Introduction
Obsessive-compulsive disorder (OCD) is a mental disorder characterized by uncontrollable, intrusive recurring obsessive thoughts and compulsive behaviors. OCD usually occurs at an early age and has a long course, which causes substantial damage to the patients’ social function and quality of life [Reference Kugler, Lewin, Phares, Geffken, Murphy and Storch1]. According to a follow-up study, only 6% of patients achieved full remission in a 2-year time frame [Reference Eisen, Pinto, Mancebo, Dyck, Orlando and Rasmussen2]. OCD has been rated as one of the top 10 disabling diseases by the World Health Organization [Reference Veale and Roberts3].
With the development of neuroimaging technology, the neural mechanism of OCD has been discovered gradually. The cortico-striato-thalamo-cortical circuits have received the most evidence-based support. In this neural network, the prefrontal cortex (PFC) (containing the orbito-frontal cortex [OFC], medial PFC [mPFC], and anterior cingulate cortex [ACC]), supplementary motor area (SMA) and premotor areas, striatum, globus pallidus, and thalamus show hyperexcitability in OCD patients [Reference de Wit, de Vries, van der Werf, Cath, Heslenfeld and Veltman4–Reference Greenberg, Ziemann, Cora-Locatelli, Harmon, Murphy and Keel7].
Cognitive abnormalities were also found in patients with OCD, including defects in memory, attention, and executive function [Reference de Geus, Denys, Sitskoorn and Westenberg8, Reference Shin, Lee, Kim and Kwon9]. OCD patients have negative attention biases and easily notice threatening information. Response inhibition (RI), one type of executive function, is considered to be the core defect of OCD and is linked to the symptom “cannot stop thinking.” RI means the ability to consciously inhibit undesirable and inappropriate thoughts and behaviors [Reference Verbruggen and Logan10, Reference Berlin and Lee11]. Therefore, RI can be divided into cognitive inhibition and behavioral inhibition. The Stroop task is usually used to measure cognitive inhibition, and the Go/No-go task or stop-signal task (SST) are used to measure behavioral inhibition. Compared with healthy control subjects, patients with OCD show more errors and longer reaction times in the Stroop task and have more errors in the Go/No-go task [Reference Bannon, Gonsalvez, Croft and Boyce12–Reference Penadés, Catalán, Rubia, Andrés, Salamero and Gastó14]. It has been confirmed that RI is correlated with the frontobasal ganglia circuit, and the pre-SMA is one of the relevant brain regions [Reference Zhang and Iwaki15, Reference Mostofsky and Simmonds16].
Pharmacotherapy and psychotherapy are the first-line treatments for OCD. Selective serotonin reuptake inhibitors (SSRIs) are widely used in clinical treatment and have been proved to be more effective than placebos [Reference Soomro, Altman, Rajagopal and Oakley-Browne17, Reference Kayser18]. Cognitive behavior therapy (CBT) combined with response prevention (ERP) is also a good treatment for OCD [Reference Öst, Havnen, Hansen and Kvale19, Reference McKay, Sookman, Neziroglu, Wilhelm, Stein and Kyrios20]. However, approximately 40% of patients have no response to these two therapies [Reference Hirschtritt, Bloch and Mathews21]. The curative efficacy of pharmacotherapy and psychotherapy is still not satisfactory; their efficacy rates range from 40 to 60% [Reference Pallanti and Quercioli22], and over 60% of patients will relapse [Reference Eisen, Sibrava, Boisseau, Mancebo, Stout and Pinto23]. Moreover, for patients who have no response to SSRIs and CBT, an increased risk of adverse events is seen in the long term [Reference Veale and Roberts3]. Therefore, it is meaningful to develop a new treatment strategy.
Progress has been continuously made in neural regulation for treating OCD. In particular, transcranial magnetic stimulation (TMS) as a safe and noninvasive treatment has a great advantage [Reference Rossi, Hallett, Rossini and Pascual-Leone24]. By creating a fast-changing magnetic field, TMS can modulate neural excitability, provoking the hyperpolarization or depolarization of surface cortical neurons [Reference Rapinesi, Kotzalidis, Ferracuti, Sani, Girardi and Del Casale25]. TMS can be divided into single-pulse TMS, paired-pulse TMS, repetitive TMS (rTMS), and theta burst stimulation (TBS) [Reference Rossi, Hallett, Rossini and Pascual-Leone24]. Among these, rTMS and TBS are used for treatment. Research has shown that high-frequency (≥5 Hz) stimulation can enhance neural excitability [Reference Sarkhel, Sinha and Praharaj26], and low-frequency (≤1 Hz) stimulation can inhibit neural excitability [Reference Speer, Kimbrell, Wassermann, Repella, Willis and Herscovitch27]. rTMS uses a repeated fixed-frequency single pulse to stimulate the cerebral cortex, while TBS embeds three burst high-frequency theta stimulations (50 Hz) into a 5 Hz persistent stimulation with an interval of 200 ms [Reference Chung, Hoy and Fitzgerald28]. Compared with rTMS, TBS is closer to the biological rhythm and can improve the induction of synaptic long-term potentiation [Reference Suppa, Huang, Funke, Ridding, Cheeran and Di Lazzaro29, Reference Blumberger, Vila-Rodriguez, Thorpe, Feffer, Noda and Giacobbe30]. TBS can be further divided into continuous theta burst stimulation (cTBS) and intermittent theta burst stimulation (iTBS). The former has an inhibitory effect, while the latter has an active effect on the cerebral cortex.
In recent years, rTMS has showed a beneficial effect on OCD [Reference Lusicic, Schruers, Pallanti and Castle31]. The mPFC, OFC, and SMA are the three main stimulation targets. According to two meta-analyses, high frequency (HF) stimulation over the mPFC could improve OCD symptoms [Reference Lusicic, Schruers, Pallanti and Castle31, Reference Zhou, Wang, Wang, Li and Kuang32]. The efficacy of dTMS (HF) and iTBS over the mPFC has also been shown [Reference Carmi, Alyagon, Barnea-Ygael, Zohar, Dar and Zangen33–Reference Carmi, Tendler, Bystritsky, Hollander, Blumberger and Daskalakis35]. Therefore, the mPFC is not an ideal target for cTBS, which is a type of low-frequency stimulation. Moreover, the OFC is a challenging target, as the location of stimulation is close to the brow bones. According to Rehn et al.’s [Reference Rehn, Eslick and Brakoulias36] meta-analysis, SMA is a promising rTMS target for OCD. Therefore, SMA was selected as the target in this study.
To explore the treatment efficacy of cTBS over the bilateral SMA in patients with OCD, this study developed a randomized sham-controlled trial, which included a 4-week treatment period and a 4-week follow-up period.
Methods
Study design
This study was a clinical, randomized, single-blind sham-controlled trial conducted in Shanghai Mental Health Center with active enrollment from 2019 to 2021. The study was approved by the local ethics institutional review board (IORG0002202) and registered at http://chictr.org.cn (ChiCTR1900026020). All of the patients signed informed consent forms.
