Introduction
Studying the host-seeking behaviours of disease vectors can provide pivotal information for not only understanding fundamentals of host–parasite biology, but also in designing effective public health interventions. Ticks are responsible for transmitting a greater variety of pathogens than any other arthropod group (Jongejan and Uilenberg, Reference Jongejan and Uilenberg2004), are the chief source of vector-borne diseases in wildlife and domestic animals and are second only to mosquitoes in transmitting human diseases (De La Fuente et al., Reference De La Fuente, Estrada-Pena, Venzal, Kocan and Sonenshine2008). As emerging tick-borne diseases are discovered, and as ticks expand their ranges, it is increasingly important to study tick behaviour in response to pathogen infection. Considering their wide host range which apart from domestic animals and humans, it also includes several wildlife species that present an important role in the spreading of ticks and tick-borne pathogens (Bezerra-Santos et al., Reference Bezerra-Santos, Mendoza-Roldan, Thompson, Dantas-Torres and Otranto2021b). For example, the lone star tick, Amblyomma americanum, is an aggressive human-biting tick that is a competent vector of many pathogens that pose a known threat to public health, including Rocky Mountain Spotted Fever (RMSF), human monocytic ehrlichiosis, tularaemia and heartland virus (Childs and Paddock, Reference Childs and Paddock2003). Amblyomma americanum is predicted to expand its geographic range northwards from the South and Central United States with changing climatic conditions (Sagurova et al., Reference Sagurova, Ludwig, Ogden, Pelcat, Dueymes and Gachon2019). Given the widening distribution and vectorial capacity of A. americanum, it is more critical than ever to study host-seeking behaviour across different habitats.
Amblyomma americanum is found in a large variety of habitat types, based mainly on the presence and movement of vertebrate hosts (Koch and Burg, Reference Koch and Burg2006; Willis et al., Reference Willis, Carter, Murdock and Blair2012). Larger populations of A. americanum are often found in canopied, shaded areas which have an overall wooded habitat providing abundant leaf litter (Semtner and Hair, Reference Semtner and Hair1973; Koch, Reference Koch1984; Koch and Burg, Reference Koch and Burg2006) potentially to reduce water loss while engaging in host seeking in shaded areas (Hair et al., Reference Hair, Sauer and Durham1975; Jaworski et al., Reference Jaworski, Sauer, Williams, McNew and Hair1984; Koch, Reference Koch1984; Needham and Teel, Reference Needham and Teel1991). Tick host-seeking behaviour is called ‘questing’ and refers to a stereotyped series of behaviours where ticks climb tall grasses and other vegetation, halt and extend their forelegs and wait to attach to a passing host (Holderman and Kaufman, Reference Holderman and Kaufman2013; McClure and Diuk-Wasser, Reference McClure and Diuk-Wasser2019). The duration and height at which ticks quest can influence host selection (Randolph and Storey, Reference Randolph and Storey1999; Randolph, Reference Randolph2004) but continued questing at greater heights can pose a desiccation risk (Needham and Teel, Reference Needham and Teel1991; Portugal et al., Reference Portugal, Wills and Goddard2020). In Texas, Amblyomma mixtum ticks quested at greater heights in experimental chambers when relative humidity was held at 95% as compared to 56% (Beck and Orozco, Reference Beck and Orozco2015). However, Schulze et al. found the opposite pattern in A. americanum ticks in New Jersey, where ticks spent the most time questing when temperatures were high and humidity was low (Schulze et al., Reference Schulze, Jordan and Hung2001). In contrast to both of these studies, Lane et al. did not find any consistent pattern of activity with nymphal Ixodes pacificus in regards to temperature, humidity or time of day (Lane et al., Reference Lane, Mun, Peribáñez and Stubbs2007). In addition, prescribed burns have been suggested as a mitigation strategy for ticks, so we also sought to test whether tick behaviour differs between habitats with varying burn history (Davidson et al., Reference Davidson, Siefken and Creekmore1994; Willis et al., Reference Willis, Carter, Murdock and Blair2012; Gleim et al., Reference Gleim, Conner, Berghaus, Levin, Zemtsova and Yabsley2014). Although extrinsic factors of the environment play a large role in tick-questing behaviour, intrinsic factors such as pathogen infection also influence host-seeking behaviour (Benelli, Reference Benelli2020). However, few studies address the joint effects of habitat type and infection status on questing behaviour in A. americanum.
