Gastric cancer is among the top ten causes of cancer-related deaths for both men and women in many countries, especially in Asia. A total gastrectomy is a major abdominal surgical procedure, which is frequently undertaken for malignant gastric diseases and usually causes much stress to patients. For most gastrectomised patients with gastric diseases, preoperative malnutrition often occurs and adequate post-operative oral intake is achieved late(Reference Delany, Demetriou and Teh1, Reference Bragelmann, Armbrecht and Rosemeyer2). Enteral and parenteral nutritional support is necessary for such patients. Although studies have shown that early enteral feeding seems to be well tolerated by patients(Reference Braga, Gianotti and Andrea3), most surgeons use the parenteral route to administer nutrients before and after a gastrectomy due to preoperative obstruction or post-operative ileus. The optimal parenteral formulation for patients undergoing a total gastrectomy is still being investigated.
Glutamine (Gln) is an abundant free amino acid in the circulation. It has traditionally been considered a non-essential amino acid, but is now known to be conditionally essential following critical illness and injury(Reference Parry-Billings, Evans and Calder4, Reference Wilmore5). Previous studies demonstrated numerous benefits of Gln administration in the experimental models of serious metabolic stress, including attenuated proinflammatory cytokine expression, improved gut barrier function and enhanced immune cell function(Reference Kudsk, Wu and Fukatsu6–Reference Yeh, Hsu and Yeh10). A meta-analysis of several studies reviewed by Novak et al. (Reference Novak, Heyland and Avenell11) concluded that in surgical patients, Gln supplementation demonstrated a trend towards a benefit in reducing infectious complications and the length of the hospital stay. The mortality benefit was even more striking in the trials that used Gln in the parenteral route(Reference Novak, Heyland and Avenell11). However, the efficacy of parenteral Gln administration on major surgery as total gastrectomy has not been evaluated.
Major surgery has been shown to result in an inflammatory reaction. Inflammatory mediators including adhesion molecules and chemokines are expressed to recruit inflammatory cells to the site of injury(Reference Driscoll, Carter and Hassenbein12–Reference Matsukawa, Hogaboam and Lukacs15). Besides, surgery is known to impair immune response(Reference Lennard, Shenton and Borzotta16), and the Th2 cytokine is associated with operation-induced immunosuppression(Reference Ayala, Deol and Lehman17). Previous studies performed by our laboratory showed that Gln supplementation reduced adhesion molecule expression and neutrophil infiltration into tissues(Reference Yeh, Hsu and Yeh10); besides, a more balanced Th1/Th2 response was found during sepsis when Gln was administered(Reference Yeh, Hsu and Yeh18). However, the effect of Gln on leucocyte adhesion molecule expression and inflammatory cell recruitment after major surgery has not clearly been elucidated. We hypothesised that parenterally infused Gln would reduce leucocyte adhesion molecule expression and release of chemokines responsible for inflammatory cell recruitment after surgery. β2 Integrins (CD18) are surface proteins expressed on leucocytes and are important in the adhesion of leucocytes to activated endothelium(Reference Springer19). Integrins are reported to be good markers of leucocyte activation in the inflammatory mechanism(Reference Smith, Joneckis and Parise20). Cytokine-induced neutrophil chemoattractant (CINC)-1 and macrophage inflammatory protein (MIP)-2 play important roles in mediating neutrophil recruitment to the site of injury(Reference Takano and Nakagawa13, Reference Greenberger, Strieter and Kunkel14). Monocyte chemotactic protein (MCP)-1 is a chemotactic and activating factor for mononuclear phagocytes. MCP-1 is also involved in recruiting peripheral blood leucocytes to the peritoneal cavity(Reference Matsukawa, Hogaboam and Lukacs15). In order to determine the possible roles of Gln in modulating inflammatory mediators in a surgical condition, the levels of these adhesion molecules and chemokines were measured. Also, the regulatory role of Gln on the Th1/Th2 response after major surgery was evaluated in the present study.
Materials and methods
Animals
Male 7-week-old Wistar rats weighing 180–210 g at the beginning of the experiment were used. All rats were housed in temperature- and humidity-controlled rooms and were allowed free access to standard rat chow for 7 d before the experiment. The present study was approved by the National Taiwan University Hospital Animal Care Committee. The care of the animals was in full compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1985), as reviewed by the Animal Care and Use Committee of the National Taiwan University Hospital.
