copper1996; Jin and Copper, Reference Jin and Copper2000). Major diversification of Silurian-type athyrides, atrypides, and pentamerides began later in the Aeronian and Telychian.
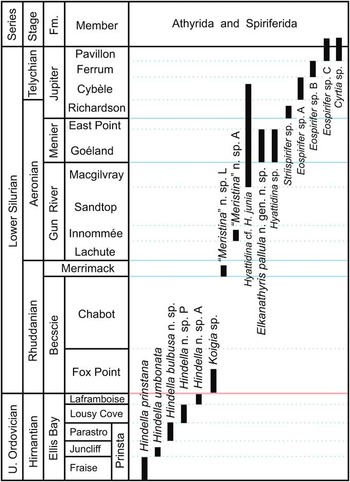
Figure 1 Stratigraphic ranges of the early hindellides Hindella, Koigia, Hyattidina, and Elkanathyris n. gen. across the Ordovician-Silurian boundary, Anticosti Island, eastern Canada. The early spiriferide occurrences are also shown for reference.
Considerable confusion exists about the richly fossiliferous transitional Ordovician-Silurian boundary interval on Anticosti, and where to draw the boundary itself (Copper et al., Reference Copper, Jin and Desrochers2013). The drastic environmental changes were reflected by critical evolution of the tropical marine faunas, such as those well preserved in the carbonate platforms of Baltica and Laurentia. Different species of spire-bearing athyrides and atrypides have, in the past, been variously assigned to the Late Ordovician or early Silurian, or sometimes to both. This study aims to clarify the morphology, evolution, and distribution of such key taxa in the Hirnantian and Rhuddanian, and provide an update and revision of the taxonomy proposed in the Treatise (Alvarez and Rong, Reference Alvarez and Rong2002).
Athyrides were late arrivals in the spire-bearing brachiopod orders in Laurentia and Baltica during the Late Ordovician, and did not become major components of the benthic shelly fauna until the Hirnantian. On Anticosti Island, the genus Hindella (Figs. 2, 3) was an abundant component of the brachiopod fauna and locally formed shell beds in the Ellis Bay Formation. In their interpretation of athyride evolution, Alvarez et al. (Reference Alvarez, Rong and Boucot1998, p. 834–835) regarded Dayia, with laterally directed spiralia and a simple jugum, as derived from Lissatrypa via lateral compression of the muscle field, thus regarding the orientation of the spiralia as insignificant, although the spiralia in these two genera have opposite directions (Copper, Reference Copper1986; Copper and Gourvennec, Reference Copper and Gourvennec1996). They also viewed the laterally directed double spiralia of the Coelospirinae as compatible with atrypoid affinities (Alvarez et al., Reference Alvarez, Rong and Boucot1998, p. 836). These authors assigned Hindella to the Silurian Meristellinae (superfamily Meristelloidea), and considered Cryptothyrella as its junior synonym. The Rhuddanian (early Silurian) genus Koigia, common in Estonia and on Anticosti, was allocated to their new subfamily Whitfieldelllinae, to which they gave a range of Ashgill through late Silurian (Alvarez et al., Reference Alvarez, Rong and Boucot1998, p. 836). Thus Alvarez et al. (Reference Alvarez, Rong and Boucot1998) abandoned the earlier name Hindellinae (Schuchert, Reference Schuchert1894), used formally for the earliest athyrides by most Russian workers. We have found no evidence for any Katian–Rhuddanian Meristellinae, nor Whitfieldellinae on Anticosti Island, where athyrides are generally abundant and well preserved at numerous localities.
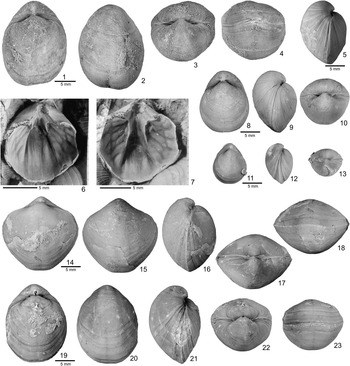
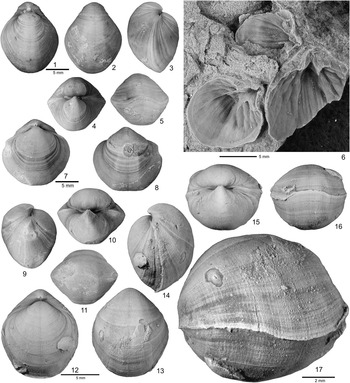
Figure 2 Hindella species from the Ellis Bay Formation (Hirnatian), Anticosti Island. (1–7) Hindella umbonata (Billings, Reference Billings1862); GSC 137675 (1–4) and GSC134359 (5), Juncliff Member, locality A814, Prinsta River (loc. A814); (6, 7) GSC 59097, interior of pedicle valve viewed at two different angles, Junction Cliff (A4, type locality). (8–13) Hindella bulbusa n. sp., Parastro Member, Parastrophinella Bluff (loc. A48); (8–10) GSC 137679, paratype; (11–13) GSC137680, paratype. (14–23) Hindella prinstana (Billings, Reference Billings1862), Fraise Member; (14–18) GSC 137666, wider variety; (19–23) GSC 137667, elongate form.
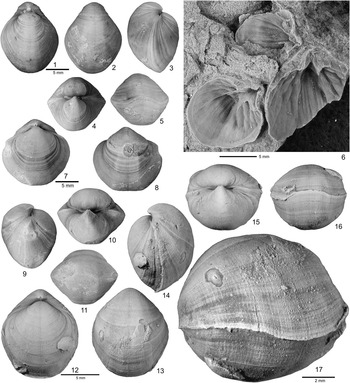
Figure 3 Hindella bulbusa n. sp. from Parastrophinella Bluff (loc. C720 = A48), Anticosti Island. (1–5) GSC 137671, holotype; (6) GSC 137681, paratype slab, interiors of ventral valves; (7–11) GSC 137670, paratype, subrhomboidal shell; (12–17) GSC 131790, well-preserved shell showing capillae in anterior part of shell.
Modzalevskaya (Reference Modzalevskaya1979, fig.1; 1996) treated the classification of more-advanced athyrides of Silurian and Devonian age, including genera with a complex jugum (or jugal saddle and loops and “arcuate jugal plates”, such as Glassina, Greenfieldia, Protathyris, and Pseudoprotathyris). Such complex jugal structures and accessory lamellae are unknown or have not been described in athyrides of Late Ordovician and earliest Silurian (Rhuddanian) age, and did not become common until Wenlock–Ludlow time. Modzalevskaya (Reference Modzalevskaya1979) also pointed out that the subfamily Protathyridinae Boucot, Johnson, and Staton, Reference Boucot, Johnson and Staton1965 represents a junior synonym of the Athyrinae. Grunt (Reference Grunt1989, fig. 41) included Hindella along with Koigia, Hyattidina, and Cryptothyrella (and others) within the Hindellinae as an ancestral group, although she did not discuss the simplicity of the brachidium as a key character. We effectively concur with that analysis.
In this study, the early athyride taxonomy adopted in the last Treatise (Alvarez and Rong, Reference Alvarez and Rong2002) is revised, based on internal structures revealed through serial sections. Although Alvarez (Reference Alvarez1999) cautioned against the use of serial sections alone to reconstruct internal structures, we employ serial sections and acetate peels of pristinely preserved calcitic whole shells from Anticosti Island, especially when the serial-section peels were taken at close intervals (0.1 mm). These have proven accurate. Early athyrides have been described as having a smooth shell, but we discovered that many have a finely capillate shell. Hindella is the direct ancestor of Rhuddanian Koigia, an earliest Silurian recovery taxon, and both belong to the subfamily Hindellinae. This description probably fits all simple ancestral Ordovician athyrides (once their brachidia become better known), and their immediate descendants with such features. Complex juga and their extensions appear to have evolved first in the mid to late Aeronian (middle early Silurian). We note from serial sections that the subfamily Hyattidininae (Sheehan, Reference Sheehan1977), and its type genus Hyattidina, which first arrives in the Gun River Formation (Aeronian) of Anticosti, has a very simple jugum, laterally directed spiralia, and crura unconnected with the spiralia and jugum. This is basically like that of Hindella (Copper, Reference Copper1986, fig. 9). Alvarez et al. (Reference Alvarez, Rong and Boucot1998, p. 841), in contrast, assigned these to the family Hyattidinidae and superfamily Athyridoidea. Herein, we consider that Hyattidina is most closely related to the subfamily Hindellinae.
Stratigraphic distribution of Hindellidae on Anticosti Island
Hindella is the only athyride genus known to date in the Upper Ordovician (Hirnantian) of Anticosti (Copper et al., Reference Copper, Jin and Desrochers2013)—there are no earlier athyrides in the underlying Katian Vaureal Formation (see Fig. 1). It first appeared in the lower beds of the Ellis Bay Formation, without any predecessors on Anticosti or in North America. It must have evolved from athyrides of mid–upper Katian age, such as those known in the Anderken Formation (Dulankara strata) of Kazakhstan (Nikitin et al., Reference Nikitin, Popov and Holmer1996, Reference Nikitin, Popov and Bassett2006). Within the Ellis Bay Formation, Hindella evolved rapidly from the earliest species Hindella prinstana, through H. umbonata, and three younger species in the Parastro and Lousy Cove members, with the largest elongate shell at the top of the reefal Laframboise Member marking the last appearance of the genus (Fig. 1).
Alvarez and Rong (Reference Alvarez and Rong2002) reported the genus Whitfieldella from the Late Ordovician, although the validity of this lower range of this largely Silurian genus seems questionable. With the Hindellinae separated from the Meristellinae, as proposed in this study, the first appearance of Meristella and Meristina would be in the late Telychian or later. This agrees with the evolutionary scenario suggested by Schuchert (Reference Schuchert1894) and Modzalevskaya (Reference Modzalevskaya1985, Reference Modzalevskaya1996) that the Hindellinae (excluding Whitfieldella and Meristina) form a natural ancestral group in the order Athyrida. In comparison, the more derived subfamily Didymothyrinae (Modzalevskaya, Reference Modzalevskaya1996), with complex umbonal blades curved from the jugal saddle, first appears much higher in Telychian strata of the Jupiter Formation, coeval with its occurrence in Baltica.
Koigia occurs in the basal Becscie Formation, within ~1 m of thin- to medium-bedded, hard, micritic mudstone and wackestone that overlie the Laframboise reefs at the O-S boundary (Copper and Jin, Reference Copper and Jin2014). It is a much smaller-shelled athyride compared to Hindella in the Ellis Bay Formation, although their internal structures are similar. Koigia is locally abundant, alongside other Rhuddanian brachiopods, such as Zygospiraella, Becscia, and Viridita. In Estonia, the type species of Koigia also occurs in the basal Rhuddanian (Rubel, Reference Rubel1970 initially cited it as Hindella).
