Introduction
There is growing interest in factors contributing to the genesis of eating disorders (EDs), supported by the great impact on patients’ quality file and burden [Reference Ágh, Kovács, Supina, Pawaskar, Herman and Vokó1]. The etiology of EDs is multifactorial due to the presence of predisposing, precipitating, and perpetuating factors that allow the onset and maintenance of the disorders [Reference Culbert, Racine and Klump2].
Recently, research has focused on the microbiota [Reference Ursell, Metcalf, Parfrey and Knight3] and its composition, with 100 trillion microbial cells residing in different human body areas [Reference Lederberg and McCray4]. The two phyla “Bacteroidetes” and “Firmicutes” represent about 90% of the bacterial populations identified, whereas the remaining 10% is mainly composed of Actinobacteria and Proteobacteria [Reference Rinninella, Raoul, Cintoni, Franceschi, Miggiano and Gasbarrini5,Reference Arumugam, Raes, Pelletier, Le Paslier, Yamada and Mende6]. The phylum Firmicutes is represented by more than 200 different genera: Lactobacillus, Bacillus, Enterococcus, Ruminococcus, and Clostridium. Bacteroidetes essentially consists of two predominant genera, the Bacteroides and the Prevotella. Bifidobacterium belongs to the Actinobacteria. A preponderance of Lactobacilli has been detected in the area of the stomach and duodenum and Streptococci at the jejunal level, whereas the ileocolic regions show a profound heterogeneity of bacterial species, including Lactobacilli, Escherichia coli, and other Enterobacteria, Enterococci faecalis, Bacteroides, Bifidobacteria, Peptococci, Petostreptococci, Ruminococci, and Clostridia [Reference Rinninella, Raoul, Cintoni, Franceschi, Miggiano and Gasbarrini5]. The composition of microbiota is not stable during life: presents rapid changes from early childhood, stabilizes in adulthood, and then deteriorates in old age [Reference Yatsunenko, Rey, Manary, Trehan, Dominguez-Bello and Contreras7,Reference Falony, Joossens, Vieira-Silva, Wang, Darzi and Faust8]. Different factors contribute to both lifetime variation and stability of the gut microbiota (i.e., age, sex, ethnicity, geographical location, environment, climate, delivery mode, breastfeeding, weaning, body mass index (BMI), exercise, smoking, alcohol, drugs, and diet) [Reference Jernberg, Löfmark, Edlund and Jansson9,Reference Dethlefsen and Relman10].
Evidence highlighted that the alteration in the normal microbial composition, called dysbiosis, may contribute to the development of EDs when associated with a specific genetic susceptibility [11–16], and several putative mechanisms have already been identified. Furthermore, nutritional rehabilitation represents one of the essential focuses for EDs, and the intake of macronutrients can significantly affect the composition of microbiota [Reference Scott, Gratz, Sheridan, Flint and Duncan17,Reference Singh, Chang, Yan, Lee, Ucmak and Wong18], reducing dysbiosis. To date, therapeutic strategies that can correct the microbiota are represented by fecal microbiota transplantation (FMT) [Reference Wouw, Schellekens, Dinan and Cryan19], but the use of prebiotics and probiotics to restore microbiota alterations has also been proposed [Reference Requena, Martínez-Cuesta and Peláez20,Reference Larroya-García, Navas-Carrillo and Orenes-Piñero21].
A recent research and systematic review demonstrated that gut dysbiosis may represent hallmarks in AN [Reference Di Lodovico, Mondot, Doré, Mack, Hanachi and Gorwood22] suggesting the potentially interesting therapeutic targets.
Nevertheless, there are no review focusing on the other ED as bulimia nervosa (BN) or binge eating. Thus, in order to fill this gap, we aimed to update and critically analyze the existing literature on the possible role of altered microbiota in the etiopathogenesis and treatment of patients with EDs.
Methods
This systematic review was done according to Participants Intervention Comparator and Outcome (PICO) methodology, and quality was measured by means of Grading of Recommendations Assessment, Development and Evaluation (GRADE) [Reference Guyatt, Oxman, Schünemann, Tugwell and Knottnerus23]. Structured question: Does dysbiosis play a role in the pathophysiological development and outcome of EDs?
Inclusion criteria
-
- Participants. The review considered studies that included participants diagnosed with anorexia nervosa (AN), BN, binge eating disorder (BED), or ED not otherwise specified.
-
- Intervention(s). This review considered studies that evaluate qualitative and quantitate microbiota analysis in EDs with/without a pathogenesis implication and studies that evaluate microbiota dysbiosis in EDs with/without the use of probiotics/prebiotics/microbiota transplantation.
-
- Comparator(s). This review considered studies that compare the intervention in outpatients and inpatients to other ED or health control (HC) group.