Participants
OCD patients (N = 61) were recruited in Shanghai Mental Health Centre through recommendations of psychiatrists and posters placed in hospital hall. Ultimately, 54 patients met the inclusion and exclusion criteria (Figure 1).
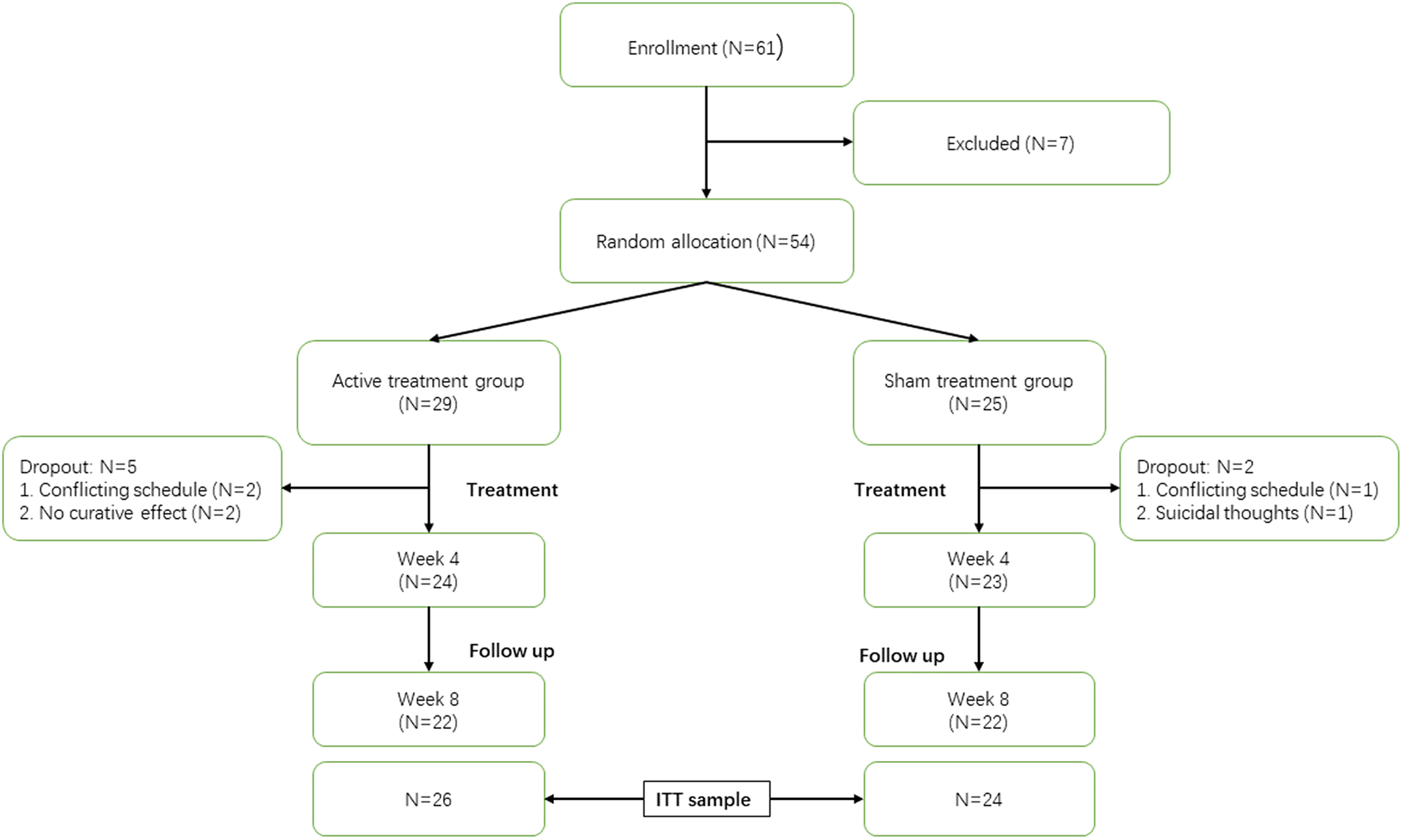
Figure 1. CONSORT diagram. Week 4 represents the end of cTBS treatment; week 8 represents the end of follow-up; ITT, intention to treat.
Inclusion criteria
All patients were treated for obsessive and compulsive symptoms and were diagnosed by psychiatrists to ensure that they met the OCD criteria of the DSM-5. Age was limited to 18 ~ 54 years old, and education level was limited to above middle school. Patients must have a score between 16 and 31 on the Yale–Brown obsessive-compulsive scale (16 ≤ YBOCS ≤ 31), because patients with severe OCD symptoms could not complete the study.
Exclusion criteria
Patients were assessed by the MINI (International Neuropsychiatric Interview) to eliminate patients with any psychiatric comorbid disorders. Patients who had a high risk of suicide or had severe physiological diseases and neurological disorders were excluded. Moreover, drug abusers and patients with ear diseases or those who used hearing aids were not allowed to participate in the research. Those who had received TMS treatment but had no significant effectiveness were also excluded.
Treatment
Participants were divided into two groups: the active (cTBS) group and the sham (control) group. The active group received cTBS for a month. There were five cTBS treatments a week (from Monday to Friday), and 20 cTBS treatments in total. Treatment was administered by a Ma GPro X100 device (Maga Venture) with a Cool-MCF-B65 butterfly coil. According to the 10/20 International EEG Positioning System, the coil was placed anterior to the apex of the skull and located 15% of the distance between the nasion and the occipital eminence. The coil was 45° from the SMA, and treatment was performed on one side and then the other side instead of treating the bilateral SMA at the same time.
The resting motor threshold (RMT) was quantified as the minimum stimulus intensity at which 5/10 of the single pulse trials to the contralateral primary motor cortex elicited a threshold electromyogram response (50 mV in the peak-to-peak amplitude) in the contralateral abductor pollicis brevis. The detailed parameters of cTBS were as follows: the intensity of stimulus was 110% RMT; the frequency of trains was 50 Hz and the number of pulses was three; and the frequency of intertrain intervals was 5 Hz and pulses number was 200. Every treatment contained 1,200 pulses and lasted for 48 s. The average stimulator intensity was 40% RMT in both groups.
The treatment of the sham group was the same as active group. The only difference was that the coil was flipped 180° in the sham group. The device also made the same sound but could not stimulate the brain. The medication regimen of the participants at baseline was maintained throughout the cTBS treatment and follow-up periods.
Assessment
The reduction rate of the YBOCS score was used as the primary outcome. Hamilton depression scale (HAMD24) and Hamilton anxiety scale (HAMA14) scores were used as secondary outcomes. A ≥ 35% reduction in YBOCS score was defined as “treatment response,” also known as “full response,” and a ≥ 25% reduction in YBOCS score was defined as “partial response” [Reference Mataix-Cols, Fernández de la Cruz, Nordsletten, Lenhard, Isomura and Simpson37].