Study protocol and operative procedures
Rats were randomly assigned to one control group and two experimental groups, with each group containing twenty rats. The average weights among the groups were adjusted to be as similar as possible. After overnight starvation, all rats (including the control and experimental groups) were anesthetised with intraperitoneal pentobarbital sodium (50 mg/kg), and the right internal jugular vein was cannulated with a Silastic catheter (Dow Corning, Midland, MI, USA) under sterile conditions. At the same time, a total gastrectomy was performed in the experimental groups, while the control group received a sham operation (Sham) group. Sham was performed by a laparotomy with a 4 cm midline incision. The stomach was pulled out and placed back, and then the cavity was closed in two layers. In experimental groups, a total gastrectomy was carried out through a 4 cm midline incision, as described by Ohta et al. (Reference Ohta, Motohashi and Sakai21). Briefly, the stomachs of the rats were removed after ligation of most of the blood vessels around the stomach, which supplied blood, and an end-to-side anastomosis was carried out between the cut edge of the oesophagus and the upper jejunum, 5 cm distal from the ligament of Treitz. Finally, the cavity was sutured closed in two layers. Half of the rats in each group (n 10) were killed 1 or 3 d (days 2 and 4 of TPN) after the operation. TPN was begun immediately after the operation and was maintained for 2 or 4 d in the different groups according to the killing schedule of the rats. All basal TPN solutions were isonitrogenous (6·82 mg/ml) and identical in nutrient composition except for the difference in the amino acid content. The control group and one of the experimental groups were infused with Conv TPN group. The other experimental group was supplemented with Gln group (Dipeptiven; Fresenius Kabi, Bad Homburg, Germany), which provided 25 % of the total amino acid nitrogen in the TPN solution. The amount of Gln administered was 2·97 g/kg body weight (BW)/d. According to the BW of individual rat, it would be 0·53–0·62 g Gln/rat. The TPN provided 1129·68 kJ/kg BW/d (270 kcal/kg BW/d), and the energy (kJ):N (g) ratio was 493·71:1. The energy density was 3·89 kJ/ml (0·93 kcal/ml). The energy distribution of the TPN solutions in the groups was 58 % from glucose, 22 % from protein and 20 % from fat (Lipovenous 10 %; Fresenius AG; Table 1). The TPN solution was refilled daily and infused for 24 h at room temperature. Two millilitres per hour were administered on the first day, and then the rats received (200·832–238·488 kJ/d (48–57 kcal/d)) according to individual BW. The infusion rate was maintained with a Terufusion pump (model STC-503; Terumo, Tokyo, Japan). The TPN solution without fat was prepared every other day in a laminar flow hood, and the fat emulsion was added daily just before use.
Table 1 Composition of the total parenteral nutrition solution (ml/l)
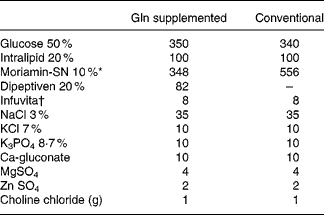
Gln, glutamine.
* From Chinese Pharmaceuticals, Taipei, Taiwan. Contents (mg/dl): Leu 1250, Ile 560, Lys acetate 1240, Met 350, Phe 935, Thr 650, Trp 130, Val 450, Ala 620, Arg 790, Asp 380, Cys 100, Glu 650, His 600, Pro 330, Ser 220, Tyr 35 and aminoacetic acid (Gly) 1570.
† From Yu-Liang Pharmaceuticals, Taoyuan, Taiwan. Contents per ml: ascorbic acid, 20 mg; vitamin A, 660 IU (198 μg retinol); ergocalciferol, 40 IU (1 μg); thiamine HCl, 0·6 mg; riboflavin, 0·72 mg; niacinamide, 8 mg; pyridoxine HCl, 0·8 mg; d-panthenol, 3 mg; dl-α-tocopheryl acetate, 2 mg; biotin, 12 μg; folic acid, 80 μg cyanocobalamin, 1 μg.
Measurements and analytical procedures
Rats in the respective groups were killed 1 or 3 d after surgery. Animals were anesthetised with intraperitoneal pentobarbital sodium (50 mg/kg BW). A middle abdominal incision was made, and 10 ml of PBS was intraperitoneally injected to elute the peritoneal cells. After harvesting the peritoneal lavage fluid, rats were killed by drawing arterial blood from the aorta. Blood samples were collected in tubes containing heparin. Fresh whole blood was used for the flow cytometric analysis.