-
- Outcomes. This review considered studies that evaluated if dysbiosis accounts for eating symptoms, maintenance, or treatment of the disorders. Various instruments are likely to be used to measure these outcomes. This review focused on those using validated questionnaires/tools as patient-reported outcome measures, measures of mood, anxiety, and eating psychopathological symptoms.
-
- Types of studies. To present a complete overview of the literature, we included randomized and nonrandomized, qualitative, and quantitative studies with and without comparison groups, case reports, and observational studies with any sample size.
Exclusion criteria
Studies were excluded in the following cases: studies on animals; patients with EDs due to other medical conditions or induced by substances; pregnant or postpartum women; patients with digestive disease (i.e., inflammatory bowel disease, irritable bowel syndrome, and coeliac disease); patients undergoing other psychiatric and/or metabolic treatments that could modify affectivity, weight, and appetite; or patients receiving nonstandard medications or any other therapy (i.e., antibiotics or steroids). Handbooks, manuals, editorials, letters to editor, reviews, or meta-analyses were also excluded. If duplicated data were found, datasets with the highest number of participants were included. Only eligible publications meeting the inclusion criteria have been included and cited in this review.
Search strategy
Articles published up to August 1, 2020, were retrieved from PubMed, EMBASE, PsychINFO, and the Cochrane Library, on human data without language or time restriction, based on the PICO strategy.
Gray literature and unpublished studies were also considered. The following MESH search strings were used: (Microbiota OR dysbiosis OR gut microbiota OR intestinal microbiota OR gastrointestinal microbiota OR microbial metabolites OR microbial peptides OR microflora OR probiotics OR prebiotics OR FMT) AND (EDs OR feeding OR anorexia OR AN OR bulimia OR BN OR binge eating OR BED) AND (etiopathogenesis OR cause OR etiology OR pathogenesis OR pathophysiological).
Selected articles were reviewed independently by two authors (EAC and PDA), who screened the titles and abstracts and read the full texts of any articles that met the eligibility criteria. In case of any disagreement, consensus was reached through discussion. All relevant original publications obtained from the literature search were identified, and the full texts were retrieved and reviewed. The reference lists were screened, and additional data were included. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria and recommendations were followed to improve the clarity and plainness of the review process [Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche and Ioannidis24]. Figure 1 shows the research strategy.
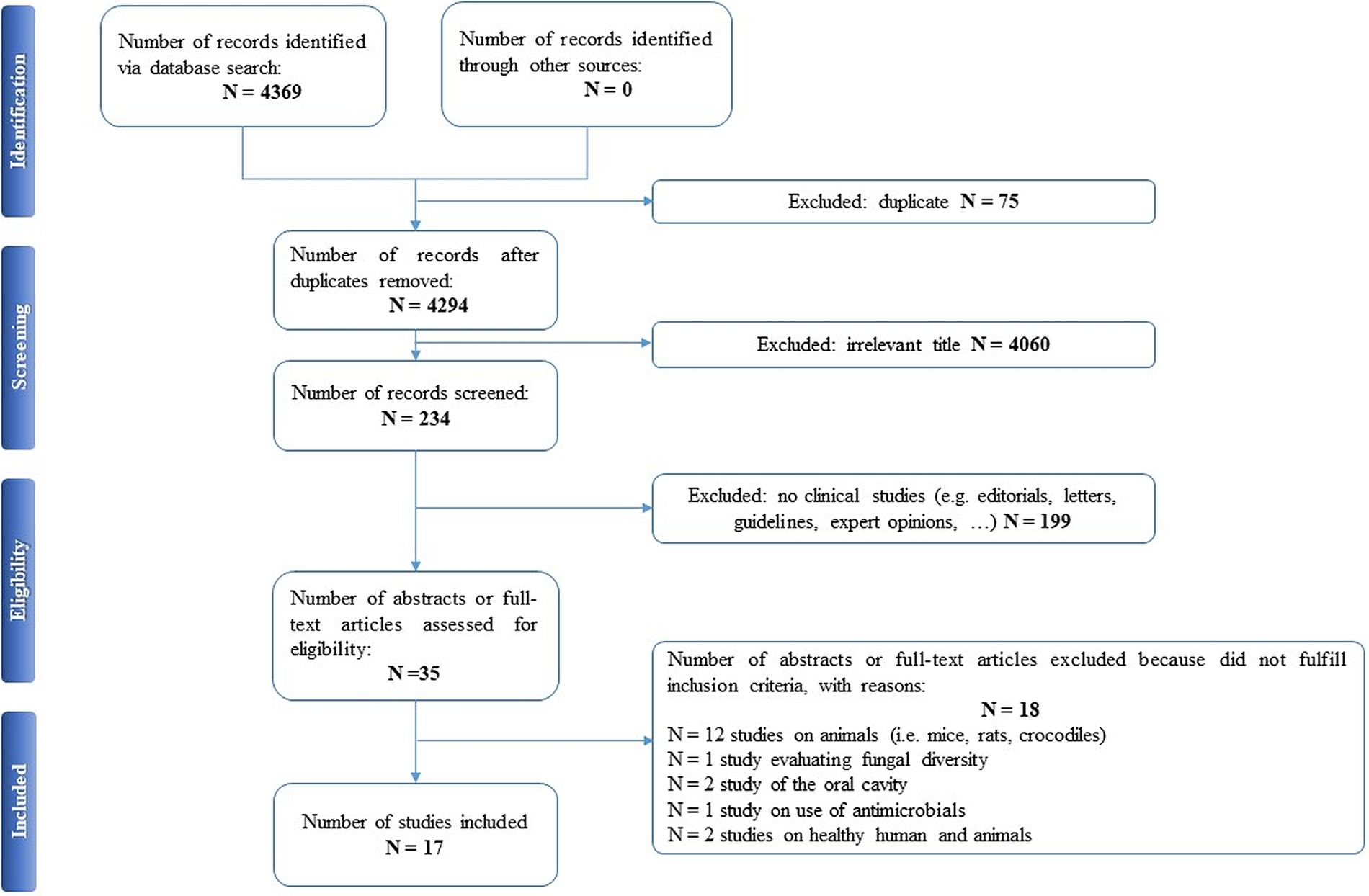
Figure 1. The PRISMA flow chart.