The OBQ44 (Obsessive Belief Questionnaire, OBQ44) was used to measure the severity of obsessive belief, which was one of the exploratory outcomes in this study. Moreover, behavioral tests, including the Stroop task, SST and probabilistic reasoning task (PRT), were also regarded as exploratory outcomes. The Stroop task and SST were used to explore the effect of cTBS on RI. PRT, a paradigm which simulates the process of decision-making during evidence accumulation [Reference Yang and Shadlen38, Reference Lin, Nie, Zhang, Chen and Yang39], was used to explore the effect of cTBS on decision-making.
We developed the treatment satisfaction scale (TSS) to evaluate patients’ satisfaction with the treatment. The adverse events questionnaire (AEQ) was developed to investigate adverse events during 1 month of cTBS treatment. The TSS score, AEQ score, and dropout rate were included as important parameters to evaluate the safety of cTBS treatment. For a detailed experimental workflow, please see Figure 2.
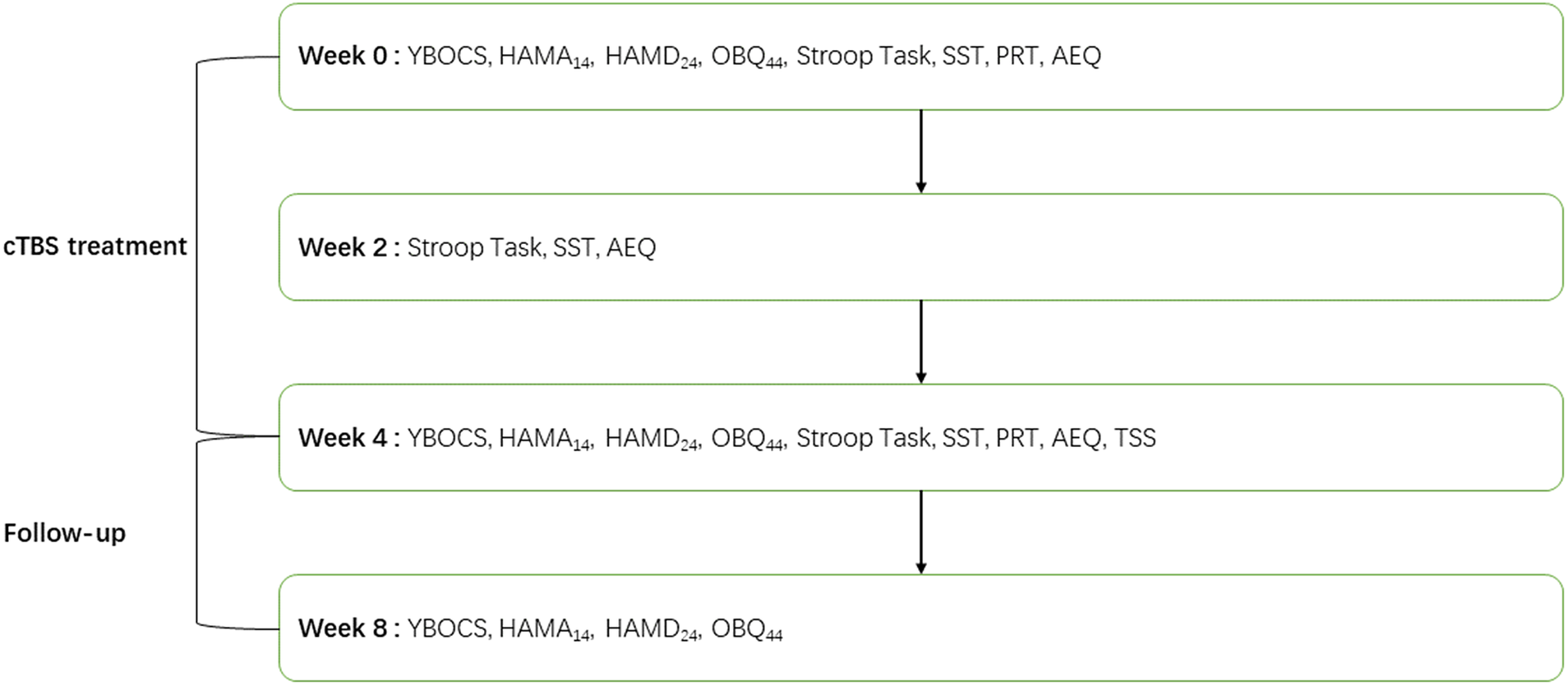
Figure 2. Experimental workflow. AEQ, Adverse events questionnaire; HAMD24, Hamilton depression scale; HAMA14, Hamilton anxiety scale; OBQ44, obsessive belief questionnaire; PRT, probabilistic reasoning task; SST, stop-signal task; TSS, treatment satisfaction scale; YBOCS, Yale-Brown obsessive-compulsive disorder scale.
Blinding
At first, patients were randomly assigned to the active or sham group through a random series number generated by a computer. As both groups received the same treatment process, the patients were blinded to the treatment conditions. Moreover, assessors were also blinded to the treatment conditions. Every patient was assigned a unique number, and only the experimenters and cTBS operators knew the detailed information.
Statistical analysis
To ensure the reliability of result, the intention-to-treat (ITT) analysis set includes all participants who had received at least one active or sham treatment, using last observation carried forward to fill missing values.
All statistical analyses were worked on SPSS, version 24.0 (IBM Corp., Armonk, NY). Both independent T-test (two-tailed) and Pearson χ2 test were used to compare the difference of demographic variables and clinical features at baseline between the active group and sham group. Response rate of outcomes and satisfaction for treatment between the two groups were compared by independent T-test (two-tailed) or Fisher exact test for effective differences. Two-ways repeated measures ANOVA was used to analyze the main effect and interactive effect of Group (active group, sham group) and Time (week 0, 4, 8). Mauchly sphericity test was essential to make sure the data satisfy the sphericity hypothesis before the two-ways repeated measures ANOVA. If the data not satisfied the sphericity hypothesis, the Greenhouse–Geisser adjustment was needed. The confidence interval was set as 95%.
Results
Twenty-four patients in active group completed cTBS treatment and 23 patients in the sham group completed sham cTBS treatment. Up to week 8, both the active group and sham group had 22 patients who completed the follow-up (Figure 1). The ITT sample included 50 patients, among whom 26 patients were included in the active group and 24 patients were included in the sham group.
Baseline assessment
Table 1 presents the demographic and clinical features of the participants at baseline. There were no significant differences between the two groups in terms of demographic features. There was also no significant difference at baseline between the two groups on the YBOCS, HAMD24, HAMA14, and OBQ44 scores.
Table 1. Demographic data and clinical characteristic in baseline.
