During psychotic episodes people with schizophrenia often have difficulties with awareness of their own actions and recognition of other individuals’ actions, evident from beliefs of alien control (Reference Daprati, Franck and GeorgieffDaprati et al, 1997). Such difficulties, like echopraxia (see e.g. Reference Chapman and McGhieChapman & McGhie, 1964) and activity delusions (patients feel that they influence others to act; Reference Stanghellini and Rossi MontiStanghellini & Rossi Monti, 1993), could be related to dysfunction of the mirror neuron system, which matches executed and observed motor actions (Reference Rizzolatti, Fadiga and MatelliRizzolatti et al, 1996). The core system comprises the inferior frontal gyrus (Broca's region in the left hemisphere), the inferior parietal lobule and the primary motor cortex.
Here we tested whether people with schizophrenia would show abnormalities in the motor cortex part of their mirror neuron system during observation and execution of finger movements. Earlier studies have indicated abnormal motor cortex function in patients with schizophrenia compared with healthy participants (reviewed by Reference Spence and RobbinsSpence, 2003). We applied a well-established method to monitor motor cortex ∼20 Hz magnetoencephalographic (MEG) activity (Reference Hari, Forss and AvikainenHari et al, 1998). In response to electrical median nerve stimuli, this ∼20 Hz rhythm is first transiently and bilaterally suppressed, and then 200–400 ms later is strongly enhanced (Fig. 1a), probably reflecting cortical inhibition (Reference Salmelin and HariSalmelin & Hari, 1994; Reference Chen, Corwell and HallettChen et al, 1999). Consequently, the size of the ‘rebound’ reflects the functional state of the primary motor cortex; for example the rebound is abolished when the person manipulates an object (Reference Hari, Forss and AvikainenHari et al, 1998).
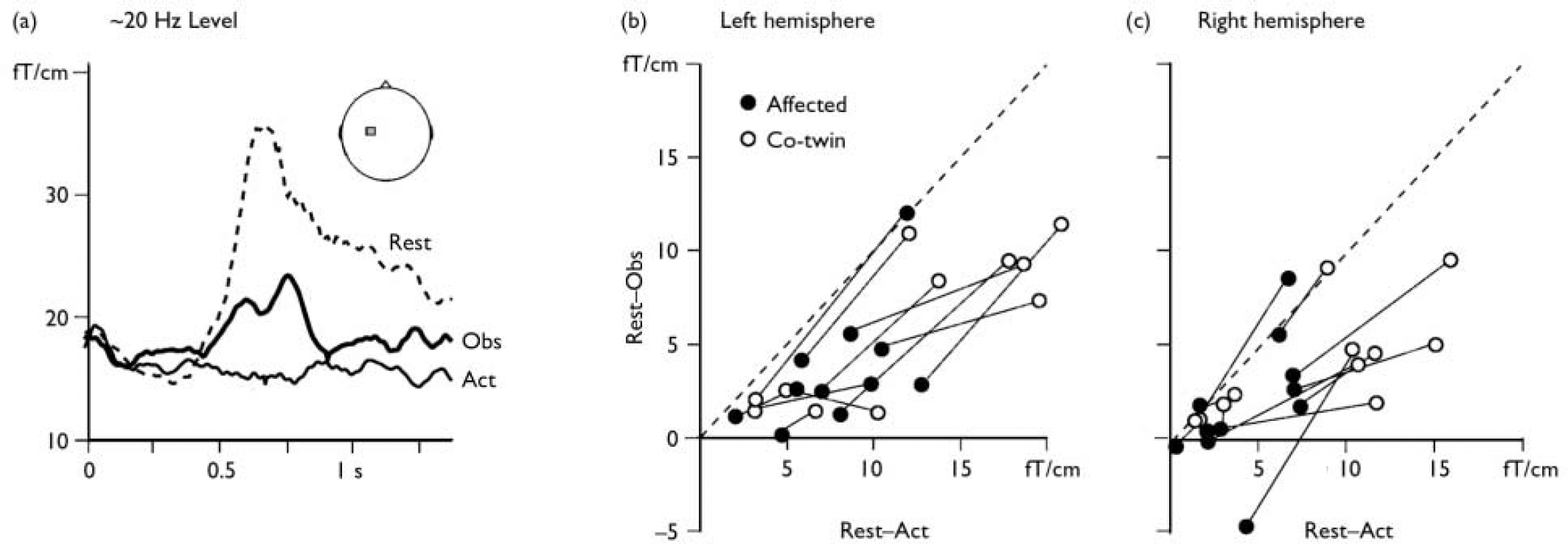
Fig. 1 (a) The level of the ∼20 Hz rhythm recorded from the left motor cortex (see inset) in a representative participant after right median nerve stimulation (0 ms). In the ‘rest’ condition the ∼20 Hz rhythm is blocked for about 400 ms after which it shows a ‘rebound’ enhancement as a signature of motor cortex stabilisation. In the ‘action’ condition (Act) the motor cortex is active during the participant's own finger movements and no rebound is observed. In the ‘observation’ condition (Obs) the rebound is of lower amplitude than during rest, indicating partial activation of the motor cortex during action observation. (b) (c) The ∼20 Hz reactivity in all participants, quantified as the difference between rest and observation and displayed as a function of the difference between rest and action. In accordance with earlier studies on healthy individuals, nearly all data points are to the right of the diagonal (dashed line), meaning that the suppression of the ∼20 Hz rebound is less marked in the observation than the action condition. In most cases (see text for statistics), values for the twin with manifest disease (•) are lower (on both axes) than for the non-affected twin (○, data for twin pairs connected with lines), indicating lower motor cortex reactivity during both action observation and action execution.