Assessment of quality
The quality of the studies was assessed using the GRADE approach [Reference Guyatt, Oxman, Schünemann, Tugwell and Knottnerus23]. Two reviewers (EAC and CSG) used GRADE approach to assess the quality of evidence from five items of research limitations, inconsistency, indirectness, inaccuracy, and publication bias.
The quality of evidence was rated as “high,” “moderate,” “low,” or “very low” based on GRADE rating standards. “High” quality of the evidence indicates that future research is very unlikely to change existing evidence, “moderate” indicates that future research may change the results, “low” level indicates that future research is likely to change the evaluation results, having an important impact on existing evidence, whereas “very low” indicates highly uncertainty about the existing evidence. In this review, GRADE ratings ranged from moderate to low or very low quality of evidence. The quality assessment was finally reviewed and agreed by the whole review team.
Results
Our search strategy resulted in 4,369 papers. After removing duplicates and irrelevant titles, only 234 articles were considered sufficiently relevant to warrant abstract review. Among them, 199 were excluded, because they were editorials, letters, reviews, systematic reviews, meta-analyses, guidelines, expert opinions, or different interventions, leaving 35 to be assessed for eligibility, with relevant references within these publications also identified and reviewed. Following the screening of literature according to inclusion and exclusion criteria, 18 studies were excluded, because they did not fulfill the inclusion criteria; the remaining 17 were included in the review. Table 1 describes the characteristics of these 17 studies.
Table 1. Main characteristics of included studies.
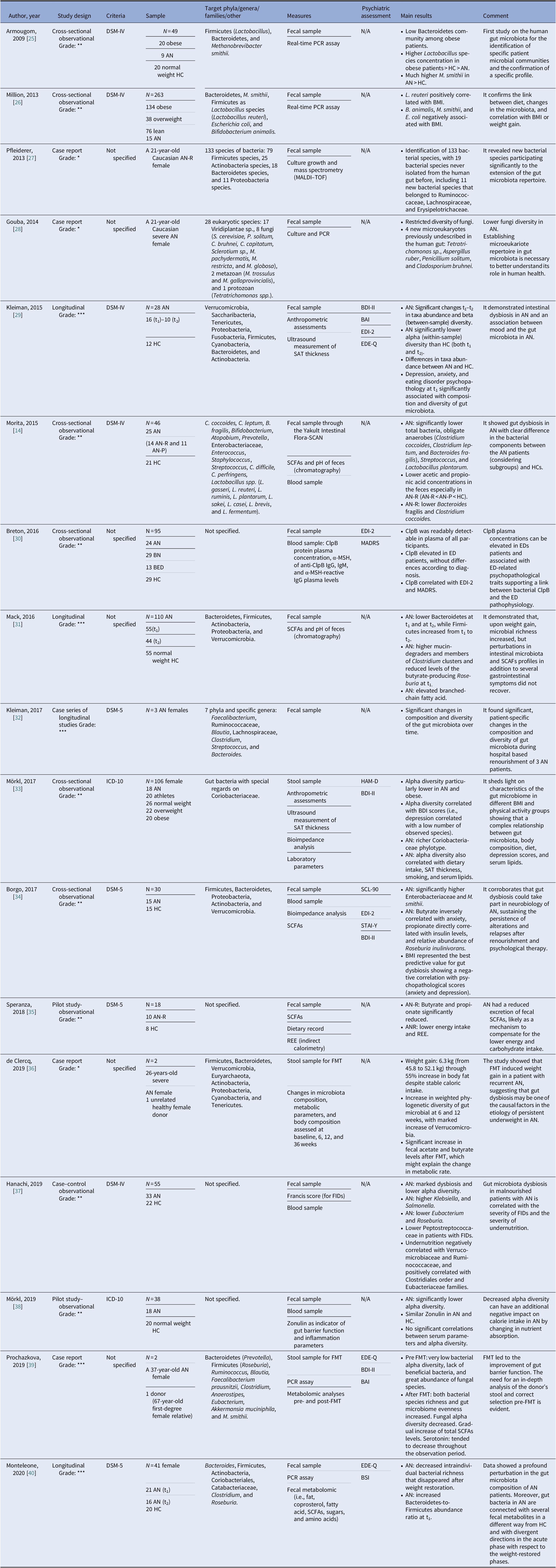
Notes: GRADE: *very low; **low; ***moderate; ****high.