So far, Cryptothyrella has not been found on Anticosti Island, although some athyride specimens from the Aeronian Gun River and Menier formations (Copper and Jin, Reference Copper and Jin2012; Copper et al., Reference Copper, Long and Jin2012) may be assignable to the genus, pending further study. The type species, Cryptothyrella quadrangularis (Foerste, Reference Foerste1906), as seen in Ohio, is characterized by an unusually large and prominently elongate shell (30–40 mm in length). The Rhuddanian ‘Cryptothyrella benthic community’, recognized by Cocks and McKerrow (Reference Cocks and McKerrow1973, p. 293) for the platform setting in Laurentia and Baltica, has not been observed on Anticosti Island, nor has its presence been confirmed in the Rhuddanian of Laurentia.
Hyattidina is very abundant in the upper Gun River, Menier, and Jupiter formations, ranging from mid-Aeronian to mid-Telychian. The genus is absent from the older Becscie, Merrimack, and lower Gun River formations (Fig. 1). It retained the simple jugum and brachidium of Hindella, but has a well-developed fold and sulcus.
The early athyride shelly community on Anticosti Island
The carbonate sediments of Anticosti were deposited in the northern paleotropical latitudes, on a platform to ramp flanking the southeast side of Laurentia (Copper, Reference Copper2002; Cocks and Torsvik, Reference Cocks and Torsvik2011). Strata are undeformed with dips <2° today. During the Late Ordovician and early Silurian, Baltica was directly to the east at a similar paleolatitude, with an ocean ~1000–1500 km wide separating it from Laurentia. To the northeast, Siberia was mostly north of the paleoequator. In the Anticosti Basin, siliciclastic sediments were rare, and consisted of episodic storm-generated or seismic deposits, marked by slumped beds, mostly during the late Katian and Hirnanian. Wet coastal climates created an epeiric sea of mixed salinities (similar to the Java and Arafura epicontinental seas today) that affected the distribution of shelly and coral faunas in the early Silurian (Edinger et al., Reference Edinger, Copper, Risk and Atmojo2002). Facies differences between the east and west ends of Anticosti reflect a curving shoreline along the 200 km long outcrop belt.
In such a carbonate-dominated depositional setting of the Anticosti Basin, athyrides formed common shell clusters, or extensive shell beds. Their minute pedicle, as indicated by the small apical to trans-apical foramen, and common co-occurrences with small and delicate bryozoans or broken shells suggest that they anchored on skeletal clasts in the sediments. In the reefal Laframboise Member, Hindella was generally rare or only locally abundant (such as locality A1161), but in the reefal East Point Member (Aeronian) athyrides are generally common. This may indicate a later adaptation to shallow-water, higher-energy, reefal settings.
The athyrides were more common in mid-shelf settings, and rare in deeper waters of the Clorinda-Dicoelosia community found in the Menier and Jupiter formations. Hindella habitats varied from deeper muddy seafloors (alongside solitary rugosans or bryozoans), to somewhat shallower, but still low-energy, carbonate substrates (where it was commonly monospecific), and extending into shallow and high-energy reefal settings. In the Parastro Member, a relatively small form of Hindella (H. bulbusa n. sp.) occurs as a common component of the Parastrophinella pentameride association (Jin and Copper, Reference Jin and Copper2008).
The small shells (<7 mm in width) of Koigia occur commonly in higher energy, storm-influenced settings represented by the Becscie Formation, especially in the lower Fox Point Member, associated with other small-shelled taxa (e.g., Becscia and Viridita; see Jin and Copper, Reference Jin and Copper2010). The small shells usually show various degrees of distortion, damage, and disarticulation, with common geopedal structures in conjoined shells, indicating rapid burial by micritic mud during storms (for more discussions on the depositional environments, see Copper and Jin, Reference Copper and Jin2014). Koigia may be regarded as an opportunist that thrived immediately after the Late Ordovician mass extinction events, but athyrides became scarce in the upper Becscie Formation (Chabot Member) when the monotypic, large-shelled Virgiana brachiopod community became dominant.
On Anticosti, Hyattidiina and Elkanathyris n. gen. occur as common components of the Pentamerus community, indicating a mid-shelf depositional environment (Jin, Reference Jin2008). They may also be associated with rich and diverse atrypides. This agrees with the treatment of the Hyattidina community as equivalent to the Pentamerus or Stricklandia communities in the Welsh Borderlands (Cocks and McKerrow, Reference Cocks and McKerrow1973). It is relatively rare in the Eocoelia community.
Materials and methods
The basis of the paper is the stratigraphic work that covers ~2000 Anticosti localities, located on metric grid maps and with GPS coordinates (Copper et al., Reference Copper, Jin and Desrochers2013; Copper and Jin, Reference Copper and Jin2014, Reference Copper and Jin2015). The large brachiopod collection is stored at the Geological Survey of Canada, Ottawa. Well-preserved, pristine, calcitic specimens were serially sectioned with a Croft Parallel Grinder. Acetate peels were taken at 0.1 mm intervals, mounted between 35 mm glass slides, and examined and photographed under microscope. To prepare the serial section drawings, the peels were projected to a scale of x16 or x20, with the main internal features traced in ink and then scanned. The technique for reconstruction of the brachidia uses peels transposed into a view of the dorsal shell interior, employing the plane of symmetry as orientation (technique described in papers from 1967 and earlier, accurate to within a millimeter, and available from first author).
Repositories and institutional abbreviations
Figured specimens are housed in the Type Collections of the Geological Survey of Canada (GSC), Ottawa, the Cincinnati Museum Center (CMC-IP), Cincinnati, and Ohio State University (OSU), Columbus, Ohio. General collections are also stored in the GSC, with a number prefixed with “A” or “C” to denote the Paul Copper Collection from Anticosti Island.
Systematic paleontology
Order Athyrida Boucot, Johnson and Staton, Reference Boucot, Johnson and Staton1964
(nom.transl. Athyridae Davidson, Reference Davidson1881; =Incerti ordinis Nikiforova and Rzhonsnitskaya, Reference Nikiforova and Rzhonsnitskaya1960, part; ex Athyridoidea Boucot, Johnson, and Staton, Reference Boucot, Johnson and Staton1964, part)
Remarks
Boucot et al. (Reference Boucot, Johnson and Staton1964) recognized that the family name Athyridae Phillips, Reference Phillips1841, used for a group of Leptaena species known at that time, was invalid because the genus Athyris M’Coy, Reference M’Coy1844 was established later and, therefore, the family name could not have been derived from the genus Athyris. Boucot et al. (Reference Boucot, Johnson and Staton1964, Reference Boucot, Johnson and Staton1965) accordingly assigned the authorship of the Athyridae to M’Coy (Reference M’Coy1844), but changed the family name to Athyrididae without any explanation. Later, Alvarez et al. (Reference Alvarez, Brime and Brunton1980) identified the error in Boucot et al. (Reference Boucot, Johnson and Staton1964) (i.e., although M’Coy [Reference M’Coy1844] erected the genus Athyris, he assigned it to the family Delthyridae, but retained Phillips’ use of Athyridae for Leptaena and “Producta”). Alvarez et al. (Reference Alvarez, Brime and Brunton1980) thereby assigned the authorship of Athyridae to Davidson (Reference Davidson1881), who was the first to include Athyris in the family Athyridae. As in Boucot et al. (Reference Boucot, Johnson and Staton1964, Reference Boucot, Johnson and Staton1965), however, Alvarez et al. (Reference Alvarez, Brime and Brunton1980) and Alvarez and Brunton (Reference Alvarez and Brunton1993) recommended the use of Athyrididae instead of Athyridae.
In terms of ICZN provisions, it should be noted that Athyridae Phillips, Reference Phillips1841 and Athyridae Davidson, Reference Davidson1881 are effectively homonyms because the name was used for different genera of brachiopods. In this instance, the suppression of the senior homonym is warranted because neither Phillips (Reference Phillips1841) nor M’Coy (Reference M’Coy1844) derived the family name Athyridae from the genus Athyris (from the Greek, thyra, door, or its diminutive thyris, small opening, referring to the pedicle opening of the shell). As a result, Athyridae Davidson, Reference Davidson1881 becomes a valid name by default, and there is no justification to change the family name to Athyrididae, or to change the order name to Athyridida. According to ICZN (1999, Article 29.3.1.1), if the genitive singular stem of a noun ends in -id, these two letters should be elided bofore adding the family suffic -idae. An unelided form can be retained only if it has been in prevailing use. Therefore, even if the Greek word thyris is regarded as a latinized noun, and its genitive singlular stem is thyrid-, a proper family name is still Athyridae, as originally used by Phillips (Reference Phillips1841) and Davidson (Reference Davidson1881). Because Athyridae Davidson, Reference Davidson1881 is a valid family name that has been in use for over a century, we argue that its change to Athyrididae by Boucot et al. (Reference Boucot, Johnson and Staton1964) and subsequent use (Athyrididae, Athyridoidea, Athyridida, etc.) should be avoided.
In light of the discussions above, we propose to retain the name Athyridae (and hence Athyrida), as we have done in this study. A detailed discussion will be suitable for an ICZN Opinion note.
Family Hindellidae Schuchert, Reference Schuchert1894
(nom. transl. Hindellinae Schuchert, Reference Schuchert1894)
Subfamily Hindellinae Schuchert, Reference Schuchert1894
Genera assigned
As emended in this study, the subfamily Hindellinae Schuchert, Reference Schuchert1894 includes the genera listed below.
Hindella, Davidson, Reference Davidson1882.—
Hirnantian, Late Ordovician, Anticosti, Canada.
Cryptothyrella Cooper, Reference Cooper1942.—
Aeronian, Llandovery, mid-western USA.
Tschatkalia Nikiforova, Reference Nikiforova1964.—
Llandovery, Chatkal Mountain Range, Siberia.
Koigia Modzalevskaya, Reference Modzalevskaya1985.—
early Rhuddanian, Estonia, and Anticosti Island, Canada (simple brachidia as in Hindella).
Genera questionably assigned
For most of the genera below, the precise nature of the jugum is not yet known, although some are superficially similar to Hindella, such as the genera from North China (Fu, Reference Fu1982).
Hyattidina Schuchert, Reference Schuchert1913.—
Aeronian, Llandovery, North America; the genus has a brachidium similar to that of Hindella, and the subfamily Hyatidininae is considered a junior synonym of Hindellinae.
Colongina Breivel and Breivel, Reference Breivel and Breivel1970.—
Early Devonian, eastern slope of Urals; designated by Grunt (Reference Grunt1986, p. 25) as a hindelline, but its brachidia are unknown; doubtful assignment considering its much younger age; possibly an atrypide.
Apheathyris Fu, Reference Fu1982.—
Katian, Ningxia, North China (smooth, biconvex, rectimarginate shell, brachidia unknown).
Weibeia Fu, Reference Fu1982.—
Katian, Shaanxi, North China (smooth shell, weak fold and sulcus, brachidia unknown).
Argella Menakova and Nikiforova, Reference Menakova and Nikiforova1986.—
Pridoli, upper Silurian, Zeravshan Range, Tadzhikistan; elongate smooth shell, with simple brachidia like Hindella and Hyattidina, but posterior internal structures unclear (Alvarez and Rongs, Reference Alvarez and Rong2002).