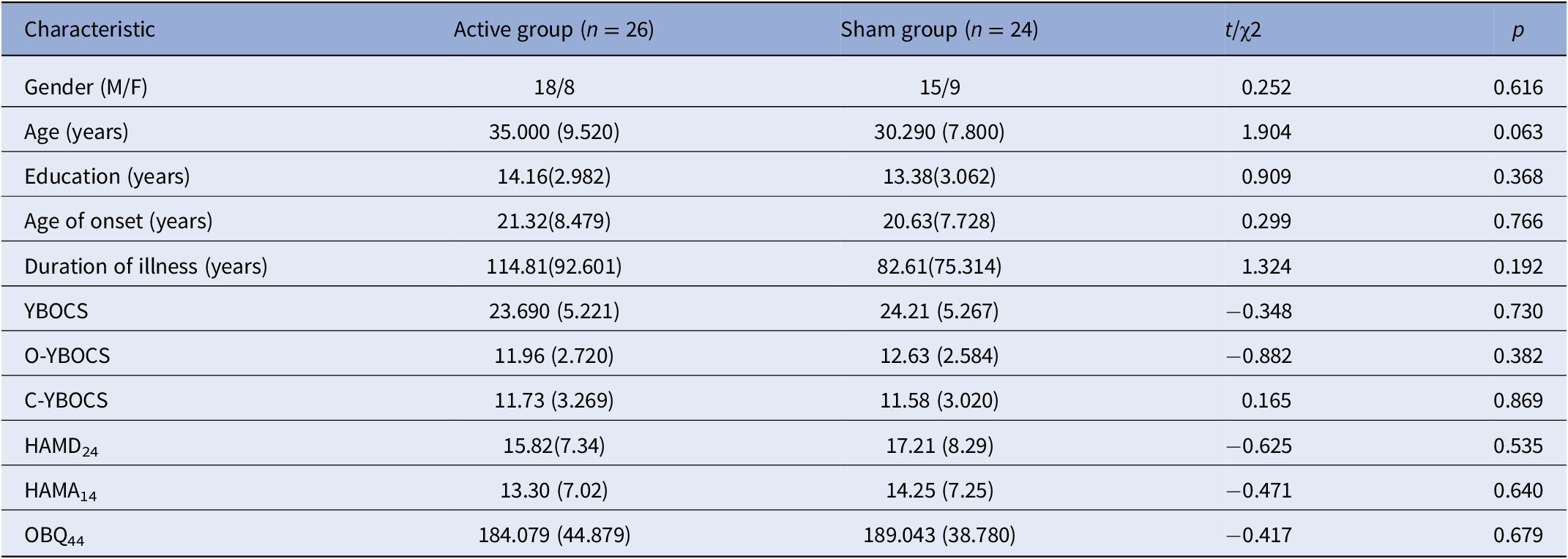
Abbreviations: C-YBOCS, compulsion sub-score of Yale–Brown obsessive-compulsive scale; HAMD24, Hamilton depression scale; HAMA14, Hamilton anxiety scale; OBQ44, obsessive belief questionnaire; O-YBOCS, obsession sub-score of Yale–Brown obsessive-compulsive scale; YBOCS, Yale-Brown obsessive-compulsive disorder scale.
Primary outcomes
All the interactions of Time × Group on the YBOCS, O-YBOCS (obsession sub-score of the Yale–Brown obsessive compulsive scale), and C-YBOCS (compulsion sub-score of the Yale–Brown obsessive compulsive scale) were not statistically significant. All the Time effects of the YBOCS, O-YBOCS, and C-YBOCS were statistically significant. For detailed results, please see Table 2.
Table 2. Effect of cTBS treatment on primary and secondary outcomes.
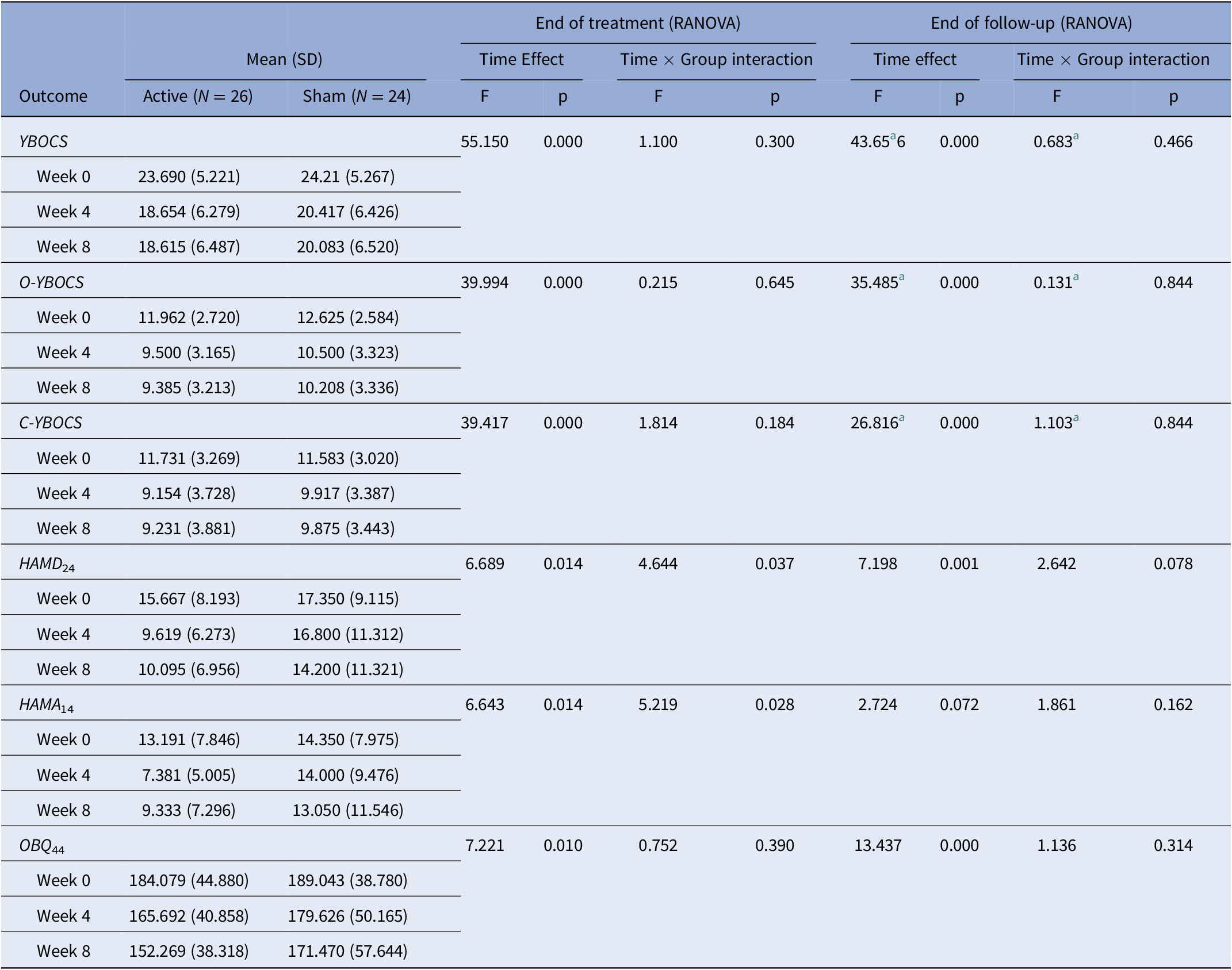
Note: End of treatment (RANOVA), two-ways (Group × Time (week 0, 4)) repeated measures ANOVA; end of follow-up (RANOVA) = two-ways (Group × Time (week 0, 4, 8)) repeated measures ANOVA.