Abbreviations: α-MSH, α-melanocyte-stimulating hormone; AN, anorexia nervosa; AN-P, anorexia nervosa purging type; AN-R, anorexia nervosa restricting type; BAI, Beck Anxiety Inventory; BED, binge eating disorder; BDI-II, Beck Depression Inventory; BMI, body mass index; BN, bulimia nervosa; BSI, Brief Symptom Inventory; ClpB, caseinolytic peptidase B; EDE-Q, Eating Disorder Examination-Questionnaire; EDI-2, Eating Disorder Inventory-2; FIDs, functional intestinal disorders; FMT, faecal microbiota transplantation; HC, healthy control; HAM-D, Hamilton Rating Scale for Depression; IgM/IgG, immunoglobulins M/G; MADRS, Montgomery–Asberg Depression Rating Scale; MALDI, matrix-assisted laser desorption/ionization; PCR, polymerase chain reaction; REE, Resting Energy Expenditure; SAT, subcutaneous adipose tissue; SCFAs, short-chain fatty acids; SCL-90, Symptom Checklist-90; STAI-Y, state-trait anxiety inventory.
Although most of the studies used the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Armougom, Henry, Vialettes, Raccah and Raoult25,Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Hanachi, Manichanh, Schoenenberger, Pascal, Levenez and Cournède37] and DSM-5 [Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Speranza, Cioffi, Santarpia, Del Piano, De Caprio and Naccarato35,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40]) or the International Classification of Diseases (ICD-10) [Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Mörkl, Lackner, Meinitzer, Gorkiewicz, Kashofer and Painold38], some studies did not specify according to which criteria the diagnosis was made [Reference Pfleiderer, Lagier, Armougom, Robert, Vialettes and Raoult27,Reference Gouba, Raoult and Drancourt28,Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39]. We included seven observational studies [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Armougom, Henry, Vialettes, Raccah and Raoult25,Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Hanachi, Manichanh, Schoenenberger, Pascal, Levenez and Cournède37], three longitudinal studies [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40], four case reports [Reference Pfleiderer, Lagier, Armougom, Robert, Vialettes and Raoult27,Reference Gouba, Raoult and Drancourt28,Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39], one case series of three cases that was also longitudinal in its design [Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32], and two pilot studies [Reference Speranza, Cioffi, Santarpia, Del Piano, De Caprio and Naccarato35,Reference Mörkl, Lackner, Meinitzer, Gorkiewicz, Kashofer and Painold38]. The samples included mostly patients with a diagnosis of AN. Only one study evaluated BN and BED patients [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30]. All studies included a control group matched for age and gender [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40], with the exception of case studies [Reference Pfleiderer, Lagier, Armougom, Robert, Vialettes and Raoult27,Reference Gouba, Raoult and Drancourt28,Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32] and studies on FMT cases [Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39]. A group of overweight and/or obese participants were collected in three studies [Reference Armougom, Henry, Vialettes, Raccah and Raoult25,Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33]. And FMT was analyzed in two studies that presented case reports of female patients with chronic and severe AN [Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39].
The gut microbiota was examined from stool samples in all included studies. Other assessments comprised SCFAs [Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Speranza, Cioffi, Santarpia, Del Piano, De Caprio and Naccarato35], fecal pH [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31], culture growth and mass spectrometry [Reference Pfleiderer, Lagier, Armougom, Robert, Vialettes and Raoult27], anthropometric measures, ultrasound measurement of subcutaneous adipose tissue thickness [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33], caseinolytic peptidase B (ClpB) protein concentrations [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30], or fecal metabolomics [Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40]. Regarding psychiatric measures, the Eating Disorder Inventory (EDI-2), Beck Depression Inventory (BDI-II), Montgomery–Asberg Depression Rating Scale (MADRS), Hamilton Rating Scale for Depression, Eating Disorder Examination-Questionnaire, State-Trait Anxiety Inventory, Symptom Checklist-90, Brief Symptom Inventory, and Beck Anxiety Inventory scores were evaluated in relation to changes in microbiota composition [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40].