Cyclorhynchia Baranov, Reference Baranov1994.—
Katian, Tscherkidium Beds, Selennyakh Range, NE Siberia (jugum unknown, and requiring assessment, but with laterally directed spiralia of three whorls); Copper (Reference Copper2002, p. H1472) incorrectly synonymized it with the atrypoid Cyclospira, which lacks a jugum, but has medially directed spiralia, the opposite to that of Cyclorhynchia.
Kellerella Nikitin and Popov in Nikitin et al., Reference Nikitin, Popov and Holmer1996.—
Anderken Formation, Dulandkara Stage (mid–late Katian), Chu-Ili, Kazakhstan; its short, disjunct jugal processes differ from those found in typical hindellides.
Nikolaispira Nikitin and Popov in Nikitin et al., Reference Nikitin, Popov and Holmer1996.—
Anderken Formation, Dulandkara Stage (mid–late Katian), Chu-Ili, Kazakhstan; its short, disjunct jugum differs from that found in typical hindellides described in this study.
Elkanathyris n. gen. (herein).—
Jupiter Formation, Aeronian–Telychian, Anticosti Island.
Diagnosis (emended herein)
Smooth or gently plicate, commonly with capillae, biconvex; small, distinct interarea, with minute deltidial plates, and apical to trans-apical foramen. Ventral valve mostly with relatively thick prismatic apical callus, deeply impressed muscle scars, and deep groove in the hinge plate. Dorsal valves with simple, arched jugum and medially aligned crura unconnected to the brachidium, with hooked terminations of the jugal blades. Laterally directed spiralia, <12 whorls. Loops, accessory lamellae, and extensions of the jugum absent.
Occurrence
Late Ordovician (late Katian) and early Silurian (Llandovery), Laurentia, Baltica, Siberia, Tadzhikistan, ?Kazakhstan, and ?North China. Katian hindellide genera have not been reported from Laurentia. Some primitive or ancestral athyrides from the mid–upper Katian of Kazakhstan and North China, such as Nikolaispira and Kellerella, are regarded as possible ancestral hindellides. During the Hirnantian, hindellides diversified to become a group of prominent and abundant brachiopods in tropical environments worldwide, forming extensive shell beds. The family may have survived into the late Silurian as Argella. An Early Devonian record is uncertain.
Remarks
The order Athyrida is characterized by (1) medial crura oriented along the plane of symmetry, (2) laterally directed spiralia (some with double spiralial lamellae), and (3) a simple or complex jugum connecting the spiralia. The earliest forms, as represented by the Late Ordovician and early Silurian Hindellinae, have a smooth or finely capillate, impunctate shell, a simple jugum, and single, flat spiralial lamellae. Another consistent early feature is that the crura and brachidia approach each other at a sharp angle, but do not fuse. During the late Silurian and Devonian, the spiralium evolved double parallel whorls, developed from jugal extensions, or by transforming from U-shaped, trough-like spiral lamellae to double lamellae, as in the Anoplothecidae. Evolution of the brachidia demonstrates that by Aeronian–Telychian (mid-Llandovery) time, some athyrides developed complex jugal stems or extensions, such as in the Meristellinae and Whitfieldellinae.
The lateral projection of spiralia in athyrides and spiriferides suggests that they had a different feeding strategy, with feeding currents (from the spiral base inwards), opposite to that in atrypides (with medially or dorsally directed spiralia, and feeding current from the base outwards; see Copper, Reference Copper1986, figs. 8, 9). Their Ordovician stratigraphic record shows that the original single spiral whorl stood in the central plane of symmetry, as seen in the Katian protozyginids (Copper, Reference Copper1977). Nikiforova and Rzhonsnitskaya (Reference Nikiforova and Rzhonsnitskaya1960) combined the superfamily “Athyracea” under Incerti Ordinis, somewhere between spiriferides and terebratulides (in that sense, they should be accredited with raising the athyrides to ordinal status because nearly all of the families they assigned are recognized as true athyrides today). Under the suborder Athyridoidea, Boucot et al. (Reference Boucot, Johnson and Staton1964;=suborder Athyrididina in Boucot et al., Reference Boucot, Johnson and Staton1965), however, excluded many groups that are recognized as athyrides today, such as the Athyrisinoidea, Retzioidea, Dayioidea, Anoplothecidae, and Kayseriidae. Thus the “Incerti Ordinis” of Nikiforova and Rzhonsnitskaya (Reference Nikiforova and Rzhonsnitskaya1960) matches more closely the order Athyrida as defined herein.
Externally, it is difficult to distinguish many athyride taxa with smooth or capillate shells, due to their strong homeomorphy. Copper (Reference Copper1986) reconstructed the spiralia and jugum of Hindella for the first time, based on topotype material, and demonstrated that there was no skeletal connection between the crura and spiralium in either Hindella or Hyattidina, although there should have been soft tissue to hold them together in vivo. It is primarily the Russian workers (e.g., Modzalevskaya, Reference Modzalevskaya1985, Reference Modzalevskaya1996; Grunt, Reference Grunt1989) who have clarified the nature of the lophophore-supporting skeletal structures, and established evolutionary relationships between genera and subfamilies. A key to their understanding lies in the earliest subfamily, the Hindellinae.
Schuchert (Reference Schuchert1894, Reference Schuchert1897), who named the Hindellinae, visualized them as encompassing Early Ordovician and Silurian athyrides possessing a simple jugum, although he unwittingly included later genera that are now known to be well outside that group, such as Anoplotheca and Coelospira, with double spiralial lamellae and a very complex jugum. Later, Schuchert (Reference Schuchert1928) revised his classification, and confined the Hindellinae to four smooth-shelled genera: Hindella, Hyattidina, Greenfieldia, and Whitfieldella (the latter two were moved to other subfamilies later). At that time, he also assigned the Hindellinae to the family Meristellidae of Waagen (Reference Waagen1883).
Nikiforova and Rzhonsnitskaya (Reference Nikiforova and Rzhonsnitskaya1960) and Menakova (Reference Menakova1964) accepted the Hindellinae as a subfamily, and included it, besides Hindella, Whitfieldella, and Hyattidina, in the family Nucleospiridae. This is close to the Hindellinae defined in this study, except that we exclude the hindellines from the later Wenlock nucleospirids. An early origin for the Nucleospiridae is uncertain, although there are relatively flat, smooth athyrides, such as “Athyris” lara Billings (Reference Billings1866) in the Merrimack Formation of late Rhuddanian age. “Athyris” solitaria Billings Reference Billings1866 from the same strata belongs to the smooth atrypide genus Cerasinella Copper, Reference Copper1995.
Sheehan (Reference Sheehan1977) abandoned the subfamily Hindellinae altogether, and allocated Hindella to the Meristellinae, and Hyattidina to a new subfamily, the Hyattidininae, both within the Meristellidae.
Modzalevskaya (Reference Modzalevskaya1985, fig. 29; 1996) proposed a comprehensive evolutionary scenario for the early athyrides of latest Ordovician–early Silurian age. She showed only Hindella in the Ordovician, but extended it into the Rhuddanian where Cryptothyrella was treated as a synonym. For the Rhuddanian, Modzalevskaya listed three genera: Koigia, Hyattidina, and Tschatkalia, and grouped them into the Hyattidinae. She did not use the subfamily Hindellinae Schuchert, Reference Schuchert1894, but assigned Greenfieldia to the younger Didymothyrinae, and Hindella to the Meristellinae. Notably, Modzalevskaya (Reference Modzalevskaya1985) showed that the Meristellinae, Meristinae, and the genus Whitfieldella (and thus Whitfieldellinae) appear first in the Wenlock, characterized by the presence of a more complex jugum. In a series of elaborate diagrams, Modzalevskaya (Reference Modzalevskaya1985, figs. 7–19) made detailed comparisons of the jugum in a range of genera for the first time, demonstrating that complex juga evolved later, and first appeared in such late Telychian–Wenlock genera as Meristella, Meristina, Didymothyris, and Collarothyris. On Anticosti Island, such complex juga first appeared in the mid-Aeronian athyrides (work in progress).
Grunt (Reference Grunt1986, Reference Grunt1989) adopted the Hindellinae of Schuchert (Reference Schuchert1894), and included in it nine genera, confining Cryptothyrella to the early Silurian. She employed Sheehan’s Reference Sheehan1977 partial serial sections for “Hindella umbonata” from Junction Cliff. Grunt (Reference Grunt1986) followed Schuchert (Reference Schuchert1928) in assigning the Hindellinae to the family Meristellidae. She elevated the Didymothyrinae to family status and, on the basis of their complex jugum, placed it under the superfamily Athyridoidea.
Dagys (Reference Dagys1996) reclassified the Order Athyrida (no author assigned) into three suborders, the Retziidina, Koninckinidina and Athyrididina, and did not recognize the subfamily Hindellinae.
Alvarez and Rong (Reference Alvarez and Rong2002, p. H1556) elevated the Hyattidininae to family status, but did not mention the subfamily Hindellinae, and assigned Hindella to the family Meristellidae within the superfamily Meristelloidea, and transferred the smooth-shelled Hyattidina Schuchert, Reference Schuchert1913 to the superfamily Athyridoidea (herein we assign Hindella and Hyattidina to the same family, Hindellidae). They did not discuss the lack of skeletal connection between the crura and brachidium, nor the simple jugum, in such early athyrides. Davidson’s (Reference Davidson1882) reconstruction of the Hindella brachidium (shown in Alvarez and Rong, Reference Alvarez and Rong2002, p. H1564, fig. 1063v) incorrectly shows fused crura.
In the revised Treatise, Alvarez and Rong (Reference Alvarez and Rong2002) assigned various early athyride genera (e.g., Hindella, Hyattidina, and Koigia) with a simple jugum into different families, abandoning the name Hindellinae. Herein, we propose to treat the Hindellinae as a natural group of early athyrides, and raise it to family status, the Hindellidae Schuchert Reference Schuchert1894, characterized by a simple jugum and crura that may or may not directly connected to the spiralia. These early forms may have a smooth or capillate shell surface. These hindellides may have evolved from the older athyrides, such as Nikolaispira Nikitin and Popov in Nikitin et al., Reference Nikitin, Popov and Holmer1996 and Kellerella Nikitin and Popov in Nikitin et al., Reference Nikitin, Popov and Holmer1996, from the Anderken Formation (Dulankara Stage, mid–late Katian) of Chu Ili, Kazakhstan (see also Popov et al., Reference Popov, Nikitin and Sokiran1999, Reference Popov, Cocks and Nikitin2002; Nikitin et al., Reference Nikitin, Popov and Bassett2006). These Kazakh forms show more primitive characters, such as short, spine-like jugul processes that are not medially connected. The subfamily Hyattidinae, therefore, is subsumed in the family Hindellidae on account of their jugum and brachidium that resemble those of Hindella, Koigia, Cryptothyrella, and Elkanathyris n. gen. (see descriptions of these genera below).
Genus Hindella Davidson, Reference Davidson1882
Type species
Athyris umbonata Billings Reference Billings1862; Juncliff Member, Ellis Bay Formation, Hirnantian, Anticosti Island.
Species assigned
The following species are assigned to Hindella:
Athyris umbonata Billings, Reference Billings1862.—
Type species (see below).