Abbreviations: C-YBOCS, compulsion sub-score of Yale–Brown obsessive-compulsive scale; HAMD24, Hamilton depression scale; HAMA14, Hamilton anxiety scale; OBQ44, obsessive belief questionnaire; O-YBOCS, obsession sub-score of Yale–Brown obsessive-compulsive scale; YBOCS, Yale-Brown obsessive-compulsive disorder scale.
a Correction for nonsphericity.
The treatment response rate (full response: a reduction ≥35% in the YBOCS score) at week 4 was 23.1% (6/26) in the active group and 16.7% (4/24) in the sham group, and there was no significant difference between the two groups (χ2 = 0.321, p = 0.571). The treatment response rate at week 8 was 26.9% (7/26) in the active group and 16.7% (4/24) in the sham group, and there was no significant difference between the two groups (χ2 = 0.765, p = 0.382). For detailed results, please see Table 3 and Figure 3.
Table 3. Response rate after cTBS treatment and follow-up.
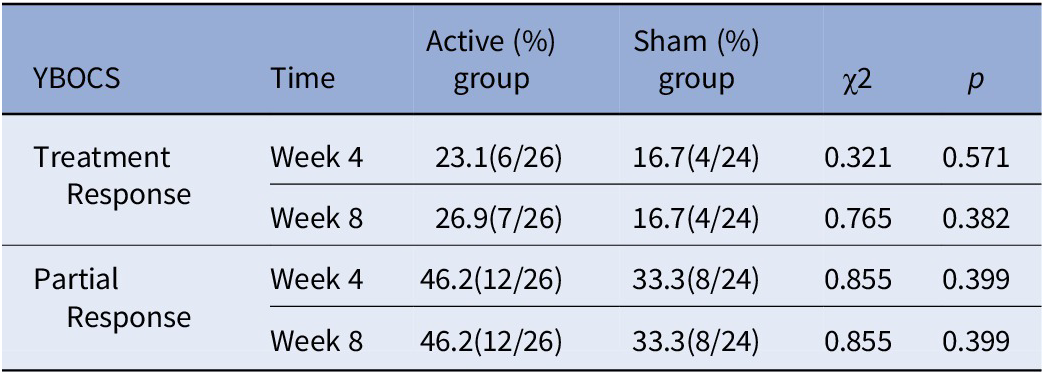
Note: Treatment response, a reduction ≥35% in the YBOCS score; partial response, a reduction ≥25% in the YBOCS score.
Abbreviation: YBOCS, Yale–Brown obsessive-compulsive scale.
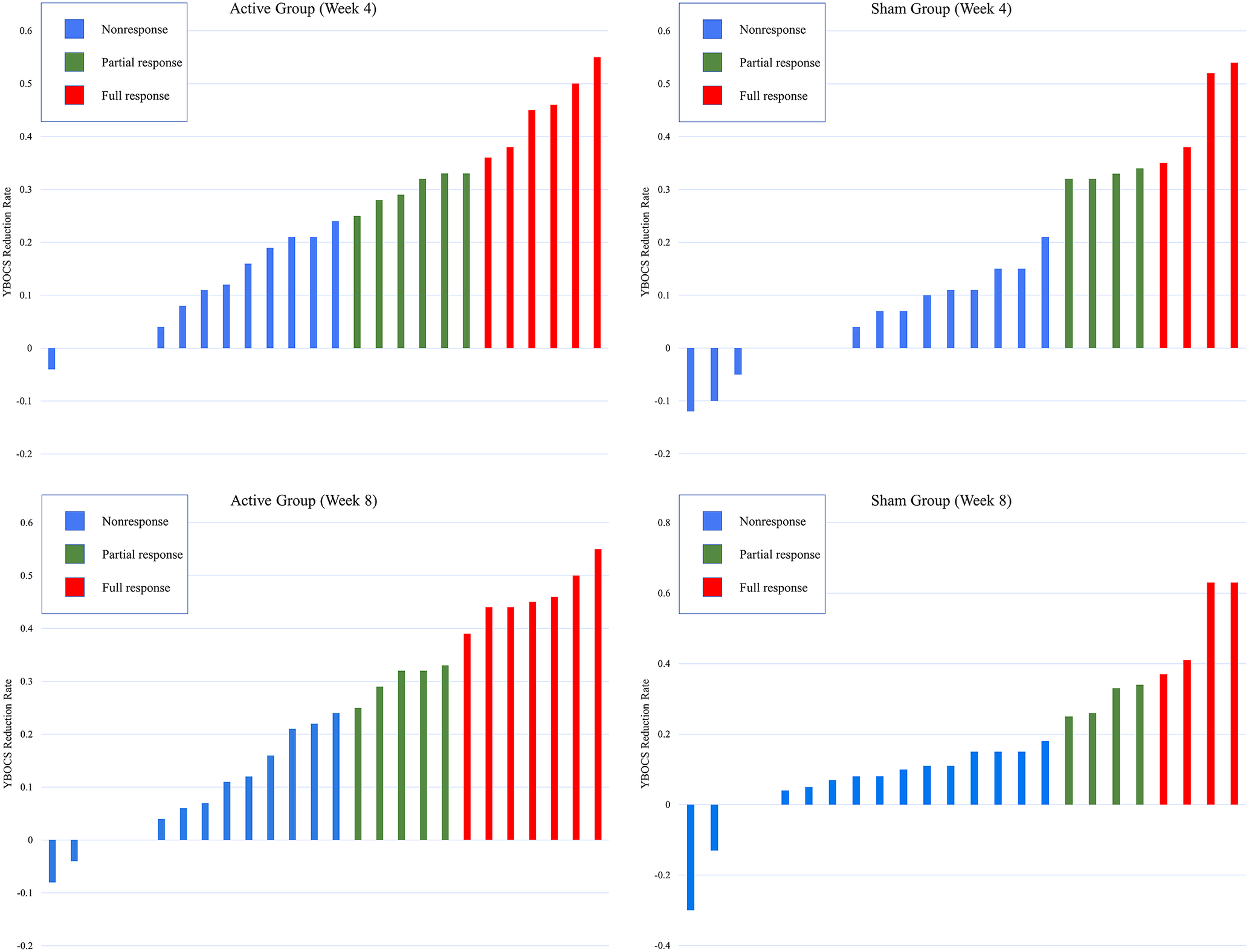
Figure 3. Individual distribution of full responders and nonresponders according to the reduction rate of YBOCS at week 4 and week 8. YBOCS, Yale-Brown obsessive-compulsive scale; week 4: YBOCS reduction rate = [YBCOS (week 0)−YBCOS (week 4)]/YBCOS (week 0); week 8: YBOCS reduction rate, [YBCOS (week 0)−YBCOS (week 8)]/YBCOS (week 0).