In AN, significant changes in the quality, quantity, and composition of gut microbiota were found during weight modification. Alpha diversity was lower during the phase of weight loss [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,37–40], resulting in a reduction of Firmicutes [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14] and SCFAs [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Speranza, Cioffi, Santarpia, Del Piano, De Caprio and Naccarato35] and the increase of Bacteroides, Actinobacteria, Enterobacteriaceae, and Methanobrevibacter smithii. A re-established Firmicutes/Bacteroides (F/B) ratio and an increase of SCFAs levels were reported during renourishment and weight gain [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32]. Data on BN and BED are limited but point in the direction of low alpha diversity and increase Firmicutes and Enterobacteriaceae. Interestingly, elevated ClpB concentration produced by E. coli suggested a role in stimulation and an autoimmune response, affecting the melanocortin system that regulates feeding behavior [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30]. The FMT led to an increased number of bacterial producing SCFAs, alpha bacterial diversity, richness, and gut microbiome evenness increased in the AN patient [Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39]. For an overall view, see Figure 2.

Figure 2. Main changes in gut microbiota composition in EDs.
Discussion
Microbiota homeostasis is essential to promote a healthy gut, ensuring its structural [Reference Ottman, Smidt, de Vos and Belzer41], immune protective [Reference Capurso42], and metabolic functions [43–45]. The imbalance between pathogenic and symbiotic or commensal species, so-called dysbiosis, seems to contribute to the development of EDs through several mechanisms [11–16] (see Supplementary Figure 1). This systematic review analyzed the contribution of dysbiosis to the pathophysiological development of EDs and the restored microbial balance as a possible treatment for EDs.
We found noteworthy evidence that quality and quantity of gut microbiota change in different way according to phase of AN disorder as well as indirect measures of microbiota diversity. SCFAs levels, lower during the restriction phase, increase during renourishment and weight regain. Unfortunately, only few studies had a longitudinal design, and results of cross-sectional studies should be read with attention, considering the absence of a control group. The role of microbiota in the etiology of BN and BED should be better deepened, conducting longitudinal trials. According to a transdiagnostic model of EDs, it would be interesting to identify possible markers of switch from AN to BN or BED, and vice versa, and to use them as therapeutic targets.
Gut–brain axis
The two-way communication network between microbiota and the Central Nervous System [Reference Wouw, Schellekens, Dinan and Cryan19,Reference Collins, Surette and Bercik46] is finely controlled and regulated by neurotrophic substances (e.g., gamma-aminobutyric acid, melatonin, serotonin, catecholamines, acetylcholine, and histamine) synthesized by the intestinal microflora that “mimic” the endogenous molecules physiologically produced by our body [Reference Strandwitz47]. Several authors have underlined the importance of the role of gut microbial balance for metabolism and appetite regulation [Reference Lam, Maguire, Palacios and Caterson11,48–50]. Dysbiosis alters the hypothalamic–pituitary–adrenal axis [Reference Waterson and Horvath51], the hunger/satiety regulatory system [Reference Lam, Maguire, Palacios and Caterson11,Reference Temko, Bouhlal, Farokhnia, Lee, Cryan and Leggio52,Reference Cone53], and mood [Reference Kokare, Dandekar, Singru, Gupta and Subhedar54]. Neuropeptide-like proteins produced by altered microbiota in EDs can simulate the endogenous appetite and satiety hormones [Reference Lam, Maguire, Palacios and Caterson11,Reference Temko, Bouhlal, Farokhnia, Lee, Cryan and Leggio52] and cause a cross-reaction of immunoglobulin produced in stressful conditions (as in EDs) [Reference Inui, Chen and Meguid55], such as autoantibodies against α-melanocyte-stimulating hormone (α-MSH). Studies have shown that high stress and psychopathological symptoms in patients with AN and BN are directly related to an increase in these anti-α-MSH antibodies [Reference Fetissov, Hamze Sinno, Coquerel, Do Rego, Coëffier and Gilbert50,Reference Fetissov, Hallman, Oreland, Af Klinteberg, Grenbäck and Hulting56,Reference Sinno, Rego, Coëffier, Bole-Feysot, Ducrotté and Gilbert57] that act directly on the arcuate nucleus and on appetite regulation [Reference Fetissov and Déchelotte58] (see Supplementary Figure 2).
Anorexia nervosa
The possible role of the microbiota in the pathogenesis of AN has become a topic of recent interest [Reference Kleiman, Carroll, Tarantino and Bulik12,Reference Ruusunen, Rocks, Jacka and Loughman13], considering the close relation with gut microbiota composition according to the dietary intake in both the short term [Reference David, Maurice, Carmody, Gootenberg, Button and Wolfe59] and the long term [Reference Wu, Chen, Hoffmann, Bittinger, Chen and Keilbaugh60]. Microbial diversity seems to change during diet restriction and also during weight regain.