Athyris prinstana Billings, Reference Billings1862.—
Prinsta Member and its stratigraphic equivalent to the west, Fraise Member, Ellis Bay Formation (see Copper et al., Reference Copper, Jin and Desrochers2013).
Athyris turgida Shaler, Reference Shaler1865.—
Probable junior synonym of H. prinstana (see below).
Anomites terebratulinus Wahlenberg, Reference Wahlenberg1818.—
Upper Boda reef-capping limestone, Hirnantian.
Atrypa cassidea Dalman, Reference Dalman1828.—
Borenshult, Ostergötland, Sweden, Dalmanitina Beds, Hirnantian.
Whitfieldella ovoides, Savage, Reference Savage1913.—
Bryant Knob Formation, Hirnantian herein (the age of the Bryant Knob is debated because some have dated it as early Rhuddanian).
Whitfieldella speciosa Savage, Reference Savage1913.—
Edgewood Group (Amsden, Reference Amsden1974 synonymized it with W. ovoides).
Meristina crassa incipiens Williams, Reference Williams1951.—
Cyrn-y-brain Formation, Hirnantian, Denbighshire, U.K.
Hindella kiaeri Sheehan, Reference Sheehan1977.—
Nesoya, Asker Region, Oslo, “calcareous sandstones”, likely Hirnantian.
Hindella bulbusa n. sp.—
Parastro Member, Ellis Bay Formation (this study).
Species questionably assigned
Hindella shianensis Reed, Reference Reed1912; Horizon 5, Shian, Pin Valley, Himalayas, precise age unknown (Hirnantian?); interior unknown, but the elongate shell resembles H. umbonata.
Diagnosis
Relatively small to medium sized, smooth or capillate, globose, biconvex shell with incurved beak, apical to transapical foramen, small distinctive interarea, and minute deltidial plates; gently folded anterior commissure, rare median ventral groove. Internally, ventral muscle scars deeply incised, flanked by prominent dental plates and dental cavities, and vascular markings and ovarian pits; apical ventral cavity partially infilled by prismatic callus, leaving shallow groove; dental plates relatively straight, subparallel to plane of symmetry. Crura short and delicate, diverging slightly laterally; umbonal blades equally short and hooked; simple jugum postero-medial, gently arched posteriorly; spiralia with 6–8 whorls, laterally directed.
Occurrence
A Hirnantian age for the genus is confirmed in Laurentia, Baltica, and South China (Rong, Reference Rong1984). The Ashgill–Llandovery age was given by Alvarez and Rong (Reference Alvarez and Rong2002) because they synonymized Aeronian Cryptothyrella Cooper, Reference Cooper1942, with Hindella. There has been confusion about the age of the Ellis Bay Formation, but recent studies have confirmed its Hirnantian age based on microfossils, megafossils, geochemistry, and sequence stratigraphy (Achab et al., Reference Achab, Asselin, Desrochers and Riva2013; Copper et al., Reference Copper, Jin and Desrochers2013; Mauviel and Desrochers, Reference Mauviel and Desrochers2016). On Anticosti Island, Hindella is the only athyride genus in the Hirnantian, co-occurring with Hirnantia, but it is absent lower in the Katian, or higher in the Silurian.
In Estonia, Hindella occurs in the Hirnantian Porkuni Stage. This distribution matches that of the type Hirnantian in the UK, where the species Hindella incipiens occurs (Harper and Owen, Reference Harper and Owen1996). The Estonian “Hindella crassa (Sowerby)” is given a Juuru (early Rhuddanian) age by Modzalevskaya (Reference Modzalevskaya1985, p. 46), but its affinity should be re-examined because it may be Koigia.
“Cryptothyrella” terebratulina (Wahlenberg, Reference Wahlenberg1818) from the Boda Limestone of Sweden was given a Late Ordovician age by Sheehan (Reference Sheehan1977); we consider it as true Hindella. Brenchley et al. (Reference Brenchley, Marshall, Hints and Nolvak1997) suggested that the Boda Limestone was Katian, but Webby (Reference Webby2002) indicated that the top of the Boda mounds stopped growth in the mid-Hirnantian. The species comes from the upper part or tops of the Boda mounds and should be of Hirnantian age. Sheehan (Reference Sheehan1977) identified Hindella crassa (Sowerby, Reference Sowerby1839) from the Hirnantian Dalmanitina Beds of Sweden. This suggests that all species of Hindella from Baltica and the UK are of Hirnantian age, as are those of Laurentia.
Amsden (Reference Amsden1974) identified “Cryptothyrella” ovoides (Savage, Reference Savage1913) from the Bryant Knob Formation and assigned it to the Edgewood Group. Amsden (Reference Amsden1974) tentatively assigned the Bryant Knob (=Leemon Formation) to the early Llandovery, which should be reconsidered as Hirnantian because it shares nearly all the shelly fauna of the underlying Noix Formation, which has the genus Hirnantia as a component. Sheehan (Reference Sheehan1977, p. 25) referred the Edgewood “Cryptothyrella” ovoides to the Silurian (its external morphology is that of Hirnantian Hindella). More recently, Bergström et al. (Reference Bergström, Saltzman and Schmitz2006) re-dated the Leemon and Girardeau limestones of the Edgewood Group as Hirnantian.
Remarks
There has been considerable confusion between Hindella and other homeomorphic athyrides that occur in the Ordovician-Silurian boundary interval. The deeply incised ventral muscle scars have been used as one criterion for Hindella, but these are similar in other early athyrides, and are also quite variable. Sheehan (Reference Sheehan1977) distinguished Hindella from Cryptothyrella mostly on external morphology: Hindella was noted to have a prominent beak with commonly well-developed growth lines and a transapical foramen. We note that these features occur in most hindellines. Hindella with prominent concentric growth lines are rare amongst Anticosti shells. Sheehan (Reference Sheehan1977, p. 25) also remarked that the muscle fields were “more divergent” in Cryptothyrella, and the “cardinalia more robust.” Sheehan’s (Reference Sheehan1977) diagnosis, however, was based on different species assignments compared to what we propose in this study. For example, we assign the Hirnantian species Anomites terebratulina to Hindella, whereas he assigned it to Cryptothyrella. In our re-assessment of the type species of Aeronian Cryptothyrella, we show radial capillae on the shell surface (see description under that genus), which are also observed in some species of Hindella.
Herein, the internal architecture of the brachidia and dental apparatus are given primary taxonomic importance. Detailed serial sections of both Hindella and Cryptothyrella, demonstrate that Hindella differs from Cryptothyrella in its straight, almost vertically aligned dental plates, much less prismatic callus in the ventral apex, short and blunt teeth, a distinctive hinge plate, and median septum reaching to the hinge plate, forming the appearance of a “septalium” in globose, adult shells. In Hindella, the umbonal blades are short and weakly hooked close to the short crura (in contrast to the long crura and “walking-stick-shaped” umbonal blades in Cryptothyrella), the jugum is arched towards the posterior.
Davidson (Reference Davidson1881, Reference Davidson1882), who described the genus Hindella based on specimens sent by Billings from the Junction Cliff locality on Anticosti, named it after the British geologist, George Hinde. Reconstruction of the shell spiralia and jugum was carried out by Norman Glass (Davidson, Reference Davidson1882, p. 130), and showed the lateral orientation of the spiral lophophore, and a single continuous brachidium starting with the crura. Hall and Clarke (Reference Hall and Clarke1894, p. 64, figs. 46–51) copied, with sketches of the jugum and the internal umbonal area, and assumed the brachidia to continue, albeit at right angles from the crura. As shown in our serial sections, the curved umbonal blades of the brachidium are not connected to the crura, but approach as curved hooks close to the crura. During life, there must have been some connecting tissue that suspended the spiralia and jugum within the shell cavity, or else the spiralia would have been loose. The soft tissue endured long enough for the lophophore supports to be left more or less in their life orientation as mud infilled the shells, with spirals pointing to the sides of the shells. In atrypides there is no such crural-brachidial structure, as the laterally positioned crura continue into the spiralial lamellae, with no sharp angle of closure. This is, de facto, a fundamental distinction from the atrypides, as shown in Copper (Reference Copper1986).
Alvarez and Rong (Reference Alvarez and Rong2002) regarded Hindella and the junior genus Cryptothyrella as indistinguishable, and subsumed Hindella in the subfamily Meristellinae, thus combining forms with a complex and simple jugum. Cocks (Reference Cocks2008) adopted the 2002 Treatise synonymy of Hindella and Cryptothyrella, referring them back to the subfamily Meristellinae. At the same time Cocks assigned a Llandovery age to Hindella angustifrons (Salter, Reference Salter1851), H. crassa (Sowerby, Reference Sowerby1839), and H. furcata (Sowerby, Reference Sowerby1839), although labelling only crassa as Hindella. The only taxon remaining in the Hirnantian was Hindella incipiens (Williams, Reference Williams1951). Sheehan (Reference Sheehan1977), and Modzalevskaya (Reference Modzalevskaya1985) labelled crassa and cassidea as Hindella, but Hiller (Reference Hiller1980) referred the species to Cryptothyrella. More recently, Niemeyer et al. (Reference Niemeyer, Alvarez, Boucot and Bruna2010) assigned some Llandovery specimens (mostly steinkerns) from Chile to “Hindella crassa incipiens”, but the Chilean shells appear to have a somewhat more complex jugum, as shown in the serial sections by these authors, than the typical Hirnantian Hindella from Anticosti Island. Thus, the species crassa has zigzagged between two generic names. Specimens of such athyrides in the UK are rare, and poorly preserved as siliciclastic molds and casts, without brachidia, and thus muscle scar and hinge identifications are debatable. This leaves the Anticosti record of pristinely preserved shells with full brachidia, and Hindella cassidea (Dalman, Reference Dalman1828), as some of the few species that are true Hirnantian Hindella.
Hindella umbonata (Billings, Reference Billings1862)
1862 Athyris umbonata Reference BillingsBillings, p. 144, figs. 121a, b.
1863 Athyris umbonata; Reference LoganLogan, p. 317, figs. 331a, b.
1865 Athyris umbonata; Reference ShalerShaler, p. 69.
1866 Athyris umbonata; Reference BillingsBillings, p. 46.
1882 Hindella umbonata (Billings); Reference DavidsonDavidson, p. 130.
1894 Hindella umbonata; Reference Hall and ClarkeHall and Clarke, pl. 41, figs. 26, 27, 29, 30.
1928 Hindella umbonata (Billings); Reference TwenhofelTwenhofel, p. 221, pl. 20, figs. 21–23.
non 1977 Hindella umbonata; Reference SheehanSheehan, pl. 1, figs. 26–28.
Types
Billings (Reference Billings1862, p. 144) established the species based on specimens from “Junction Cliff, Anticosti, Division 1.” In modern stratigraphy, this locality at western Anticosti Island exposes the Juncliff Member, Ellis Bay Formation, as well as the underlying the recessive Fraise Member (see Copper et al., Reference Copper, Jin and Desrochers2013, fig. 4c, d for the type locality; Jin and Copper, Reference Jin and Copper1997 for a map), Hirnantian, latest Ordovician. Junction Cliff is readily accessible, and shows a 10 m thick upper unit of resistant, partly nodular micrite with shaly partings, with H. umbonata (Juncliff Member) and an underlying recessive lower unit of shales and limestones (Fraise Member, with H. prinstana). Here, the distinctive large elongate shell of H. umbonata can be easily distinguished from the smaller, rounded, globose shell of the older species, H. prinstana. The restricted type locality (C718) is defined here as the east end of Junction Cliff (UTM 20, 0396180E, 5519840N), where H. umbonata occurs in the resistant upper ledges of the lower Juncliff Member.