Analysis of CD11a/CD18 distributions in lymphocytes and polymorphonuclear neutrophil expressions of CD11b/CD18
Hundred microlitres of fresh blood were incubated with 10 μl of fluorescein isothiocyanate-conjugated monoclonal mouse anti-rat CD11a and phycoerythrin-conjugated mouse anti-rat CD18 (Serotec, Oxford, UK) antibodies for 15 min at 4°C. Afterwards, red blood cells were lysed with lysing buffer (Serotec). The proportions of CD11a/CD18 expressed on lymphocytes were analysed by flow cytometry (Coulter, Miami, FL, USA). Fluorescence data were collected, and the results are presented as a percentage of CD11a-presenting cells in 1 × 105 lymphocytes. To determine CD11b/CD18 expressions on polymorphonuclear neutrophils (PMN), fluorescein isothiocyanate-conjugated mouse monoclonal anti-rat CD11b and phycoerythrin-conjugated mouse anti-rat CD18 (Serotec) antibodies were added to 100 μl of the PMN suspension. Fluorescence data were collected on 1 × 105 viable cells and also analysed by flow cytometry (Coulter). The results are presented as a percentage of CD11b-presenting cells in 1 × 105 PMN. Lymphocytes and PMN were gated on the basis of their forward- and side-scatter profiles and analysed for the expressions of CD11a/CD18 and CD11b/CD18, respectively.
Analysis of lymphocyte IL-4 and interferon-γ expressions
Populations of lymphocyte IL-4 and interferon (IFN)-γ expressions in fresh blood were analysed by flow cytometry (Coulter). After killing the rats, 50 μl of fresh blood was immediately incubated with Leucoperm (Serotec) to fix and penetrate the leucocytes, and then 10 μl of fluorescein-conjugated mouse monoclonal anti-rat IFN-γ (Serotec) and 5 μl phycoerythrin-conjugated mouse monoclonal anti-rat IL-4 (Serotec) antibodies were incubated together for 30 min. Lymphocytes capable of IL-4 and IFN-γ expressions were assessed using dual intracellular cytokine staining and flow cytometry (Coulter). Lymphocytes were gated on the basis of their forward- and side-scatter profiles. The results are presented as a percentage of cytokine-producing cells in 1 × 105 lymphocytes.
Monocyte chemotactic protein-1, macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant-1 levels in peritoneal lavage fluid
MCP-1, MIP-2 and CINC-1 concentrations were measured by a quantitative sandwich enzyme immunoassay kit (BioSource, Camarillo, CA, USA; Amersham Pharmacia Biotech, Amersham, Buckinghamshire, UK). A monoclonal antibody specific for rat MCP-1, MIP-2 and CINC-1 was precoated onto a microplate. All procedures followed the manufacturer's instruction. The detection limits were 8, 1·5 and 1·3 pg/ml for MCP-1, MIP-2 and CINC-1, respectively.
Statistical analysis
Data are expressed as the means and standard deviations. Differences among groups with different diets were analysed by one-way ANOVA using Duncan's test. The paired t test was used to compare the difference between 1 and 3 d post-operatively in the same group. P < 0·05 was considered statistically significant.
Results
There were no differences in the initial BW and BW after TPN administration for 3 d among the groups (data not shown).
Lymphocyte CD11a/CD18 and polymorphonuclear neutrophils CD11b/CD18 expressions
There were no differences in lymphocyte CD11a/CD18 expressions among the three groups on any post-operative days. However, Conv group presented lower CD11a/CD18 expression on post-operative day 3 than day 1. The Sham group had the lowest CD11b/CD18 expressions among the three groups on post-operative day 1. The Conv group had higher CD11b/CD18 expressions than the other two groups on post-operative days 1 and 3. The CD11b/CD18 expressions in the Conv and Gln groups on post-operative day 3 were lower than those on post-operative day 1 (Table 2).
Table 2 Lymphocyte CD11a/CD18 and polymorphonuclear neutrophils CD11b/CD18 expressions among the sham and experimental groups 1 and 3 d after the surgery
(Mean values and standard deviations for ten rats in each group)
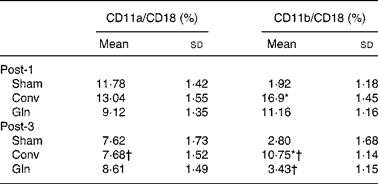
Post-1, experimental group 1 d after surgery; Sham, sham operation; Conv, conventional; Gln, glutamine; Post-3, experimental group 3 d after surgery.
* Mean values were significantly different from the other groups on the same post-operative day (P < 0·01).
† Mean values were significantly different from the same group on post-operative day 1 (P < 0·05).
Lymphocyte interferon-γ and IL-4 distributions
The Gln group had lower IFN-γ and IL-4 distributions than the other two groups on post-operative day 3. The percentage of IL-4 in the Gln group was lower on day 3 than that on day 1 (Table 3).