Microbial diversity during diet restriction, renourishment, and weight regain
Significant changes in the composition of gut microbiota (in terms of phyla quality and quantity) on the basis of caloric intake were evaluated in patients with AN during weight loss [Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Hanachi, Manichanh, Schoenenberger, Pascal, Levenez and Cournède37], renourishment [Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40], and weight gain [Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26]. A significant decrease in Firmicutes levels [Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Hanachi, Manichanh, Schoenenberger, Pascal, Levenez and Cournède37], especially Roseburia [Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34], Lactobacillus, Streptococcus [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14], and Clostridium [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Hanachi, Manichanh, Schoenenberger, Pascal, Levenez and Cournède37], was found in patients with AN compared to healthy controls (HCs), independently of subtype (restrictive or binge/purge) or any compensatory behavior [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14]. The majority of studies included in this review showed that alpha diversity in AN patients was lower [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,37–40] and suggested to interfere with nutrient absorption and calorie intake [Reference Mörkl, Lackner, Meinitzer, Gorkiewicz, Kashofer and Painold38]. In humans, a lower F/B ratio was reported in underweight subjects compared to normal-weight subjects [Reference Koliada, Syzenko, Moseiko, Budovska, Puchkov and Perederiy61]. A diet with low carbohydrate or low lipid intake, as with that of AN patients, can favor an increase in Bacteroides [Reference Fava, Gitau, Griffin, Gibson, Tuohy and Lovegrove62], Actinobacteria, Proteobacteria, Enterobacteriaceae [Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34], E. coli [Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34], and M. smithii [Reference Armougom, Henry, Vialettes, Raccah and Raoult25]. M. smithii (methane producers) seem to increase significantly in the intestinal epithelium of malnourished patients, providing a more efficient and increased supply of energy and calories [Reference Armougom, Henry, Vialettes, Raccah and Raoult25,Reference Carr, Kleiman, Bulik, Bulik-Sullivan and Carroll63,Reference Hoffmann, Dollive, Grunberg, Chen, Li and Wu64]. Indeed, studies have demonstrated that acute malnutrition is characterized by gut dysbiosis and that the malnutrition phenotype can be transmitted via the intestinal microbiota in a gnotobiotic mouse model [Reference Smith, Yatsunenko, Manary, Trehan, Mkakosya and Cheng65,Reference Gehrig, Venkatesh, Chang, Hibberd, Kung and Cheng66]. The imbalance in excess of commensal species in the patient’s intestine is a result of the restriction in food intake and may also account for the maintenance of the disorder. This microbial dysbiosis may interact with a nutrient-deficient diet affecting energy metabolism and causing the persistence of malnourishment. A reduction in microbial biodiversity also leads to an alteration of the immune system and to the assimilation and accrual of calories from food [Reference Ruusunen, Rocks, Jacka and Loughman13,Reference Lippert, Kedenko, Antonielli, Kedenko, Gemeier and Leitner67]. On the other hand, the systemic inflammation, as a result of the increased gut permeability, [Reference Bercik, Collins and Verdu68] and an altered neuronal activity are reported in various psychiatric disorders [Reference Karakuła-Juchnowicz, Pankowicz, Juchnowicz, Valverde Piedra and Małecka-Massalska15,Reference Qin, Wu, Block, Liu, Breese and Hong69], and the same mechanism may be proposed for AN. More recently, 11 completely new bacterial species [Reference Pfleiderer, Lagier, Armougom, Robert, Vialettes and Raoult27] and 4 new microeukaryotic communities never previously found in humans [Reference Gouba, Raoult and Drancourt28] were identified. Future studies should investigate new species and their contribution to gut microbiota stability.
Patients’ BMI and physical activity also showed a complex relationship with gut microbiota: patients with AN, similar to individuals during intense physical activity [Reference Zhao, Zhang, Hu, Huang, Yuan and Zou70], have shown high levels of Coriobacteriaceae in their stool samples [Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33]. It has also been hypothesized that moderate and controlled exercise can provide an improvement in gut inflammation, decrease intestinal permeability, and positively regulate the composition of microbiota enhancing the number of beneficial microbial species [Reference Monda, Villano, Messina, Valenzano, Esposito and Moscatelli71].
Nutritional rehabilitation represents one of the essential focuses for EDs and has an impact on the composition of microbiota. The intake of macronutrients, such as fatty acids, proteins, and carbohydrates, especially fibers, can significantly affect the gut microbiota composition [Reference Scott, Gratz, Sheridan, Flint and Duncan17], facilitating the onset of dysbiosis [Reference Singh, Chang, Yan, Lee, Ucmak and Wong18] or restoring it. Weight regain with unbalanced diet, as high-lipid nutrition, in AN patients showed a reduction in Firmicutes and an elevation of Bacteroides and Ruminococci [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31], an increase of mucin-degrading bacteria, and a reduction of butyrate-producing bacteria [Reference Herpertz-Dahlmann, Seitz and Baines72]. Interestingly, the microbiota changed qualitatively and quantitatively, and microbial richness increased after weight regain in patients with AN [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Kleiman, Glenny, Bulik-Sullivan, Huh, Tsilimigras and Fodor32], but in some patients the trend of changes in alpha diversity was lower compared to HCs [Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40] and gastrointestinal symptoms did not recover at the end of 3 months treatment [Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31]. This may be due to a diet rich in fibers and suggests that energy derived from macronutrients is crucial for modest alpha diversity. Another explanation may be that microbial richness is related to colonic transit time [Reference Roager, Hansen, Bahl, Frandsen, Carvalho and Gøbel73]. It is well known that patients with AN often suffer from constipation and this could affect the measures for alpha diversity.