The original type lot of H. umbonata Billings in the GSC type collection consists of six specimens, labelled “GSC 2284, GSC2284a–e” collected by J. Richardson from “Junction Cliff.” The shell figured by Billings (Reference Billings1862), GSC2284, listed as the holotype, is lost. The remaining five shells, GSC 2284a–e are not assignable to the species, with four belonging to H. prinstana, and one resembling H. bulbusa n. sp. Richardson (Reference Richardson1857) collected brachiopods from the lower Fraise to Parastro members, stretching from Junction Cliff to Parastrophinella Bluff (the latter being the type locality of Parastrophinella reversa and Hindella bulbusa n. sp.). Thus, Richardson’s “Junction Cliff” collection is a mixture of three species of Hindella from the Fraise, Juncliff, and Parastro members. This makes it impossible at this time to name a neotype for the lost H. umbonata “holotype” because the precise collecting locality or horizon is unknown for the extant types. This is left for a later revision, and complete description of all Hindella from the Hirnantian Ellis Bay Formation. Based on our new collections, the exact localties and stratigraphic positions of the three Hindella species from Junction Cliff to Parastrophinella Bluff can, however, be clearly defined.
Occurrence
In addition to the type locality, collections of Hindella umbonata were also made from the following sites exposing the Juncliff Member:
C692 (=C701, A4a). Laloutre road, ~4.2 km south of main road. Light-gray weathering, thin- to medium-bedded micrites to coquinites, with abundant Hindella umbonata, rare Eospirigerina, Mendacella; middle-upper Juncliff Member. Map 12E/13, UTM 20, 53640E, 13150N.
C693. Laloutre road, ~4.2 km south of main road. Lithology and fauna similar to C692, but ~2 m higher stratigraphically. Hindella umbonata less common, more nested; upper Juncliff Member. Map12E/13, UTM 20, 53650E, 13120N.
C721 (=A1, A426). Port Menier Quarry at Cap Blanc, south side of Port Menier, next to shoreline facing Ellis Bay. Light green-gray micrites at top of quarry (~3–4 m thick), capping recessive shale. Upper 1–2 m of quarry section consists of thin-bedded, gray micrite with shaly partings; H. umbonata co-occurs with abundant Barbarorthis and common Mendacella. Juncliff Member. Map 22H/16, UTM 20, 03120E, 18430N.
A813. Prinsta River, ~1 km upstream from mouth, at first sharp bend to south, north bank; 3–5 m thick section, with Hindella umbonata shellbed in recessive blue-gray shale unit, and micrite at 1.45 m above a 20 cm thick sandstone bed with giant Hormotoma, corals marking the base of the Lousy Cove Member. Map 12F/5, UTM 20, 73480E, 66540N.
A1317b. Lac Cailloux road, 4.8 km south of main road; 3 m thick section of light gray, resistant micrite, with Hindella umbonata Juncliff Member. Map 12E/13, UTM 20, 39700E, 14360N.
Remarks
Hindella umbonata is a common species of the genus on Anticosti Island, and can be readily distinguished from other congeneric species on the island by its larger (average shell width 16 mm), strongly elongate, globose shell with parallel sides. Both H. prinstana and H. bulbusa n. sp. are smaller, with average width of 12 mm and 10 mm, respectively. Hindella prinstana also has a narrower apical angle, and H. bulbusa n. sp. is pear-shaped (see below).
Hindella prinstana (Billings, Reference Billings1862)
1862 Athyris Prinstana [sic] Reference BillingsBillings, p. 145, figs. 122a, b.
?1865 Athyris turgida Reference ShalerShaler, p. 69.
1866 Athyris Prinstana; [sic] Reference BillingsBillings, p. 46 (no figures).
1894 Hindella prinstana (Billings); Reference Hall and ClarkeHall and Clarke, pl. 41, fig. 28, pl. 49, fig. 1 (specimen illustrated from the Fraise Member, in the lower unit at Junction Cliff).
1928 Hindella prinstana (Billings); Reference TwenhofelTwenhofel, p. 220, pl. 22, figs. 12, 13.
1977 Hindella umbonata; Reference SheehanSheehan, pl. 1, figs. 26–28.
Type locality and horizon
Billings (Reference Billings1862, p. 145) reported the species from “Prinsta Bay, Anticosti, Division 1”, but his original types have not been located. At this locality on the northeast coast, the species occurs in the lower Prinsta Member, stratigraphically coeval to the Fraise Member of the west coast, lower Hirnantian. This low bluff locality on the east side of the Prinsta River mouth (=A135 or A362 of the new collections; map sheet 12F/5, UTM 20, 74480E, 66450N), consists of the following units, in descending order:
-
(1) 105 cm of calcareous sandy shales with rare nodules.
-
(2) 319 cm of nodular shale and limestone. Upper 289 cm nodular shale and calcarenites with nodules at top, sandy, upper resistant ledge with Hindella prinstana, Hormotoma, and sandstones at 30 cm and 95 cm above base. Most Hindella occur ~30 cm above base at ledge in this 5 cm bed with broken Hindella, aulacerids, cup corals; units less sandy and calcarenitic at base then near top. The base of the western Prinsta Member (=Fraise Member) is at this level.
-
(3) 145 cm nodular, wavy bedded and platy calcareous sandstone (Velleda Member, Vaureal Formation).
Occurrence
In addition to the type locality, the species occurs in the localities listed below:
A134a. Prinsta River mouth, first outcrop on NW bank, ~3 m recessive, silty dark green-gray shale and sandstone interbeds at base, overlain by 2 m of nodular limestone, with loose valves of Hindella prinstana. Prinsta Member (base). Map sheet 12F/5, UTM 20, 74360E, 66570N.
A134b. Prinsta River, NW bank, ~200 m upstream, same stratigaphic level as A134a, with H. prinstana. Map sheet 12F/5, UTM 20, 74130E, 66510N.
C717. Jupiter road, ~3 km south of main road. Recessive green-brown soft shales, nodular calcarenites, with abundant Hindella prinstana and Eospirigerina. Fraise Member. Map sheet 12E/11, UTM 20, 69480E, 10370N.
A431. Anse aux Fraises. Thinly bedded, dark gray shale, with nests of Hindella prinstana, Plaesiomys, and Eospirigerina in tidal flat outcrops. Fraise Member, ~15 m above base. Map sheet 22H/16, UTM 20, 95660E, 20680N.
A432, Anse aux Fraises, tidal flat outcrop, ~150 m south of A431, with localized nests of Hindella prinstana, Parastrophinella, and Leptaena. Fraise Member (middle). Map sheet 22H/16, UTM 20, 95780E, 20430N.
A1317a. Lac Cailloux road, 4.8 km south of main road, 3–5 m lower recessive weathering shales, and brown-green soft to blocky, nodular micrite, with Vellamo, Plaesiomys, and Hindella prinstana. Fraise Member. Map sheet 12E/13, UTM 20, 39700E, 14360N.
Remarks
There are five species of Hindella in the Ellis Bay Formation, suggesting a relatively rapid evolution of Hindella during the Hirnantian. Other species occur in the higher Parastro, Prinsta, and Laframboise members. The oldest species, Hindella prinstana is smaller (~12 mm wide), and about equally as wide as long (instead of elongate, as in H. umbonata), less inflated, and with a more pronounced anterior fold. In eastern Anticosti, this is the stratigraphically lowest species of Hindella, occurring directly above sandstones of the Velleda Member of the Vaureal Formation. The elongate shells of Hindella umbonata occur upstream on the Prinsta River at locality A813 (see localities of H. umbonata). Hindella prinstana is abundant in the Prinsta and Fraise members at both ends of the island.
Shaler (Reference Shaler1865) reported his species “Athyris turgida” from “1/2 mile north of White Cliff”, which posits it within the Fraise Member, and is thus a probable synonym of H. prinstana.
The specimen illustrated by Sheehan (Reference Sheehan1977) resembles those from the lower recessive shales of the Fraise Member at Junction Cliff, and is thus assignable to H. prinstana.
Hindella bulbusa new species

Figure 4 Selected serial sections and reconstruction of the spiralium and jugum of Hindella bulbusa n. sp. Paratype, GSC 131799, Parastro Member, Ellis Bay Formation, Parastrophinella Bluff (loc. A84), Anticosti Island. Note the simple jugum in the anterior central part of the shell cavity, the hook-like attachment points of the jugal blades, and the lack of skeletal connection to the crura. Number below each serial section denotes distance (mm) from shell apex.
Types
Holotype, GSC 137671 (Fig. 3.1–3.5); figured paratypes, GSC 131790, 137670, 137679–137681 (Figs. 2, 3), and 131799 (serially sectioned specimen; Fig. 4). Parastrophinella Bluff, southwest coast of Anticosti Island, locality A84 (=C720; see Jin and Copper, Reference Jin and Copper1997). First coastal bluff scree outcrops ~700 m southeast of Junction cliff (UTM 20, 0397126E, 5518771N). Lower half of bluffs of thinly bedded micrites, shales with abundant Parastrophinella reversa in several layers (type locality), and a diverse benthic fauna (see Jin and Copper, Reference Jin and Copper2008, fig. 6C). Upper Parastro Member, Ellis Bay Formation, middle Hirnantian.
Diagnosis
Relatively small, elongate, suboval shells of Hindella, with narrow apical angle and low beak; usually prominent growth disruptions, concentric filae, and more distinctive radial capillae; gentle fold and sulcus developed towards anterior commissure. Dental plates straight, flanking wide lateral cavities; small teeth; 7 or 8 spiral whorls; simple low jugum and flat jugal arch.
Description
Shells relatively small, longer than wide, bulbous, ovoid to pear-shaped, wider anteriorly than posteriorly. Apical angle relatively narrow, rounded. Adult shells 8–10 mm wide (average = 10 mm), with average thickness of ~9 mm. Umbo strongly convex, inflated. Anterior commissure weakly plicate. Internal structures as in diagnosis.
Etymology
From the Latin, bulbus, a swell, referring to the globular, pear-shaped shell typical of the new species.
Remarks
The new species is readily distinguished from Hindella umbonata (Billings, Reference Billings1862) and H. prinstana (Billings, Reference Billings1862) of the underlying Juncliff and Fraise members by its smaller size (with average width 10 mm versus 16 mm for H. umbonata, and average thickness 9 mm versus 12 mm for the large shells of H. umbonata), slightly wider apical angle, more bulbous shape, and less robust shell wall. Hindella umbonata is strongly elongate, with parallel sides, whereas H. bulbusa n. sp. reaches its maximum width anteriorly, giving it a pear shape. The umbo of H. bulbusa n. sp. is relatively pinched, given its narrow apical angle of ~110°, versus 100° in H. umbonata. Hindella bulbusa n. sp. is common only at the western (e.g., Parastrophinella Bluff) and middle parts of Anticosti Island, and appears to be absent at the east coast.