Table 3 Distributions of lymphocyte interferon (IFN)-γ and IL-4 expressions among the sham and experimental groups 1 and 3 d after surgery
(Mean values and standard deviations for ten rats in each group)
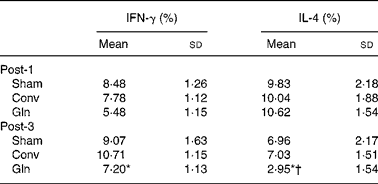
Post-1, experimental group 1 d after surgery; Sham, sham operation; Conv, conventional; Gln, glutamine; Post-3, experimental group 3 d after surgery.
* Mean values were significantly different from the Sham and Conv groups on the same post-operative day (P < 0·05).
† Mean values were significantly different from the Gln group on post-operative day 1 (P = 0·006).
Monocyte chemotactic protein-1, macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant-1 levels in peritoneal lavage fluid
MCP-1 concentrations in the Gln group were higher than those of the Sham and Conv groups; the MIP-2 levels were also higher in the Gln group than that of the Conv group, but did not differ from that of the Sham group on post-operative day 1. The CINC-1 and MIP-2 levels in peritoneal lavage fluid were significantly higher in the Conv group than those in the Sham and Gln groups on post-operative day 3. The Conv group had higher MIP-2 on post-operative day 3 than that on day 1. The concentration of MCP-1 was significantly lower in the Gln group on day 3 than that on day 1 post-operatively (Table 4).
Table 4 Concentrations of cytokine-induced neutrophil chemoattractant (CINC)-1, monocyte chemotactic protein (MCP)-1 and macrophage inflammatory protein (MIP)-2 in peritoneal lavage fluid among the control group received a sham operation (Sham) and experimental groups 1 and 3 d after surgery
(Mean values and standard deviations for ten rats in each group)
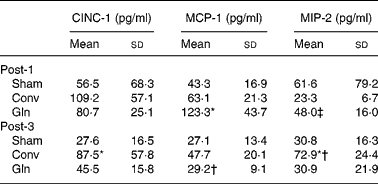
Post-1, experimental group 1 d after surgery; Conv, conventional; Gln, glutamine; Post-3, experimental group 3 d after surgery.
* Mean values were significantly different from the other two groups on the same post-operative day (P < 0·01).
† Mean values were significantly different from the same group on post-operative day 1 (P = 0·006).
‡ Mean values were significantly different from the Conv group on post-operative day 1 (P = 0·011).
Discussion
The present study investigated the effect of parenteral Gln supplementation on leucocyte integrin expressions and immune cell recruitment after major surgery. Also, a gastrectomy-induced change in inflammatory mediators was evaluated. We included a Sham group in the present study. Because the Sham group also underwent jugular cannulation and laparotomy, the only difference between the Sham and Conv groups was the gastrectomy. Therefore, the difference in immune reaction in response to gastrectomy can be evaluated. We found that a gastrectomy induced early PMN CD11b/CD18 expressions on day 1, and more neutrophils were recruited to the site of injury 3 d after surgery. This result indicated that although rats in the Sham group also underwent operation, a higher immunological response to injury was observed when major surgery as total gastrectomy was performed in the present study.
Adhesion molecule expressions are upregulated by proinflammatory cytokines and NF-κB(Reference Jersmann, Hii and Ferrante22). CD11a/CD18 are exclusively expressed on leucocytes, while CD11b/CD18 are abundant in PMN(Reference Henderson, Lim and Tessier23). Excessive expression of these integrins induces an inflammatory response and tissue injury(Reference Henderson, Lim and Tessier23, Reference Ulbrich, Eriksson and Lindbom24). We did not observe difference in CD11a/CD18 among the groups; however, we found that compared with the Conv group, parenteral Gln infusion blunted the increase in PMN CD11b/CD18 on day 1 and restored it to the Sham levels on day 3 after surgery. CD11b/CD18 is reported to be a good marker of PMN activation in the inflammatory mechanism(Reference Smith, Joneckis and Parise20). The study by Brunialti et al. (Reference Brunialti, Martins and de Carvalho25) showed that the expressions of CD11b/CD18 were significantly increased in patients with septic shock. Previous literature has revealed that Gln can inhibit inflammatory cytokine production and reduce nuclear binding/activation of NF-κB(Reference Singleton, Beckey and Wischmeyer26), and may, consequently, downregulate the expression of adhesion molecules in a surgical condition. The present result is consistent with a previous report which showed that Gln supplementation decreased neutrophil CD11b/CD18 expressions and plasma intracellular adhesion molecule-1 levels in a septic condition(Reference Yeh, Hsu and Yeh10).