Short-chain fatty acids
SCFAs are fatty acids (butyrate, acetate, and propionate) produced by the gut microbiota during the nondigestible polysaccharides fermentation (fibers and resistant starch) [Reference Tan, McKenzie, Potamitis, Thorburn, Mackay, Macia and Alt74]. The levels of SCFAs represent the indirect measurement of the microbial composition and are influenced by dysbiosis. SCFAs were lower in stool samples of AN patients [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Speranza, Cioffi, Santarpia, Del Piano, De Caprio and Naccarato35], especially in the AN-Restricter subgroup, compared to the control group [Reference Morita, Tsuji, Hata, Gondo, Takakura and Kawai14]. The reduction of SCFAs in patients with AN is a consequence of the low abundance of Roseburia [Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34]. A significant increase in both fecal acetate and butyrate levels during renourishment of AN patients [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29] and changed microbiota composition, especially increased Firmicutes, after weight regain [Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31] were demonstrated. Bacterial species richness, gut microbiome evenness, and SCFA levels gradually increased in severe or chronic AN patients and also after FMT from a healthy donor. FMT resulted in an increase of specific genera and total SCFA levels, especially butyrate-producing Roseburia, 1 year after FMT, contributing to the improvement of gut barrier function [Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39]. A beneficial role in appetite regulation [Reference Byrne, Chambers, Morrison and Frost75] as well as the involvement of SCFAs in energy homeostasis regulation has been suggested [Reference Hu, Lin, Zheng and Cheung76]. The importance of SCFAs on appetite and energy metabolism suggests SCFA modulation as new nutritional target to prevent or counteract EDs. Nevertheless, it is important to remind that the analysis of the fecal microbiota only indirectly reflects the upper intestinal flora and that the weight loss process involves consumption of metabolites instead of production [Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40]. Studies demonstrated also a significant enrichment difference in the gut microbiota composition according to the gut section [Reference Rinninella, Raoul, Cintoni, Franceschi, Miggiano and Gasbarrini5]. Analysis of these microbial metabolites will surely improve the understanding of the etiology of EDs.
Bulimia nervosa
If more data are available for AN, a dramatic lack of data is evident for BN. To date, only one study focused on microbiota changes in BN even if it is a life-threatening condition. Appetite regulation mechanisms seem modulated by changes in the microbiota even during BN [Reference Fetissov49,Reference Fetissov, Hamze Sinno, Coquerel, Do Rego, Coëffier and Gilbert50,Reference Fetissov, Harro, Jaanisk, Järv, Podar and Allik77,Reference Fetissov, Hamze Sinno, Coëffier, Bole-Feysot, Ducrotté and Hökfelt78]. ClpB was detectable in plasma of both HC and ED patients, but plasma levels were more elevated in the patients’ group. ClpB produced by E. coli is capable of “mimicking” α-MSH [Reference Oldstone79] and stimulating an autoimmune response [Reference Fetissov, Legrand and Lucas80]. IgG autoantibodies against α-MSH allow internalization of the IgG/α-MSH immunocomplex [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30]. Furthermore, this mechanism has been highlighted in both AN and BN [Reference Lucas, Legrand, Bôle-Feysot, Breton, Coëffier and Akkermann81]. Therefore, a “hunger” rather than a “satiety” effect is due to the epitope switch of the IgG forming the immunocomplex in BN patients [Reference Fetissov and Hökfelt82]. Binding occurs at the N-terminal in AN and at the C-terminal in BN, and in both cases is associated with anxiety and a high EDI-2 total score [Reference Fetissov, Harro, Jaanisk, Järv, Podar and Allik77,Reference Fetissov and Hökfelt82]. This cross-reactivity of α-MSH autoantibodies may also explain the possibility of shifting from AN to BN or BED [Reference Lucas, Legrand, Bôle-Feysot, Breton, Coëffier and Akkermann81,Reference Fetissov and Hökfelt82], and vice versa, through modulation of the melanocortin system that regulates feeding behavior. Funding new evidence on the role of microbiota and its alteration in BN is the new challenging to address. Future research should investigate composition in patients with BN compared to HCs and other EDs and evaluate change in gut microbial species in prospective studies.