Genus Cryptothyrella Cooper, Reference Cooper1942
Type species
Whitfieldella quadrangularis Foerste, Reference Foerste1906. Brassfield Formation, Aeronian, Dunkinsville (=“Duncansville” of Foerste, Reference Foerste1906), Adams County, Ohio.
Species assigned
In addition to the type species, the following species are assignable to Cryptothyrella:
Atrypa crassa Sowerby, Reference Sowerby1839.—
Goleugoed Formation, late Rhuddanian, Girvan.
Terebratula furcata Sowerby, Reference Sowerby1839.—
Bog Quartzite, Aeronian, Shropshire.
Atrypa cylindrica Hall, Reference Hall1852, p. 76, pl. 24, figs 2a–h.—
Irondeqoit Formation, basal Sheinwoodian, Niagara region, New York (strongly elongated shell with prominent capillae). The shells figured later as “Whitdieldella cylindrica Hall” by Hall and Clarke (Reference Hall and Clarke1894, pl. 40, figs. 16–22) are from the “Niagara group” (=Bisher Formation, coeval with the Irondequoit Formation; C.E. Brett, personal communication, 2017), “Hillsboro, Ohio” and have anterior plicae and capillae.
Cryptothyrella bisulcata Gauri and Boucot, Reference Gauri and Boucot1970.—
Brassfield Limestone, Aeronian, near West Union, Ohio.
Species questionably assigned
The following species require further study to establish their generic affinities:
Hemithyris angustifrons M’Coy, Reference M’Coy1851.—
Mulloch Hill Formation, late Rhuddanian, Girvan. Internal structures poorly known.
Whitfieldella subquadrata Foerste Reference Foerste1906, p. 326, pl. 1, figs. 3a–f.—
Indian Fields Formation, Aeronian, Berea, Kentucky. Regarded as junior synonym of the type species Cryptothyrella quadrangularis by Gauri and Boucot, Reference Gauri and Boucot1970.
Diagnosis
Shell medium to relatively large, elongate, globose, smooth to capillate, uniformly biconvex to bisulcate. Very small deltidial plates flanking transapical foramen in adult shells; beak incurved. Ventral umbo thickened internally by callus fill, leaving narrow medial groove; teeth small, rounded; dental plates short, with thin terminations, medially inclined, fused posteriorly through prismatic thickening, becoming discrete anteriorly; dental cavities mostly infilled with prismatic callus posteriorly. Socket plates relatively thin, but inflated apically to support dental sockets; crura thin, delicate, parallel to each other, extending along commissural plane. Umbonal blades terminated as hooks, fused to crura; jugal saddle almost flat, positioned in mid-shell; spiralia with 8–10 whorls, directed laterally.
Remarks
Based on Gauri and Boucot’s (Reference Gauri and Boucot1970) study of Cryptothyrella, Ziegler and Boucot (Reference Ziegler and Boucot1970) proposed a Cryptothyrella community for North America. The genus, however, has had a shifting taxonomic history between a valid genus Cryptothyrella to a synonym of Hindella, resulting in a confusing stratigraphic range between the Late Ordovician (Hirnantian) and early Silurian. Various species have been allocated to one or the other genus (Sheehan, Reference Sheehan1977), thus making the community analysis unreliable. Gauri and Boucot (Reference Gauri and Boucot1970) provided a single transverse section that showed large lateral cavities, ventro-medially inclined dental plates, and flat, thin, divided horizontal hinge plate, which are similar to those in the topotype shell of C. quandrangularis examined in this study. Gauri and Boucot (Reference Gauri and Boucot1970, fig. 1) did not examine the crura, jugum, or spiralia, but noted a questionable “septalium”, which is not present in the shell serially sectioned herein, although a median septum is present. More detailed internal structures were provided by Grunt (Reference Grunt1980, Reference Grunt1986, Reference Grunt1989), through serial sections of topotype material.
Cryptothyrella is externally distinct from Hindella by its notably larger and more elongate shell (commonly twice as long as Hindella), commonly with a ventral medial groove (a dorsal medial groove may also be present in some shells). Internally, the ventral apical cavity has a much thicker prismatic callus than in Hindella, and dental plates are strongly inclined ventro-medially, almostly forming a “pseudospondylium”—a feature that is not prominent in Hindella. Internally, the crura of Cryptothyrella are much longer (about twice the length), and straight anteriorly, parallel to each other. The umbonal blades form long hooks, which are double the size seen in Hindella. The mid-shell-positioned jugal saddle of Hindella is rounded, and tilted slightly to the posterior, whereas in Cryptothyrella it is flat, and more anteriorly positioned in the shell. A larger number of spiral whorls in Cryptothyrella may be related to its larger shell size (Fig. 4). A “pseudoseptalium” may be seen in Hindella sections near the dorsal umbo, but this is absent in Cryptothyrella. There is no true septalium present in either genus.
Cocks (Reference Cocks1978) assigned a loose valve (the lectotype) of Hemithyrias angustifrons Salter from the Rhuddanian Mulloch Hill Formation to Cryptothyrella, but later transferred it to Hyattidina (Cocks, Reference Cocks2008). However, Cocks (Reference Cocks1978) also assigned the holotype of Atrypa crassa Sowerby, Reference Sowerby1839, a Rhuddanian shell, to Hindella, which would make the two genera coeval in the UK. These assignments seem doubtful.
Cryptothyrella quadrangularis (Foerste, Reference Foerste1906)

Figure 5 Cryptothyrella quadrangularis (Foerste, Reference Foerste1906), three topotype specimens from the Brassfield Formation, lower Aeronian, Dunkinsville (=Duncansville of Foerste, Reference Foerste1906), Adams County, Ohio. (1–5) CMC-IP 36178, #1, a subquadrate form; (6–11) CMC-IP 36178, #2, a suboval form, showing faint capillae (11) in antero-lateral part of ventral valve; (12–16), CMC-IP 36178, #3, a slightly narrower shell, with a rectimarginate anterior commissure typical of the species.
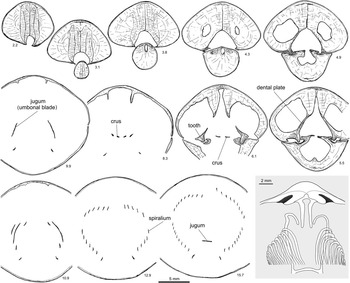
Figure 6 Serial sections and brachidium reconstruction of Cryptothyrella quadrangularis (Foerste Reference Foerste1906). OSU 18250, topotype, Brassfield Formation, lower Aeronian, Dunkinsville (=Duncansville of Foerste, Reference Foerste1906), Adams County, Ohio. Note the development of massive prismatic thickening of the ventral umbo divided by a deep groove, baso-ventrally inclined dental plates, long and straight crura, and the anteriorly positioned jugum, which differentiate Cryptothyrella from Hindella. Number below each serial section denotes distance (mm) from shell apex.
1906 Whitfieldella quadrangularis Reference FoersteFoerste, p. 327.
1906 Whitfieldella subquadrata Reference FoersteFoerste, p. 326.
1970 Cryptothyrella quadrangularis (Foerste); Reference Gauri and BoucotGauri and Boucot, p. 125, pl. 29, 30.
1986 Cryptothyrella quadrangularis; Reference GruntGrunt, p. 18, fig. 3.
1989 Cryptothyrella quadrangularis; Reference GruntGrunt, p. 39, fig. 23.
Types
Foerste (Reference Foerste1906, p. 327, pl. 1, figs. 4a–c) reported the type species from a “ravine...northeast of Duncansville, east of Sprow’s bridge…in Adams county, Ohio, …38 feet above the base of the Clinton.” Foerste (Reference Foerste1906, p. 41) further described the quadrangularis bed within a measured 54 ft (16 m) section in which it forms a “6 inch” (15 cm) layer. In modern stratigraphy, the type species is from the Brassfield Formation, lower Aeronian (C.E. Brett, personal communication, 2017). Whifieldella subquadrata Foerste, Reference Foerste1906, synonym of C. quadrangularis (see Gauri and Boucot, Reference Gauri and Boucot1970), is from the basal Plum Creek Shale, which overlies the C. quadrangularis bed and is separated from it by a minor disconformity (C.E. Brett, personal communication, 2017). The serially sectioned specimen in this study (Fig. 6) is a topotype provided by W. Ausich of Ohio State University.
Diagnosis (emended herein)
Large, elongate, strongly biconvex shells, commonly with gentle ventral sulcus, and faint radial capillae. Anterior commissure broadly uniplicate; beak incurved with obscured small deltidial lates and apical to transapical foramen. Both valves thickened by prismatic infill, marked by narrow median groove on ventral interior. Large dental cavities flanked by thin dental plates; teeth small, solid. Crura long, straight, subparallel to each other; dorsally flat hinge plates; median septum present; umbonal blades with hook-like terminations, not connected to crura; simple jugum flat, saddle-shaped; laterally directed spiralia with 8–11 whorls.
Remarks
The large, elongate shell (Fig. 5) easily distinguishes the Aeronian genus Cryptothyrella from Hirnantian Hindella and Rhuddanian Koigia. Striking are the internal massive, prismatic anterior deposits of the shell wall that would have weighted the shell in an umbo-down position during life (Fig. 6; 2.2–6.1 mm from shell apex). The jugum is simple, with a flat arch (at 15.7 mm), similar to that in Hindella, both of which share a finely capillate shell surface, although the faint capillae can be observed only on well-preserved shells. Serial sectioning in this study revealed that the crura are not fused directly to the umbonal blades that have a hook-like ending (Fig. 6).
Grunt (Reference Grunt1986) was the first to illustrate the complete internal structure of the species, with serial sections based on “Whitfieldella subquadrata” material from Indian Fields of Kentucky (Smithsonian collections). Cryptothyrella subquadrata forms a distinct marker bed in the Brassfield Formation, traceable from Kentucky to Ohio (C.E. Brett, personal communication, 2017), and its synonymy with C. quadrangularis by Gauri and Boucot (Reference Gauri and Boucot1970) is justified because the quadrate form is within the intraspecies variation of C. quadrangularis based on examination of the topotypes (e.g., Fig. 5.1–5.5). The serial sections of a shell from the original type locality (Duncansville, Ohio) of C. quadrangularis, as is shown for the first time here, display internal structures that are largely the same as those in the topotype of “W. subquadrata” as illustrated by Grunt (Reference Grunt1986, fig. 3), especially in the development of a simple jugum.
The prismatic pedicle callus that fills most of the ventral umbo, as seen in the serial sections, is also shown in Grunt (Reference Grunt1986, Reference Grunt1989). The teeth are supported by dental plates with prismatic thickening, with the blunt teeth directed inwards into opposing dorsal sockets. The dorsal hinge plate is strong, separated by a notothyrial pocket, and reinforced by prismatic layer under the crura. The crural bases are minute, imbedded in the hinge plate, and stretch to form thin, long, flat, parallel plates (Fig. 6; 5.5–6.1 mm from shell apex), narrowing anteriorly to points in the medial plane. The umbonal blades from the jugum start before the crura, extend posteriorly, forming a round arch, like a shepherd’s crook, disconnected from the crura (Fig. 6).