A major avenue of research in disease vector ecology focuses on how infection status alters vector behaviour and life history traits and, in turn, affects disease risk for hosts. For example, Ixodes ricinus ticks infected with Borrelia spp., the causative agent of Lyme disease, exhibit increased lifespan and resistance to desiccation, and as a result have been shown to spend longer periods of time questing compared to uninfected ticks (Herrmann and Gern, Reference Herrmann and Gern2015). Similarly, Ixodes scapularis nymphs infected with Borrelia burgdorferi quest at greater heights and show an increased tendency to overcome physical barriers or obstacles in order to quest (Lefcort and Durden, Reference Lefcort and Durden1996). Busby et al. found that ticks infected with Anaplasma phagocytophilum showed increased questing speeds and reduced desiccation risk (Busby et al., Reference Busby, Ayllon, Kocan, Blouin, De La Fuente, Galindo, Villar and De La Fuente2012). Since Lyme disease has been the focus of tick research for so long, most of the literature on infection status and tick-questing behaviour has focused on Ixodes ticks (Benelli, Reference Benelli2020). However, public health officials are becoming increasingly concerned about tick-borne diseases transmitted by A. americanum, and no study has yet focused on infection status and questing behaviour in A. americanum.
Rickettsia amblyommatis is very prevalent in some A. americanum populations with one study finding up to 84% prevalence (Mixson et al., Reference Mixson, Campbell, Gill, Ginsberg, Reichard, Schulze and Dasch2006). In a study conducted across Florida, R. amblyommatis prevalence was found to be between 20 and 50% depending on the time of year, along with other tick-borne pathogens with low prevalence (De Jesus et al., Reference De La Fuente, Estrada-Pena, Venzal, Kocan and Sonenshine2019). In addition, this pathogen has been detected in other Amblyomma spp. species collected from wildlife and domestic animals (Costa et al., Reference Costa, da Costa, Moraes-Filho, Martins, Soares, Ramirez, Dias and Labruna2017; Bezerra-Santos et al., Reference Bezerra-Santos, Ramos, Campos, Dantas-Torres and Otranto2021a; Mendoza-Roldan et al., Reference Mendoza-Roldan, Ribeiro, Castilho-Onofrio, Marcili, Simonato, Latrofa, Benelli, Otranto and Barros-Battesti2021). This study aimed to address the joint effects of habitat type and tick-borne pathogen infection (i.e., Ehrlichia spp. and R. amblyommatis) on A. americanum questing behaviour.
Materials and methods
Tick collection and maintenance
In total, 182 adult and nymphal A. americanum ticks were collected in the Ordway-Swisher Biological Station (OSBS) in Hawthorne, Florida, a research and extension facility of the University of Florida. OSBS contains an array of natural and altered habitat types, defined by the Florida Natural Areas Inventory classification system (Guide to the natural communities of Florida: 2010 edition, 2010). Furthermore, OSBS has a detailed history of prescribed fires across each habitat type. Ticks were collected in three habitat types: abandoned fields/pastures, successional hardwood forests and xeric hammocks. Due to the low number of ticks (n = 5) collected in the abandoned field/pasture habitat type, only the other two habitat types were considered in the analysis. One additional tick was removed from the analysis as it had a low DNA output after extraction and could not be sequenced for pathogens. Successional hardwood forests are altered landcover characterized by a closed canopy dominated by fast-growing hardwoods, a dense subcanopy and shrub layer (Guide to the natural communities of Florida: 2010 edition, 2010; Fig. 1a). Xeric hammocks are evergreen forests characterized by well-drained sandy soils, a low canopy of various oak trees and an open or shrubby understory (Guide to the natural communities of Florida: 2010 edition, 2010; Fig. 1b). A total of 82 ticks were collected from the xeric hammock habitat types and 95 from the successional hardwoods.

Fig. 1. Habitat types where ticks were collected in OSBS: (A) successional hardwood and (B) xeric hammock. Photo credits: Ordway-Swisher Biological Station.