We compared motor cortex reactivity within schizophrenia-discordant twin pairs, thereby controlling for a portion of the genetic influences on brain physiology. Some of the non-affected co-twins resembled their twin in possessing features of schizotypal personality, which further increased the suitability of this comparison for pinpointing brain abnormalities related to manifest schizophrenia.
METHOD
Participants were derived from a randomly selected subset of 335 schizophrenia-discordant twin pairs, identified (for another study) in a cohort of all 9562 pairs of same-sex twins born in Finland between 1940 and 1957 (Reference Cannon, Huttunen and LönnqvistCannon et al, 2000). The Structured Clinical Interview for DSM–III–R Axis I Disorders (patient or non-patient edition; Reference Spitzer, Williams and GibbonSpitzer et al, 1989) served for verification of the diagnoses in all participants. Interviewers were masked to zygosity and diagnostic status. A diagnosis of schizoaffective disorder, affective type in a twin with manifest disease or a psychotic disorder diagnosis in a non-affected twin led to exclusion of that twin pair (Reference Cannon, Huttunen and LönnqvistCannon et al, 2000). Eleven twin pairs (aged 49–64 years, mean age 54.4 years, s.d.=4.8, five monozygotic and six dizygotic) participated in the study after informed consent and ethics committee approval. All the participants with manifest disease were out-patients in stable clinical condition (further details in a data supplement to the online version of this report). For all pairs, zygosity was determined by DNA analysis (for details see Reference Cannon, Huttunen and LönnqvistCannon et al, 2000).
Neuromagnetic data were acquired during three experimental conditions: (a) rest – the participants rested in a relaxed state; (b) observation – the participants observed the experimenter manipulate a small object with her right-hand fingers; (c) action – the participants manipulated the small object with their right-hand fingers without seeing their own hand.
The left and right median nerves were stimulated alternately at the wrists (0.2 ms constant current pulses at intensities exceeding the motor threshold), once every 1.5 s. Signals from 204 planar gradiometers of a helmet-shaped whole-scalp neuromagnetometer (Vectorview, Neuromag, Helsinki, Finland) were analysed. Stimulus-related changes in the level of the ∼20 Hz rhythm were quantified by first filtering signals through 14–30 Hz, then rectifying them and finally averaging them time-locked to the median nerve stimuli (approximately 100 signals averaged per condition). The strength of the rebound in each condition was then quantified (from the MEG channel with the strongest rebound suppression during action observation) as the mean level from 300 ms to 1300 ms after stimuli (Reference Salmelin and HariSalmelin & Hari, 1994).
RESULTS
Figure 1a shows the ∼20 Hz motor cortex level for one participant. The rebound, peaking at 700 ms, was abolished during object manipulation and significantly suppressed during observation, as shown previously (Schnitzler et al, 1997; Reference Hari, Forss and AvikainenHari et al, 1998). Figure 1b and 1c illustrate the ∼20 Hz reactivity in all twin pairs. For both hemispheres and for both observation and action conditions, the twins with schizophrenia showed weaker reactivity of the ∼20 Hz rhythm than their non-affected co-twins (binomial test for n=11 pairs: rest–action P=0.033 and rest–observation NS in left hemisphere; rest–action P=0.006 and rest–observation P=0.006 in right hemisphere).
The rest levels of the ∼20 Hz rhythm did not differ between affected and non-affected co-twins, nor was there any statistically significant difference between the groups in the strengths of cortical responses peaking in the primary somatosensory cortex 20 ms and 35 ms after median nerve stimuli (t-test, P≥0.2). The ∼20 Hz reactivity and the dosages of antipsychotic medication were not correlated (Pearson's r=0.43, P=0.19) (further details in a data supplement to the online version of this report).
DISCUSSION
The ∼20 Hz motor cortex rhythm in the twins with schizophrenia was systematically less reactive than in their non-affected co-twins, both during action observation and execution, with no sign of an additional mirror neuron system abnormality. Since the observed effects were not correlated with medication, we attribute them to the disease itself. The similar somatosensory cortical responses and the comparable resting levels of the rhythmic activity in non-affected and affected participants render implausible any general dysfunctioning of cortical responsiveness in the patient group. The weakened ∼20 Hz reactivity, specific to clinically manifest disease in the affected twins, could be related to a deficit in motor cognition affecting both the command and the experience of action, both important for delusions of control (Reference FrithFrith, 2005). Further studies should test more extensively the functionality of motor and sensory mirroring in people with schizophrenia, focusing on subgroups displaying special abnormalities in the experience of action.
Acknowledgements
Supported by the Academy of Finland (National Centers of Excellence Programme 2006–2011), Sigrid Jusélius Foundation, and the National Institute of Mental Health, USA (MH52857). We thank Ulla Mustonen for help in recruiting the participants.




eLetters
No eLetters have been published for this article.