Cryptothyrella cylindrica (Hall, Reference Hall1852) reported from New York and Ohio, is early Sheinwoodian, thus much younger species than the Aeronian C. quadrangularis. It differs from the latter in having a more elongate shell, with well-developed dorsal fold and vental sulcus towards the anterior, forming a highly uniplicate anterior commissure, and marked by well-developed anterior capillae (Fig. 7.1–7.6), originally described as “radiating striae” by Hall (Reference Hall1852, p. 77).

Figure 7 (1–6) Cryptothyrella cylindrica (Hall, Reference Hall1852), OSU 14066, Bisher Formation, Hillsboro, Ohio. Note the presence capillae in anterior part of shell (6). Scale bar=10 mm unless noted otherwise.
Genus Koigia Modzalevskaya, Reference Modzalevskaya1985

Figure 8 Koigia from the Fox Point Member (basal Rhuddanian), Becscie Formation, Anticosti Island. (1, 2) GSC 134362, dorsal and vental views, with ventral valve partly impacted by another shell; (3, 4) GSC 134363, dorsal and vental views, with anteriorly crushed vental valve; (5) small slab showing bedding surface covered by shells of Koigia sp., small favositid corals, and conispiral gastropods, loc. A1450; (6) thin section of slab at right angles to bedding plane showing shells in resting position, loc. A313d. Note the thin-walled shells with geopedal infill and spiralia preserved.

Figure 9 Serial sections and reconstruction of Koigia sp. Specimen GSC 131800 from a coastal bluff section on the east side of the cove at Ruisseau aux Algues (loc. A314), Fox Point Member (basal 3 m), Becscie Formation. Note the lack of skeletal connection between the crura and jugal blades. Number below each serial section denotes distance (mm) from shell apex.
Type species
Hindella extenuata Rubel, Reference Rubel1970 (p. 48, pl. 25, figs. 1–9), Juuru Regional Stage, Koigi Member, Varbola Formation (basal Rhuddanian, Nestor, Reference Nestor1997); Vakhtrepa, Koigi, Estonia. See Koigia serial sections in Modzalevskaya (Reference Modzalevskaya1985, p. 38).
Diagnosis
Shell small, smooth, approximately as wide as long, moderately biconvex, with incurved beak. Ventral apical cavity with little callus. Dental plates thin, defining open lateral cavities. Dorsal valve with relatively flat hinge plates; crura short, thin, flat, not connected to jugal blades, forming sharp angle at junction with primary lamellae; jugum simple, with flat jugal saddle positioned at mid-length to posterior of shell; modest median septum connected to hinge plate; spiralia with 5–7 whorls, laterally directed (Fig. 8).
Remarks
Koigia has a small shell (usually <10 mm wide) compared to other genera of the Hindellinae (Fig. 8). Using serial sections, Rubel (Reference Rubel1970, figs. 15–17) reconstructed six whorls of laterally directed spiralia, a simple jugum, and a hooked, right-angle connection of the umbonal blades of the first spiral whorl with the crura. This type of connection is not observed in the Anticosti shells of Koigia (Fig. 9). All hindellines from Anticosti Island show a disconnection between the crura and brachidia. Thus, it is likely that this disconnection between crura and brachidia was overlooked in the Estonian material during sectioning and reconstruction. The small-shelled Koigia differs from Hindella in its larger and more distinctive lateral cavities, and thin dental plates, as well as a thinner shell wall. Lateral cavities are infilled with thick callus in the ventral apex of Hindella. The crura in Koigia are short and stubby; the simple jugal arch is rounded, versus flat in Hindella. Externally, the shell of Hindella tends to be more elongated and globose, and commonly larger. Capillae, observed in well-preserved shells of Hindella, are unknown so far in Koigia. The younger Rhuddanian genus Koigia bears similarities to its presumed Hirnantian ancestor Hindella. The smaller Koigia shells may have been an example of dwarfism immediately after the Hirnantian mass extinctions. This agrees with many other small-shelled brachiopod taxa in the basal Ruddanian strata on Anticosti Island, such as the atrypides Becscia and Zygospiraella, the orthides Isorthis and Mendacella, and the pentameride Viridita.
Genus Hyattidina Schuchert, Reference Schuchert1913
Type species
Atrypa congesta Conrad, Reference Conrad1842, New York, Clinton Group, Llandovery.
Diagnosis (emended herein)
Small, biconvex, inflated, smooth shells with strongly incurved beak, minute hollow deltidial plates, and prominent angular fold-sulcus. Internally ventral umbo with thick callus; teeth short, blunt, directed medially; dental plates relatively strong, straight, defining small lateral cavities. Dorsal hinge plate stout, divided by narrow groove, with bulbous inner socket ridges; median septum weak; crura short, not fused with but approaching umbonal blades at sharp angle, in non-touching “handshake” pattern; jugum simple, with angular saddle pointing ventro-dorsally; spiralium with 6–8 whorls, laterally directed.
Occurrence
Aeronian to Telychian, ?Wenlock.
Remarks
When proposing the genus Hyattella, Hall and Clarke (Reference Hall and Clarke1893) compared Athyris junia Billings, Reference Billings1866 with the type species H. congesta. Schuchert (Reference Schuchert1913, p. 415) renamed the genus Hyattidina because the name Hyattella was pre-occupied.
The shells of Hyattidina show considerable variability, ranging from almost smooth and round to those with an angular fold and sulcus. The brachidia, however, are quite consistently developed, with a simple jugum and laterally directed spiralia. The reconstruction of the jugum and spiralium by Hall and Clarke (Reference Hall and Clarke1894, pl. 40, fig. 26), based on a silicified shell from “Reynale’s Basin, New York”, is essentially correct, but missed the sharp angle and juxtaposition of the crura and umbonal blades (which are shown as a straight connection). Our material, presented herein, is very similar in shape and size to the type Hyattidina congesta from the “Clinton... Lockport, New York” as figured by Hall and Clarke (pl. 40, fig. 26). Alvarez and Rong (Reference Alvarez and Rong2002, p. H1556) selected a neotype from the Hall collection, which has a more prominent fold-sulcus than seen commonly in the Anticosti specimens, which are flatter, with a weaker fold. Hall and Clarke (Reference Hall and Clarke1893, p. 61; 1894, p. 767) illustrated the type species H. congesta with a simple jugum (referred to as a “loop top”), similar to that of the Anticosti species.
Alvarez and Rong (Reference Alvarez and Rong2002) described Hyattidina, and its subfamily, as lacking a median septum and a jugal saddle, and having a shell with numerous growth lines and thin dental plates. Based on the new data from this study, these criteria should be emended to describe a smooth shell (without prominent growth lines), a distinct septum, relatively thick dental plates, and a jugal saddle. The information on the shape and configuration of umbonal blades, crura, jugum and spiralia, as presented in this study, is also new. Alvarez and Rong (Reference Alvarez and Rong2002, p. H1556) used the “numerous growth lines” to assign the genus to the superfamily Athyridoidea. Our data makes such assignment doubtful. Alvarez and Rong (Reference Alvarez and Rong2002) also allocated a Ludlow age to the genus, but the type and most other species of the genus are Telychian in age, thus much older. On Anticosti, the lowermost occurrence of the genus, which is often abundant, and shell-bed forming, or packed in large nests, is in the Macgilvray Member of the upper Gun River Formation (mid-Aeronian; Copper et al., Reference Copper, Jin and Desrochers2013). It retains this abundance into to the Ferrum Member of the Jupiter Fornation (early middle Telychian; for example, see Hyattidina cf. junia, below), becoming rare in the Pavillon Member (mid-Telychian). In the richly fossiliferous Anticosti succession with abundant athyrides, Hyattidina is absent from the upper Katian through lower Aeronian strata.
Internally, the brachidia of Anticosti Hyattidina are quite similar to those of Hindella in the jugum and short crura, but differ from Koigia, which has a simpler, rounded jugum, and fewer spiral whorls. Thus there is little to distinguish the brachidia in the hyattidines and hindellines, and we thus place them in the same family Hindellidae. Hyattidina and Koigia have much less ventral apical prismatic callus than either Hindella or Cryptothyrella, suggesting that Hyattidina may have its ancestry in Rhuddanian Koigia.
Hyattidina cf. H. junia (Billings, Reference Billings1866)
Figures 10.16, 11

Figure 10 (1–15) Hyattidina sp. from the Goéland Member, Menier Formation, locality A852a; (1–5) GSC 134443; (6–10) GSC 134441; (11–15) GSC 134442. (16) Thin sections of Hyattidina cf. H. junia (Billings, Reference Billings1866) from the Cybèle Member, Jupiter Formation, coastal bluff section, just southeast of Richardson Cliff (loc. A872).
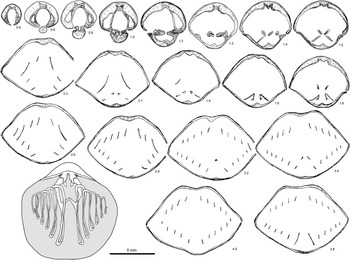
Figure 11 Serial sections and reconstructed brachidia of Hyattidina cf. H. junia (Billings Reference Billings1866). Specimen GSC 131801 from locality A163c, Cybèle Member, Jupiter Formation. Note the thin, relatively straight dental plates flanking small lateral cavities, buried median septa in both valves, minute stubby crura, hooked umbonal blades, small, and a pointed jugal saddle. Number below each serial section denotes distance (mm) from shell apex.
1866 Athyris junia Reference BillingsBillings, p. 46 (no illustrations).
?1894 Hyattella junia Billings; Hall in Reference Hall and ClarkeHall and Clarke, pl. 40, figs. 29–31.
1928 Hyattidina congesta junea Billings; [sic] Reference TwenhofelTwenhofel, pl. 30, figs 4–6.
1981 Hyattidina junea (Billings); [sic] Bolton, Reference Bolton1981, pl. 5, fig. 4.
Types
Lectotype, herein selected, GSC 2374, from “six miles east of Otter River, Anticosti Island, … Divs 2,3,4 Richardson” (Billings, Reference Billings1866, p. 47, based on Richardson’s collections). Twenhofel (Reference Twenhofel1928, p. 223 and explanation of pl. 30) illustrated the type specimen and labeled it as from “Hannah Cliff, east of Gun River, zone 2.” This places the type locality within the Macgilvray Member of the Gun River Formation (see Copper et al., Reference Copper, Long and Jin2012), where the genus becomes abundant for the first time. The species reaches its largest shell size in the Goéland Member of the Menier Formation (Copper and Long, Reference Copper and Long1990; Copper et al., Reference Copper, Long and Jin2012), and fades away in the Richardson and Cybèle members of the Jupiter Formation (Copper and Jin, Reference Copper and Jin2015).