Secondary outcomes
At week 4, both HAMD24 and HAMA14 scores had significant Time effects (HAMD24: F = 6.689, p = 0.014; HAMA14: F = 6.643, p = 0.014) and Time × Group interaction (HAMD24: F = 4.644, p = 0.037; HAMA14: F = 5.219, p = 0.028). At week 8, the Time effect of the HAMD24 and HAMA14 scores was still significant (HAMD24: F = 7.198, p = 0.001; HAMA14: F = 2.724, p = 0.072), but the Time × Group interaction of the HAMD24 and HAMA14 was not significant (HAMD24: F = 2.642, p = 0.078; HAMA14: F = 1.861, p = 0.162). Whether after treatment or after follow-up, the Time effect and Time × Group interaction were not significant in OBQ44 scores. For detailed results, please see Table 2.
Simple effect analysis (Table 4) indicated that the HAMD24 and HAMA14 scores of the active group between week 0 and week 4 were significantly different (HAMD24: D-value = 6.048, p = 0.005; HAMA14: D-value = 5.810, p = 0.004), but the HAMD24 and HAMA14 scores of the active group between week 4 and week 8 were not significantly different. Moreover, these differences were not significant for the sham group (Table 4). The changes of the HAMD24 and HAMA14 scores are displayed in Figure 4.
Table 4. Simple effect analysis of HAMD24 and HAMA14.
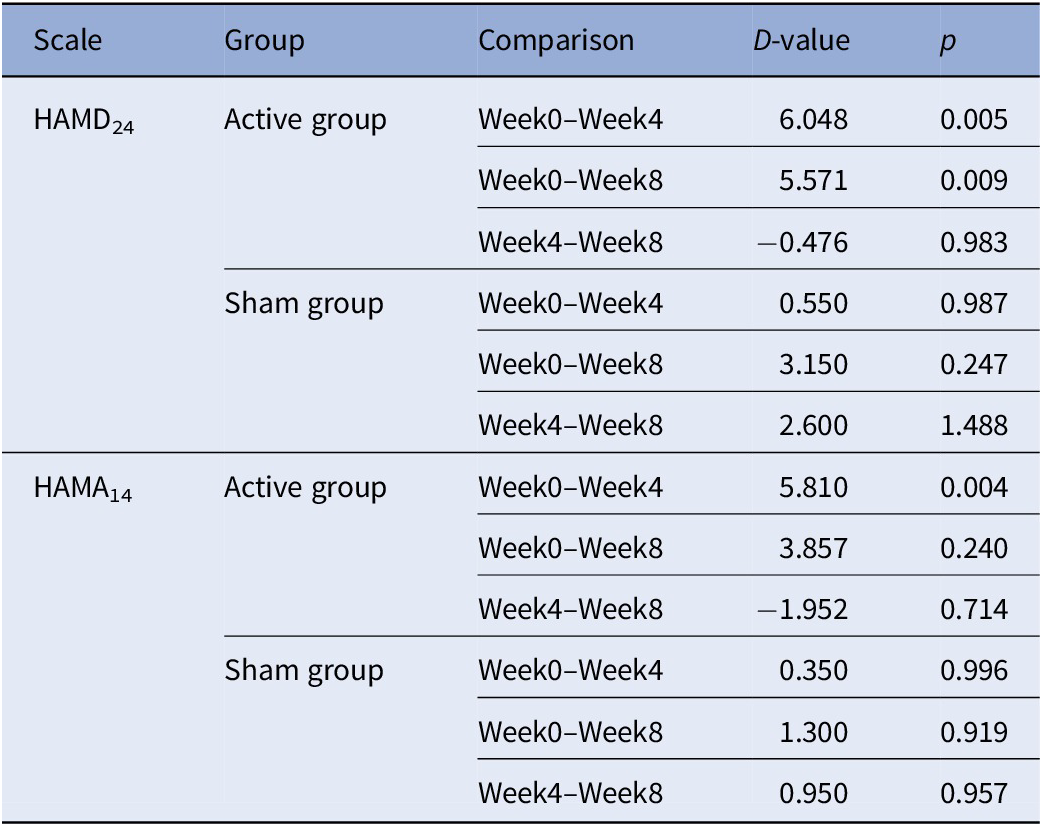
Abbreviations: HAMD24, Hamilton depression scale; HAMA14, Hamilton anxiety scale.
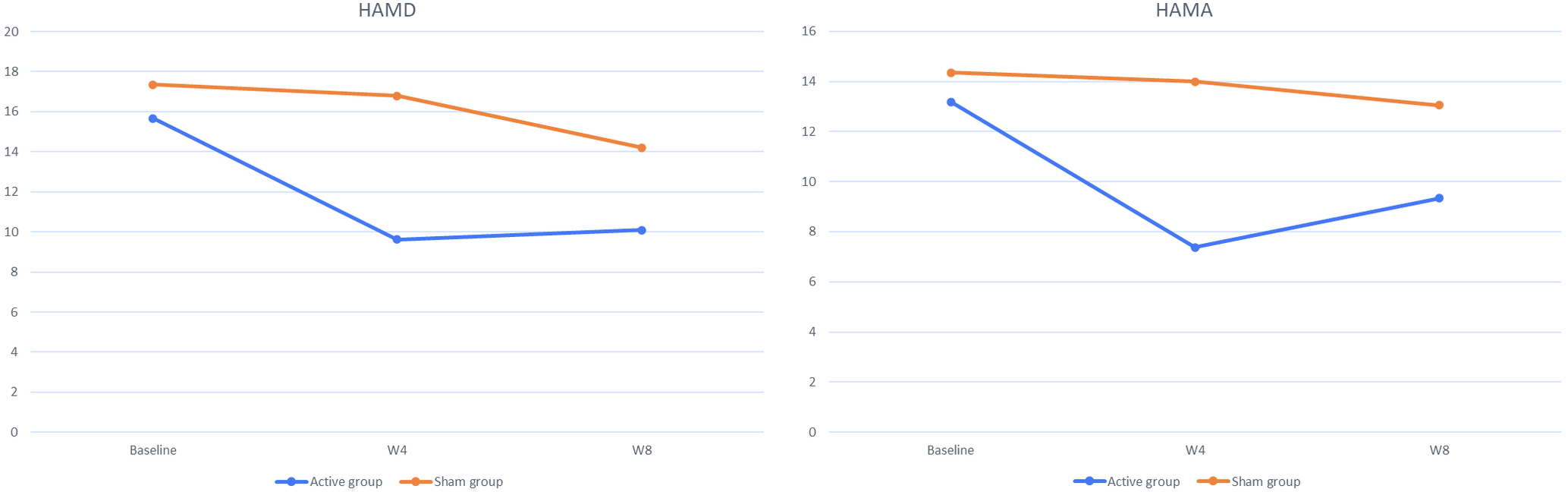
Figure 4. Changes of HAMD and HAMA scores between the active group and the sham group. Baseline, week 0; W4, week 4; W8, week 8.