To assess the effects of burn history, two sites were chosen per habitat type, one that had a prescribed burn within the last 4 years and one that had been burned more than 4 years ago. At each collection event, temperature, relative humidity and wind speed were collected using a Kestrel® 3000 Wind Meter (Table S1). Ticks were collected in the late morning to early afternoon to maximize the number of A. americanum ticks collected (Schulze et al., Reference Schulze, Jordan and Hung2001). Two collection events took place at each habitat type between July and August 2020 using a tick drag, a white canvas sheet that is dragged behind the researcher. Approximately 50 drags were completed at each time of collection and drags were checked for ticks approximately every 30 m. Ticks were placed in glass vials with mesh tops to ensure air flow and brought back to a standardized location for questing assays.
Questing assays
Questing assays were conducted outdoors on a screened-in porch in a standardized setting exposed to ambient environmental conditions. Questing assays all took place in the same location to reduce the effects of various environmental conditions, as well as to have a consistent location all individual ticks would be tested in. Ticks were tested approximately within 1–3 h after being collected. Questing assays were conducted in a clear plastic plant saucer (12 cm height; 18.5 cm diameter) with a 55 cm wooden dowel with 1 cm demarcations in the middle of the saucer held in place with crafting putty (following methods in Tietjen et al., Reference Tietjen, Esteve-Gasent, Li and Medina2020; Supplementary materials). The vial that held the tick was opened and human breath was used to activate/stimulate the tick (Vassallo and Pérez-eid, Reference Vassallo and Pérez-eid2002). The open vial was placed adjacent to the putty so that the tick could leave the vial on its own, walking onto the base of the dowel. The 10 min period began immediately upon the tick leaving the vial. Once the tick reached the dowel, the height at which each quest occurred was recorded and the total amount of time spent questing was quantified using an additional stopwatch. Questing assays were conducted for 10 min total, and questing was defined as a tick-halting motion and extending its forelegs off the dowel. Ticks were classified as non-questing if they never, halted and extended their forearms while on the dowel, this includes if a tick climbs the dowel but does not stop to extend their forearms. For example, if a tick engaged in three questing bouts during the 10-min period, each for 10 s, they were recorded as a total questing time of 30 s and the average height of the three questing bouts was recorded. Following the assay, ticks were placed in a microcentrifuge tube and frozen at −80°C.
DNA extraction and pathogen screening
DNA was extracted from 176 ticks using a Qiagen DNeasy Tissue and Blood extraction kit. Prior to extraction, each tick was placed in liquid nitrogen and promptly crushed with a pestle. Conventional polymerase chain reaction (PCR) with a pan–genus primer pair was used to screen all ticks for both Rickettsia spp. and Ehrlichia spp. Primers and protocols for screening for Rickettsia spp. were chosen based on Kidd et al. (Reference Kidd, Maggi, Diniz, Hegarty, Tucker and Breitschwerdt2008) and the research of Tucker (Reference Tucker2017) (Table 1). The primers used for the screening of Ehrlichia spp. were developed by Doyle et al. (Reference Doyle, Labruna, Breitschwerdt, Tang, Corstvet, Hegarty, Bloch, Li, Walker and McBride2005) and the protocols were based on the research of Tucker (Reference Tucker2017). The specific instructions for the protocols relating to DNA extraction and PCR can be found in the Supplementary materials. For the screening of rickettsial pathogens, the primers 107F and 299R were used to produce a 209–212 bp amplicon 5′-hypervariable region of known ompA sequences (Kidd et al., Reference Kidd, Maggi, Diniz, Hegarty, Tucker and Breitschwerdt2008; Tucker, Reference Tucker2017). For the screening of Ehrlichia pathogens, the primers, DSB-321 and DSB-671 were used to amplify the 378 bp Ehrlichia dsb gene (Doyle et al., Reference Doyle, Labruna, Breitschwerdt, Tang, Corstvet, Hegarty, Bloch, Li, Walker and McBride2005). All samples were checked for amplification via gel electrophoresis (Supplementary materials). When testing for Ehrlichia spp., DNA samples were pooled, with ten ticks in each reaction. All positive samples were nano-dropped for quantitation and to test for purity, then sent to Genewiz (Gainesville, FL, USA) for Sanger sequencing. Sequences were aligned and analysed using Geneious Prime 2020.2.4 (https://www.geneious.com) and then blasted against NCBI Genbank.