Cytokines are peptides produced by the cells of the immune system that act as mediators of the immune response. Cytokine profiles are related to the severity of different types of infection. The cytokine profiles are determined by two functional subsets of T-lymphocytes: Th1 and Th2. Th1 cytokines, including IL-2 and IFN-γ, enhance cell-mediated immunity, whereas Th2 cytokines, including IL-4 and IL-10, enhance humoral immunity. The effects of Th1 and Th2 lymphocytes are counter-regulatory(Reference DiPiro27). Studies have shown that overexpression of the Th2 cytokine, IL-4, is responsible for the immunosuppression associated with surgery(Reference Ayala, Deol and Lehman17). According to our previous observations, plasma IL-2, IL-4 and IFN-γ are not detectable in sepsis(Reference Yeh, Yeh and Lin28); therefore, we directly measured lymphocyte IFN-γ and IL-4 production to investigate the effects of Gln administration on Th1- and Th2-type responses after an operation. Yaqoob & Calder(Reference Yaqoob and Calder29) found that Th1 cytokine production depends on the concentration of Gln present in the culture medium. A previous study performed by our laboratory showed that Gln supplementation enhanced IFN-γ, suppressed IL-4 production and reversed the predominant Th2 response to a more balanced Th1/Th2 response during sepsis(Reference Yeh, Hsu and Yeh18). In the present study, we observed that compared with the Conv group, both Th1 (IFN-γ) and Th2 cytokines (IL-4) in the Gln group were suppressed and the extent of IL-4 decrement was greater than that of IFN-γ. Since overexpression of IL-4 is associated with operation-induced immunosuppression(Reference Ayala, Deol and Lehman17), determining whether a lower IL-4 distribution observed after Gln feeding has a favourable effect with gastrectomy requires further investigation.
CINC-1, MCP-1 and MIP-2 are the chemokines that exhibit activities associated with inflammatory, immune and tissue repair processes. Chemokines play important roles in mediating inflammatory cell recruitment in tissues in response to infection and injury. Standiford et al. (Reference Standiford, Kunkel and Greenberger30), using a murine model of acute bacterial pneumonia, found that depletion of MIP-2 is associated with higher early mortality(Reference Standiford, Kunkel and Greenberger30). A study performed by Matsukawa et al. (Reference Matsukawa, Hogaboam and Lukacs15) found that endogenous MCP-1 protected mice in a model of septic peritonitis. However, a previous study showed that serum MCP-1 levels are raised in septic patients and are even higher in those with severe sepsis(Reference Bossink, Paemen and Jansen31). Also, MCP-1 and CINC-1 were significantly increased in a severe inflammatory reaction as found in thermal injury and caecal ligation and perforation in rats(Reference Gauglitz, Song and Herndon32, Reference Miyakawa, Kira and Okuda33). The discrepancies among the studies may have resulted from the time required to take the measurement and the duration and location of these mediators exist in biological fluids. In the present study, we found that compared with the Conv group, the Gln group had higher MCP-1 and MIP-2 levels as soon as day 1 after surgery, indicating that Gln supplementation caused larger neutrophil and monocyte recruitment at the early stage of surgery. The chemokines were restored to the levels comparable with the Sham group at a later phase of surgery, whereas it was not with the Conv group. Gln provides a vital fuel source for the rapidly dividing leucocytes and macrophages(Reference Newsholme, Crabtree and Ardawi34). It is possible that whenever Gln is available for use, neutrophils and macrophages are activated rapidly in response to the injury as observed on post-operative day 1 and returned to the basal levels afterwards.
In conclusion, the present study showed that administration of TPN-containing Gln affected several aspects of the immune response that differed from those seen with Conv TPN. We found that, compared with the Conv group, Gln supplementation attenuated PMN integrin expression as soon as day 1 and limited lymphocyte cytokine release on day 3. In addition, Gln-enriched parenteral nutrition caused larger neutrophil and monocyte recruitment at the early stage, and this was restored to Sham levels at a later phase of surgery, whereas it was not with the Conv parenteral nutrition. This is likely that Gln-enriched parenteral nutrition induced an earlier more intensive and rapid immune response to injury than the Conv parenteral nutrition.
Acknowledgements
The funding of the present study is totally supported by research grant NSC95-2314-B-002-157 from the National Science Council, Republic of China. No financial support from any other sources. The authors are not the employees or consultants associated with any commercial companies and there is no conflict of interest in the present study. M.-T. L. contributed to the concept of the study and prepared the manuscript; S.-Y. C., S.-S. T., M.-Y. W. and M.-H. W. did the data analysis and interpreted the data; and S.-L. Y. designed the study, prepared and revised the manuscript. All authors have read and approved the final submitted manuscript.