Behavioral tests
In total, three behavioral tests were used in this study: the Stroop task, SST, and PRT. None of the parameters of the SST or PRT had statistically significant Time × Group interaction (Table 5). The Time × Group interaction of the Stroop task showed significance (F = 3.255, p = 0.045), but the results of the simple effect analysis indicated that there were no differences between any level of variables (Supplementary Table S1).
Table 5. Results of behavioral tests (Stroop task, stop-signal task).
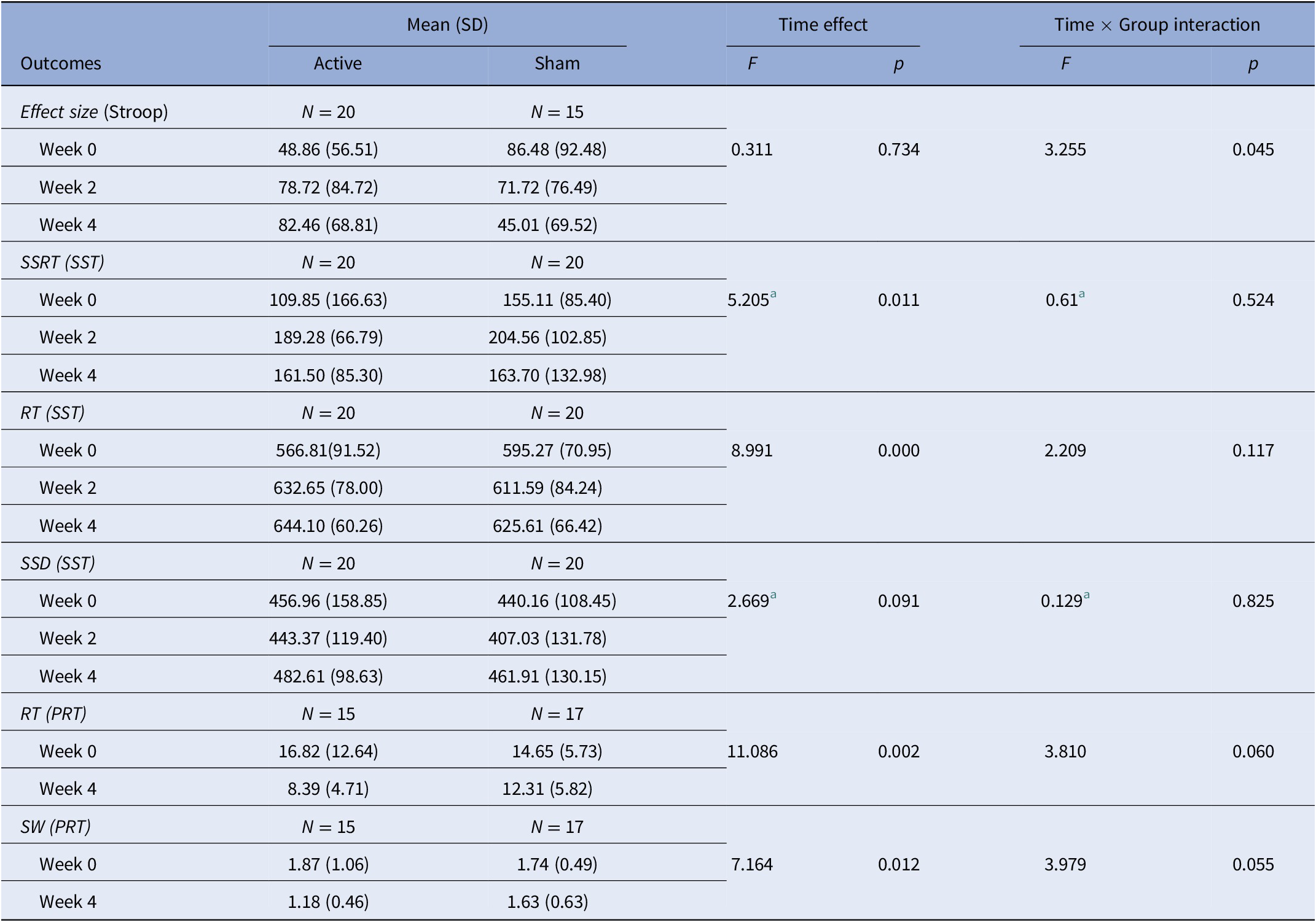
Abbreviations: PRT, probabilistic reasoning task; RT, reaction time; SSD, stop signal delay; SSRT, stop-signal reaction time; SST, stop-signal task; SW, sum weight.
a correction for nonsphericity.
Satisfaction
The TSS was administered at week 4 after the patients completed the cTBS treatment. Seven degrees were used to measure satisfaction (1, very dissatisfied; 2, dissatisfied; 3, a little dissatisfied; 4, neutral; 5, a little satisfied; 6, satisfied; 7, very satisfied). Regarding the mean degrees of treatment satisfaction, the active group received 5.06 points (SD = 0.87) and the sham group received 4.82 points (SD = 0.73), with no statistically significant difference between the two groups (t = 1.044, p = 0.302).
Adverse events and dropouts
Adverse events were reported for one patient in the active group and two patients in the sham group. One patient in the active group reported facial muscle twitching during the first two cTBS treatments. One patient in the sham group reported that his anxiety increased during the first three cTBS treatment, but gradually adapted later. One patient in the sham group reported a feeling of debility after the sixth cTBS treatment, but recovered after half an hour. The dropout rate between the two groups was not statistically significant (active group, 5/29; sham group, 2/25; χ2 = 0.289, p = 0.426).
Discussion
The results indicate that cTBS treatment over the bilateral SMA could improve OCD symptoms, but there was no statistically significant difference in treatment efficacy between cTBS and placebo treatment. This conclusion is similar to a previous study [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40]. Germaneau et al. [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40] developed the first randomized controlled trial (RCT) that treated OCD patients with cTBS, and found that cTBS treatment over the pre-SMA was not sufficient to improve OCD symptoms. In their study, the cTBS treatment (600 pulses, 70% RMT) lasted for 6 weeks, with five sessions a week. The author commented that the small number of pulses and low threshold intensity caused the failure of the cTBS treatment [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40]. Our study used a higher intensity (110% RMT) and a larger number of pulses (1,200 pulses) but also obtained negative results.
Treatment with cTBS over the SMA can significantly improve the anxiety and depression of OCD patients according to the simple effect analysis of HAMD24 and HAMA14 scores. Many studies support the opinion that theta burst stimulation over the left dorsolateral prefrontal cortex is an efficient treatment for major depressive disorder (MDD), which can significantly improve depression of MDD patients [Reference Berlim, McGirr, Rodrigues dos Santos, Tremblay and Martins41–Reference Lee, Corlier, Wilson, Tadayonnejad, Marder and Ngo43]. In 2021, a study found that cTBS over the OFC could significantly improve anxiety and depression in OCD patients [Reference Dutta, Dhyani, Garg, Tikka, Khattri and Mehta44]. Our results indicate that cTBS over the SMA may also be a significant way to improve anxiety and depression in OCD patients.