Table 1. Primers used for pathogen screening of collected ticks

Statistical analyses
To analyse a tick's likelihood to quest during the 10 min assay, a binary logistic regression in the program JMP (JMP Pro 15.0; 2019, SAS Institute) was used with habitat type, burn history, tick life stage (nymph/adult) and pathogen infection status as independent variables. To analyse average questing height and time spent questing, only ticks that quested during the 10 min assay were used (n = 73) using generalized linear models with identity link functions. Response variables were log 10 transformed to fit model assumption of normally distributed residuals, and both models included the same independent variables: habitat type, burn history, life stage and infection status. We originally included a habitat type × infection status interaction term, but it was not significant (P > 0.05), so it was removed for model simplification.
Results
Out of 176 ticks collected in OSBS, 52 tested positive for R. amblyommatis [overall prevalence = 30%; 28% (26/94) in successional hardwood habitats, 32% (26/82) in xeric hammock habitats]. The homology for these samples with R. amblyommatis varied from 90.26 to 100%. None of the ticks collected tested positive for Ehrlichia infections. No evidence was found that habitat type, burn history, infection status or life stage altered the questing likelihood for ticks during the experimental assay (all P > 0.31; Table 2). Similarly, none of our independent variables appeared to have an effect on the average height at which ticks quested (all P > 0.31; Table 2).
Table 2. Summary of general linear mixed models predicting questing propensity, average questing height and total time spent questing

Significant effects are denoted with an asterisk.
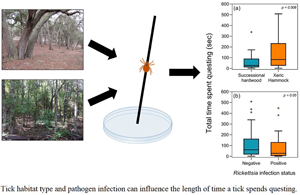
Ticks collected from xeric hammock habitats spent over twice as long (151 s on average) engaging in questing behaviour compared to ticks collected from successional hardwood forests (58 s on average) (df = 1, χ 2 = 6.99, P = 0.008; Table 2; Fig. 2A). Also, a weaker effect was detected where ticks that tested positive for R. amblyommatis infection spent less time questing compared to uninfected ticks, with infected ticks spending 85 s on average questing and uninfected ticks spending 112 s (df = 1, χ 2 = 3.85, P = 0.05; Table 2; Fig. 2B). Tick-questing duration did not differ between adults and nymphs, nor did burn history appear to affect questing duration (all P > 0.10; Table 2).

Fig. 2. Time (s) spent questing by Amblyomma americanum in (A) two different habitats or (B) when infected or uninfected. Boxplots extend from the 25th to 75th percentiles, and the midline denotes the median. Whiskers generated using the Tukey method and outliers are shown outside of the whiskers.
Discussion
A major factor in the prevention of tick-borne diseases is understanding how intrinsic and extrinsic factors influence tick host-seeking behaviour across different habitats. Here, we assessed how habitat type, burn history and infection status influenced host-seeking behaviours in A. americanum. We found infection prevalence with R. amblyommatis to be 30%, which corresponds to recent similar studies in Florida that found prevalence to be 29% (De Jesus et al., Reference De Jesus, Ganser, Kessler, White, Bhosale, Glass and Wisely2019). Although habitat, burn history and infection status did not affect the questing likelihood of a tick or the questing height, the amount of time a tick spent questing was significantly affected by the habitat type and infection status. We found that ticks collected from xeric hammock habitats spent twice as long questing compared to ticks collected from successional hardwood forests. We also found a weaker effect that ticks infected with R. amblyommatis spent less time questing compared to uninfected ticks.