Remarks
The serially sectioned specimen (Fig. 11) comes from the Cybèle Member of the Jupiter Formation, Cape Billings at the north end of Wreck Bay (locality A163, map sheet NTS 12F/4, UTM 20, 96180E, 41640N), a ~8 m thick low bluff section leading to the sea. The sampled beds include, in descending order:
A163d, thin-bedded micrites and green-gray shales within the top 1 m of section, with the rhynchonellide Platytrochalos;
A163c, 1–2 m of thinly bedded, shaly micrites, with Hyattidina; A163b, ~2 m of poorly fossiliferous gray shales and micrites, with the atrypide Clintonella and small favositid corals;
A163a basal 2 m of thin-bedded coquina, with small-shelled Gotatrypa, Coolinia, small favositids, and gastropods.
The specimen is from the northeast coast, as was the material sent by Billings to Hall and Clarke (Reference Hall and Clarke1893), but lies stratigraphically well above the smaller shells in the Macgilvray Member, Gun River Formation, on the south coast.
The specimen illustrated as Hyattella junia by Hall and Clarke (Reference Hall and Clarke1894, pl. 40) and labeled as from “East cape”, was most likely sent by Billings from the Richardson collection from bluffs east of East Point. This would place them in the Cybèle Member of the Jupiter Formation, similar to the serially sectioned specimen in this study. The strata of the Goéland Member (Menier Formation) were never sampled by either Richardson (Reference Richardson1857) or Twenhofel (Reference Twenhofel1928) because of the usually stormy northeast coastline, with its high cliffs (there was no road access at their time).
The type specimen of Hyattidina congesta (Conrad, Reference Conrad1842, as illustrated by Hall and Clarke, Reference Hall and Clarke1894, pl. 40, figs. 23–28) differs from the type of Hyattidina junia in its larger size, and smoother shell, with a prominent dorsal fold and ventral sulcus. The shell of H. junia illustrated by Hall and Clarke (Reference Hall and Clarke1894, explanation to pl. 40) came from the Cybèle Member, stratigraphically much higher than the type stratum in the Gun River Formation. The shell illustrated in 1894 as Hyattella congesta by Hall and Clarke (Reference Hall and Clarke1894) bears strong similarity to Hyattidina sp. (see below), which occurs in the Goéland Member of the Menier Formation (Aeronian). There are several undescribed species of the genus on Anticosti Island.
Hyattidina sp.
Figures 10.1–10.15, 12

Figure 12 Serial sections and reconstructed brachidia of Hyattidina sp. Specimen GSC 131802 from locality A708, Goéland Member, Menier Formation. Note the thick shell posterior wall, minute, pointed jugal arch, and slit-like dental cavities. Number below each serial section denotes distance (mm) from shell apex.
Remarks
Smooth, biconvex shells with a distinct ventral sulcus and broad dorsal fold (Fig. 10.1–10.15) are herein referred to the genus Hyattidina under open nomenclature. They occur together with Elkanathyris pallula n. gen. n. sp. in the lower Goéland Member of the Menier Formation. Hyattidina sp. differs from typical H. junia from the Gun River Formation in that the unusual umbonal blades seen in H. junia are absent in Hyattidina sp. (compare Figs. 11, 1.8–2.1 mm with Fig. 12, 2.6–3.5 mm from apex).
Elkanathyris new genus
Type species
Elkanathyris pallula n. gen. n. sp., Menier Formation, upper Goéland Member, mid-Aeronian, Llandovery; Anticosti Island.
Species assigned
Type species only.
Diagnosis
Shell small to medium sized, wider than long, posteriorly plicate, with 4–6 strong ribs; hinge line relatively straight, long. Dental plates straight, delimiting relatively small lateral cavities. Inner socket ridges strong and bulbous; umbonal blades curved in sharp juxtaposition to crura; spiralia with <12 whorls; simple jugum with pointed jugal saddle.
Etymology
After Elkanah Billings, the first Canadian paleontologist of the Geological Survey of Canada, who described numerous fossils from Anticosti Island, and Athyris, the eponymous genus of the order Athyrida.
Remarks
Large collections from shell nests in the Gun River and Menier formations of Anticosti demonstrate considerable variability in the development of coarse ribs (plicae). The strong ribs are most prominent in the apical area, clearly defined in the posterior half of most shells, but fade anteriorly and laterally, extending to the anterior margin only in some immature shells. This may be an endemic genus to Anticosti Island, and its development of apical plicae is similar to that in some unrelated brachiopods lineages on Anticosti, such as Phricoclorinda Jin and Copper, Reference Jin and Copper2000, which evolved radial and crisscross ribbing from the normally smooth Clorinda. In New York, Clinton strata (Aeronian–Telychian) contain abundant Hyattidina, but no ribbed forms are known to be related to Hyattidina.
Elkanathyris n. gen. differs from the older Hyattidina in its long straight hinge plates, bulbous inner socket ridges on the dorsal valve constrained laterally by teeth from the ventral valve, and a sharp angular jugum connecting a spiralium with up to 10 whorls. As in Hyattidina, there is a median septum buried in both valves; the crural bases are small and delicate within the bulbous inner socket ridges.
Elkanathyris pallula new genus new species
Figures 13.1–13.19, 14

Figure 13 Elkanathyris pallula n. gen. n. sp. from the Goéland Member, Menier Formation, locality A852a. (1–5) 134439, holotype; (6–10) GSC 134440, paratype; (11–14) GSC 134437, paratype; (15–18) GSC 134438, paratype, imature shell showing strong ribs; (19) shell bed with densely packed E. pallula n. sp. shells.
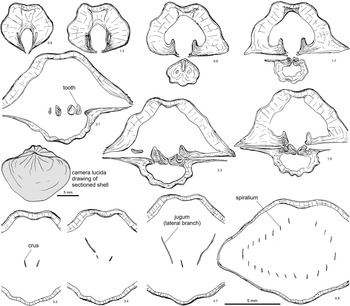
Figure 14 Serial sections of Elkanathyris pallula n. gen. n. sp. Specimen GSC 131803 from locality A708, Goéland Member (unit 5), Menier Formation. Note that the left spiralium has broken off, causing the jugum and spiralium to be displaced posteriorly towards the hinge, making it difficult to reconstruct the connection between the umbonal blades and the crura. Number below each serial section denotes distance (mm) from shell apex.
Type
Holotype, GSC 134439 (Fig. 13.1–13.5), from locality A708 (=A852a, map sheet NTS 12F/4, UTM 20, 92900E, 49400N), exposures along Sandtop gravel road, 2.4 km southeast of South Sandtop Creek. Soft-weathering, blue-gray shales and micrites, with local shell beds or lenses rich in Pentamerus, Hyattidina, Joviatrypa, Stricklandia, and Triplesia, in addition to the new species. Goéland Member, upper unit 5, Menier Formation.
Diagnosis
Small to medium sized, transversed extended, posteriorly plicate hindellide shells, with very narrow dental cavities, bulbous socket ridges, and a simple jugum with pointed jugal saddle.
Description
Shell small to medium sized, generally wider than long, subquadrate, biconvex; coarse ribs (plicae) in posterior half of adult shell, two or more medial ribs on ventral valve, single strong median rib on dorsal valve, and two or three lateral ribs; remainder of shell smooth; concentric growth lines weak or absent; hinge line relatively long, straight; beak incurved, with minute apical, or transapical foramen flanked by small, hollow deltidial plates. Ventral umbonal interior with narrow, slit-like dental cavities, leading to dorso-medially directed teeth; inner socket ridges bulbous, obscuring thin crural bases; jugum simple with pointed jugal saddle; laterally directed spiralia of 9–10 whorls.
Remarks
In this study only one new species of the new genus is described. The genus ranges through ~150 m of strata, found above and below the type stratum. Some of these hyattidinid nests, but not all, include both smooth Hyattidina and ribbed Elkanathyris n. gen. shells, which occur in the Stricklandia or Triplesia brachiopod community, with the Pentamerus community in the strata above. This suggests that the athyrides lived in relatively deeper water, mid-shelf carbonate settings (equivalent to a BA-4 setting of Boucot, Reference Boucot1975).
Population variants at the type locality include smooth shells (without the undulating ribs or plicae) that are internally identical to Elkanathyris pallula n. gen. n. sp. (Figs. 13, 14), as confirmed by serial sections of several specimens of both variants in this study. Pending a broader investigation into the internal structures of Cryptothyrella and Elkanathyris n. gen., the smooth form that co-occurs with the ribbed Elkanathyris pallula n. gen. n. sp. is assigned provisionally to Hyattidina sp. under open nomenclature.
Conclusions
An abundant and well-preserved suite of athyride brachiopods is present in the Late Ordovician (Hirnantian only) and early Silurian (Llandovery) sequence of Anticosti Island. Their stratigraphic distribution provides clues as to the change-over in shelly communities crossing the Ordovician-Silurian mass extinction boundary. Marked are rapid evolution of Hindella species during deposition of the Hirnantian Ellis Bay Formation, and their disappearance at the top of the Ordovician, alongside the last occurrence of the orthide genus Hirnantia. Hindella is replaced by the athyride Koigia in the Rhuddanian, a smaller genus that is locally abundant along with the new Early Silurian shelly fauna of Zygospiraella, Becscia and Viridita (Jin and Copper, Reference Jin and Copper2010; Copper and Jin, Reference Copper and Jin2014). In the Aeronian-Telychian, athyrides diversified further into the meristelline and whitfieldelline subfamilies that mark the Telychian through Wenlock in Laurentia.
Detailed serial sections using acetate and butyrate peels are reconstructed in three dimensions to demonstrate the nature of the calcified skeletal supports of the lophophore in these early athyrides. This sheds a new, and different, light on their rise in the Silurian. For the first time we note: (a) Hindellide brachidia lack a skeletal connection between the spiralia and the dorsal hinge crura (this is thus unlike what is normally shown in figures), (b) the umbonal blades of the brachidium and crura are bent at their tips into a hook-like structure (new discovery), and (c) the jugum of hindellides is a simple arch, either rounded or angular. Using the crura, and brachidium, we modify and simplify the existing taxonomy of early athyrides, combining such genera within the Hindellidae Schuchert, Reference Schuchert1894. The evolution of such early athyrides provides a stratigraphically useful tool that explains the westward migration of pentameride, rhychonellide, and atrypide shelly communities in the early Silurian equatorial belt of Laurentia and Baltica.
Acknowledgments
We jointly thank NSERC’s Discovery Grant Program (Natural Sciences and Engineering Research Council) for its long-term support of stratigraphic work on Anticosti Island. J. Dougherty and M. Coyne provided access and curation for the Billings collection (Geological Survey of Canada, Ottawa), in charge of the curation of the Anticosti collections, now In Ottawa, made by us since 1966. Specimens of Cryptothyrella were kindly provided for study by W. Ausich (Ohio State University) and B. Hunda (Cincinnati Museum Center, Ohio). Stratigraphic information on the Cryptothyrella occurrences in Ohio and New York was provided by C.E. Brett (University of Cincinnati) and M. Kleffner (Ohio State University). The constructive comments of journal reviewers R.-Y. Li and F. Alvarez and journal editor B. Hunda greatly helped improve the presentation and discussions. This paper is a contribution to the International Geoscience Programme (IGCP) Project 653—The onset of the Great Ordovician Biodiversification Event.