Performance on the Stroop task and SST showed no significant improvement after cTBS treatment. The hyperexcitability of the SMA is an important neural mechanism defect for OCD patients [Reference Yücel, Harrison, Wood, Fornito, Wellard and Pujol45], and extensive connections have been found between the SMA and frontal lobe [Reference Bozkurt, Yagmurlu, Middlebrooks, Karadag, Ovalioglu and Jagadeesan46]. Execution control is the characteristic function of the frontal lobe. Based on the above evidences, we supposed that the excitability of the SMA was negatively correlated with RI. The results of the Stroop task and SST indicated that RI had no significant improvement after cTBS treatment.
cTBS using the Cool-MCF-B65 butterfly coil was considered to be well tolerated by the OCD patients for the reason that no severe adverse events occurred. And most patients evaluated the cTBS treatment as somewhat satisfactory. In general, cTBS is a safe and acceptable treatment for OCD patients, which is consistent with the conclusions of previous studies [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Liu, Shao, Liao, Yang, Ma and Yang48].
SMA plays an important role in the neural mechanism of OCD [Reference Chamberlain, Fineberg, Blackwell, Robbins and Sahakian49]. The first RCT of rTMS over the SMA was conducted by Mantovani et al. [Reference Mantovani, Simpson, Fallon, Rossi and Lisanby50] in 2010, who found that the improvement of OCD symptoms in the active group was better than that in the sham group. This finding has attracted many OCD researchers’ attention to the SMA. In 2012 and 2016, Gomes et al. and Hawken et al. separately conducted the second and third RCTs of rTMS over the SMA. Both also found a significant difference in treatment efficacy between rTMS and placebo treatment [Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Gomes, Brasil-Neto, Allam and Rodrigues de Souza51]. However, three RCTs separately reported in 2016, 2018, and 2019 reached a negative result: rTMS/cTBS over pre-SMA was not an effective treatment for OCD [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Pelissolo, Harika-Germaneau, Rachid, Gaudeau-Bosma, Tanguy and BenAdhira52, Reference Arumugham, Subhasini, Madhuri, Vinay, Ravi and Sharma53]. Comparing the above six RCT studies [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Mantovani, Simpson, Fallon, Rossi and Lisanby50–Reference Arumugham, Subhasini, Madhuri, Vinay, Ravi and Sharma53] with two different conclusions, we speculate three explanations for our negative results.
Firstly, in this study, the duration and quantity of cTBS treatment may not be sufficient. After cTBS treatment (week 4), the depression and anxiety of OCD patients in the active group were significantly improved compared with that in the sham group. However, the difference between the two groups was not maintained during the follow-up period. This finding indicates that 20 sessions of cTBS can improve the emotional symptoms (depression and anxiety) of OCD patients but is not enough to improve OCD symptoms. Although a previous study already conducted 30 sessions of cTBS treatment over the SMA still obtained negative results [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40], the reference value is limited because the parameters were different from those in our study. Therefore, it is still meaningful and necessary to increase the duration and quantity of the cTBS intervention in our future study.
Secondly, the heterogeneity of the OCD patients may contribute to our negative results. Among the six RCT studies [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Mantovani, Simpson, Fallon, Rossi and Lisanby50–Reference Arumugham, Subhasini, Madhuri, Vinay, Ravi and Sharma53], there are three RCT studies (one obtained positive results and two obtained negative results) requiring the sample population must be treatment refractory OCD patients [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Pelissolo, Harika-Germaneau, Rachid, Gaudeau-Bosma, Tanguy and BenAdhira52]. And Hegde et al. [Reference Hegde, Ravi, V, Arumugham, Thirthalli and Janardhan Reddy54] suggested that rTMS over the pre-SMA may not be helpful in treatment refractory OCD patients. In our study, treatment refractory OCD patients were not identified in the sample, so there may have been an instability interference factor.
Thirdly, the stimulus parameters of cTBS in our study may not be appropriate. Stimulus parameters (including the frequency, intensity, and intervention time) have a great impact on treatment efficacy. In particular, the stimulus intensity influenced the response to cTBS treatment [Reference Sasaki, Kodama, Togashi, Shirota, Sugiyama and Tokushige55]. The RMT parameter is controversial, Germaneau et al. [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40] adopted 70% RMT, and our study adopted 110% RMT. Further studies are needed to explore the optimal parameters.
Strengths and Limitations
The current study updates knowledge on the efficacy of cTBS over the SMA for treating OCD. Compared to previous RCT studies looking at rTMS/cTBS over the SMA treatment for treating OCD [Reference Harika-Germaneau, Rachid, Chatard, Lafay-Chebassier, Solinas and Thirioux40, Reference Hawken, Dilkov, Kaludiev, Simek, Zhang and Milev47, Reference Mantovani, Simpson, Fallon, Rossi and Lisanby50–Reference Arumugham, Subhasini, Madhuri, Vinay, Ravi and Sharma53], the current study has the largest sample size.
The insufficient accuracy of neuro-navigation is an important limitation. This study adopted the 10/20 International EEG Positioning System to position the bilateral SMA, instead of a more advanced neuro-navigation system based on magnetic resonance imaging. The position deviation may lead to the deviation of cTBS stimulation, which means that not every patient’s SMA received the equal treatment.
Conclusions
The results suggested that cTBS over the bilateral SMA was a safe and tolerable treatment for OCD patients, and it could significantly improve the depression and anxiety of OCD patients, but was not enough to improve OCD symptoms in this study. Further studies are needed to improve the efficacy by increasing the duration and quantity and identifying the optimal parameters of cTBS treatments.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2022.2323.
Data Availability Statement
The data that support the findings of this study are publicly available. The corresponding author can be contacted upon reasonable request.
Acknowledgements
We are thankful to all patients and assessors (Huiling Ye, Na Lü, and Yuxin Zhou) who participated in the trial. We would also like to thank Yilin Chen for her support in the main data analysis of the probabilistic reasoning task.
Author Contributions
Conceptualization: K.W., Z.W., Q.F.; Data curation: K.W., H.H., P.L., J.C.; Formal analysis: Q.G., K.W.; Funding acquisition: Z.W., Q.F.; Methodology: K.W., H.H., P.L., J.C., J.Z., Z.W., Q.F.; Project administration: Q.F.; Resources: H.H., P.L., J.C., J.Z., Z.W., Q.F.; Writing—original draft: Q.G.; Writing—review and editing: Q.G., K.W., Q.F.
Financial Support
This work was supported by the National Natural Science Foundation of China [No. 81771460, 81671340]; Shanghai Jiao Tong University “star of Jiao Tong University” medical engineering cross research fund key project [No. YG2021ZD28]; Grant from Shanghai Hospital Development Center [No. SHDC12018115]; Key Program of Shanghai Mental Health Center [No.2019-zd02]; and Key Laboratory of Psychotic Disorders [No.13dz2260500].
Conflicts of Interest
The authors declare none.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.












Comments
No Comments have been published for this article.