Different habitat types possess a variety of environmental characteristics that can each influence a tick's host-seeking abilities. For example, a multitude of studies have shown the key roles that humidity and temperature play in tick host-seeking behaviours (Semtner and Hair, Reference Semtner and Hair1973; Schulze et al., Reference Schulze, Jordan and Hung2001; James et al., Reference James, Bowman, Forbes, Lewis, McLeod and Gilbert2013; Beck and Orozco, Reference Beck and Orozco2015), so ticks are more likely to be found in microhabitats that reduce desiccation risk. Here, we found that ticks collected from xeric hammocks spent twice as long questing as the ticks collected from successional hardwood forests. If ticks in different habitat types exhibit differences in questing behaviour within individual questing bouts (58 s vs 151 s during a 10 min observation period), this may generate important functional differences in questing behaviour across tick lifetimes. Interestingly, these differences in tick behaviour were displayed despite questing assays occurring in the same standardized location, rather than at the site of collection. Future studies should address how environmental characteristics and tick experience influence long-term physiological changes or behavioural tendencies that influence host-seeking behaviour. Reciprocal transplant studies between different habitats would be particularly helpful to address these questions. Although we measured similar levels of relative humidity, and temperature during our collection periods (Table S1), there are numerous differences in understory complexity. For example, the average wind speed in xeric hammock was higher than that of successional hardwood forests, most likely due to a more open understory (Fig. 1). We also noted that xeric hammocks had a much thicker layer of leaf litter, which can act as a protective barrier from stressors like high heat and low humidity (Semtner and Hair, Reference Semtner and Hair1973; Koch and Burg, Reference Koch and Burg2006). The most notable difference between these habitats is the lack of structural complexity in xeric hammocks compared to successional hardwoods. In I. scapularis, immature tick densities appear to increase with the density of woody vegetation and decrease with the density of herbaceous vegetation (Adler and Spielman, Reference Adler and Spielman1992). It may be that ticks in habitats with fewer suitable substrates available for questing (i.e., less vegetation and course woody debris) will quest for longer durations once a suitable substrate is discovered. That is, each questing opportunity is more valuable when there are fewer opportunities to quest. Testing tick-questing behaviour before and after the removal/addition of vegetation to natural habitats could shed light on this phenomenon. Additionally, which hosts are present in each habitat type and the host's size could play a role in their host-seeking behaviours like questing heights (Portugal et al., Reference Portugal, Wills and Goddard2020).
We found no effect of burn history on tick-questing behaviour. In both xeric hammocks and successional hardwoods, ticks were collected from sites that had been subjected to prescribed burns either within 4 years or those that had burned longer ago. Gleim et al. (Reference Gleim, Conner, Berghaus, Levin, Zemtsova and Yabsley2014) found that long-term prescribed burn histories reduced tick counts in surveys across northern Florida and southern Georgia (Gleim et al., Reference Gleim, Conner, Berghaus, Levin, Zemtsova and Yabsley2014). We are unaware of any studies which have assayed tick-questing behaviour across habitats with varying burn histories, but perhaps the effects of prescribed burn latencies on the scale of 1–7 years is not great enough to alter tick host-seeking behaviour. Given that prescribed burns have widespread use in tick control programmes (Davidson et al., Reference Davidson, Siefken and Creekmore1994; Gleim et al., Reference Gleim, Conner, Berghaus, Levin, Zemtsova and Yabsley2014), more studies are needed to identify how burn history and habitat characteristics interact to influence tick host-seeking behaviours.
How vector behaviour changes when carrying human pathogens is a major focus of vector-borne disease studies. Much research has focused on the effects of infections on Ixodes tick behaviour, but far fewer studies have focused on A. americanum despite its increasing population densities and expanding range (Sagurova et al., Reference Sagurova, Ludwig, Ogden, Pelcat, Dueymes and Gachon2019; Benelli, Reference Benelli2020). Here, we found that ticks infected with R. amblyommatis spent less time engaging in host-seeking behaviours compared to their uninfected counterparts. Romashchenko et al. (Reference Romashchenko, Ratushnyak, Zapara, Tkachev and Moshkin2012) found that Borrelia-infected I. persulcatus ticks quested at greater heights but were also significantly slower-moving than the uninfected ticks (Romashchenko et al., Reference Romashchenko, Ratushnyak, Zapara, Tkachev and Moshkin2012). Interestingly, Lefcort and Durden (Reference Lefcort and Durden1996) found that Borrelia-infected I. scapularis adults were less active and quested at lower heights (Lefcort and Durden, Reference Lefcort and Durden1996). Although both of these studies were looking at infections with B. burgdorferi, they were not looking at the same species of tick. This difference in species could have accounted for this difference in questing activity found. This change in activity levels in infected ticks also has been correlated with an increased resistance to heat stress (Alekseev and Dubinina, Reference Alekseev and Dubinina2000; Busby et al., Reference Busby, Ayllon, Kocan, Blouin, De La Fuente, Galindo, Villar and De La Fuente2012; Herrmann and Gern, Reference Herrmann and Gern2015). It is possible that infection with R. amblyommatis could have an effect on physiological aspects of A. americanum and this could lead to greater heat stress expression or greater risk of desiccation. Physiological impacts on the vector such as these could lead to differences in questing capabilities which impact transmission dynamics. Given that we collected ticks from the wild, assessed their behaviour and then sequenced them for pathogens, the risk of type-II error from small/uneven sample sizes is possible. Future studies could utilize artificial infections in lab-reared ticks to generate more even sample sizes between infected and uninfected ticks.
An important aspect of this research is understanding the relationship between disease risk and human recreational land use. The habitat types that ticks were collected from, xeric hammock, successional hardwood forest and abandoned fields/pastures were chosen with human land use in mind. Xeric hammock habitat types are commonly used as hiking trails, as they have open spaces that are easy to traverse (Friend, Reference Friend2004). Interestingly, ticks from xeric hammocks may pose a greater risk of tick-borne diseases as ticks found in this area spent twice as long questing. Future studies could collect ticks on hiking trails cut through successional hardwood forests to test for differences in questing behaviour between trails and intact forests. Interestingly, although we originally sought to collect ticks from abandoned fields/pastures, these sites were removed from analyses due to the small number of ticks obtained from this habitat type. This is fortunate as this habitat type is often used for recreation (Plieninger et al., Reference Plieninger, Hartel, Martín-López, Beaufoy, Bergmeier, Kirby, Montero, Moreno, Oteros-Rozas and Van Uytvanck2015). Given that A. americanum is an increasingly important disease vector in Florida and elsewhere, future studies should incorporate host-seeking behaviour into investigations of human disease risk across habitats when designing management plans for recreational spaces. Additionally, future research could perform quantitative PCR to determine if behavioural changes associated with infection in ticks are intensity dependent. As another caveat, having removed ticks from their original habitat and conducting the questing assay elsewhere exposes the ticks to novel environmental variables that could alter their questing behaviour. Comparing our results to studies where ticks are tested in situ would be helpful. Additionally, adding a transparent barrier between the assay chamber and the researcher could assist in preventing any breath/CO2 from the researcher onto the ticks which could encourage the ticks to quest.
This study provides a foundation for understanding the potential joint effects of extrinsic factors (habitat type and burn history) and intrinsic factors (pathogen infection) on tick host-seeking behaviour. Although studies on R. amblyommatis infection influencing tick traits are still developing, there is evidence that this infectious agent can potentially complicate RMSF diagnoses in human hosts (Barrett et al., Reference Barrett, Little and Shaw2014; Karpathy et al., Reference Karpathy, Slater, Goldsmith, Nicholson and Paddock2016). Thus, it is important to understand the ways in which R. amblyommatis can affect a primary vector, A. americanum, so we can better understand how this pathogen is spread to humans, wildlife and domesticated animals. Future research should focus on the physiological basis of infection-induced changes to vector behaviour in the A. americanum–R. amblyommatis system. Additionally, this should be tested in ticks collected from a variety of habitat types so that we can better link vector behaviour with human disease risk across different habitat types.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001554.
Acknowledgements
We thank David Buttrick for help with fieldwork and Andrew Rappe for guidance in the Ordway. This research was supported by the Ordway Swisher Biological Station.
Author contributions
EAR and CNK conceived and designed the study. EAR, CET and BJ conducted data collection. CNK and EM supervised the project and provided resources and guidance. CNK performed statistical analysis. EAR wrote the manuscript with editing and final approval from CET, BJ, EM and CNK.
Financial support
This work was supported by the H. Jane Brockmann Graduate Research Award awarded to EAR.
Conflict of interest
The authors declare none.