Binge eating disorder
Studies regarding BED are still lacking. However, the relationship between microbiota and BED remains in the shadows, awaiting further research. A similar but opposite mechanism to that already described in AN has been hypothesized due to the cross-reactivity of IgG toward α-MSH in overweight [Reference Fetissov, Hamze Sinno, Coquerel, Do Rego, Coëffier and Gilbert50,Reference Fetissov and Hökfelt82] and obese patients [Reference Lucas, Legrand, Bôle-Feysot, Breton, Coëffier and Akkermann81]. Plasma concentrations of ClpB in patients with AN, BN, and BED were higher compared to HCs, without any significant differences according to diagnosis, suggesting a link between bacterial ClpB and EDs [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30]. Serum concentrations of inflammatory cytokines and growth factors seem related to dysfunctional eating behaviors at the extremes of BMI, including BED [Reference Caroleo, Carbone, Greco, Corigliano, Arcidiacono and Fazia83]. As noted, BED is very often associated with numerous comorbidities, especially obesity [Reference Iqbal and Rehman84], and patients with BED and obesity exhibited an unfavorable metabolic and inflammatory profile related to their characteristic eating behaviors [Reference Succurro, Segura-Garcia, Ruffo, Caroleo, Rania and Aloi85]. It is evident that microbiota in obesity, similar to AN, differs in comparison to healthy, normal-weight subjects [Reference Million, Angelakis, Maraninchi, Henry, Giorgi and Valero26,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33]. A diet rich in lipids is able to raise levels of Firmicutes and Proteobacteria and decrease levels of Bacteroides [Reference Murphy, Velazquez and Herbert86], thus leading to an increased F/B ratio [Reference Ley, Turnbaugh, Klein and Gordon87]. A lower alpha diversity has been demonstrated in gut microbiota in both underweight and obese patients, with a significant correlation with BDI score indicating greater levels of depression [Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33]. In this light, the evidence of dysbiosis in gut microbiota with extreme BMI could justify its possible role in BED. In a transdiagnostic view of EDs [Reference Birmingham, Touyz and Harbottle88], the hypothesis of neuroinflammation and gut dysbiosis in the etiology of EDs should be screened. Recently, a new a continuum model was presented, suggesting that changes in proinflammatory cytokines, serotonin levels, and microbiota cause shifts in EDs [Reference Rantala, Luoto, Krama and Krams89].
Therapeutic approach: FMT
FMT is a new and promising therapy, already indicated in the treatment of diarrhea caused by Clostridium difficile [90–92], inflammatory bowel disease and ulcerative rectocolitis [Reference Gianotti and Moss92], autoimmune diseases, allergic syndromes [Reference Borody and Khoruts93], neurological syndromes such as Parkinson’s, multiple sclerosis, and fibromyalgia, as well as metabolic diseases (i.e., obesity, insulin resistance, and metabolic syndrome). The first FMT was performed in 2018 on a 26-year-old patient with severe restrictive AN after 2 years of standard therapies. Following FMT from a healthy donor, at a 36-week follow-up, the patient had gained 6.3 kg. The authors hypothesized that the patient’s microbiota remodeling could be due to the increased number of bacterial elements producing SCFAs, detected in the donor sample, in comparison with the basal levels recorded in the recipient [Reference de Clercq, Frissen, Davids, Groen and Nieuwdorp36]. More recently, another case report detailed the use of FMT on a 37-year-old female with severe chronic AN from a 67-year-old first-degree female relative donor [Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39]. Pre- and post-treatment analyses showed changes in gut microbiota composition: alpha bacterial diversity, richness, and gut microbiome evenness increased in the patient; and the fungal alpha diversity decreased, persisting for 1 year. Restoration of the F/B ratio can rehabilitate homeostasis or regulate the composition of the gut microbiota and correct abnormal responses of the mucosal immune system to chronic gut inflammation [Reference Ghouri, Richards, Rahimi, Krill, Jelinek and DuPont94]. Despite bias, the results remain encouraging and indicate that, if replicated in a controlled trial, FMT may represent a new line of treatment in patients with AN; perhaps its use could be studied in other EDs. Although these results are promising, it should be taken into account that FMT is also associated with adverse events such as diarrhea, constipation, infections, and others not yet known.
Microbiota and outcome
Gut–brain interplay is fundamental, and numerous intestinal microflora have an important role through the synthesis of neurotransmitters [Reference Strandwitz47]. Unfortunately, there is little evidence supporting the associations between the intestinal microbiota and depression or anxiety disorders in humans [Reference Naseribafrouei, Hestad, Avershina, Sekelja, Linløkken and Wilson95,Reference Foster and McVey Neufeld96]. In this systematic review, few studies have cleared up the relationship between dysbiosis and psychopathology in AN [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39,Reference Monteleone, Troisi, Fasano, Dalle Grave, Marciello and Serena40], with only one study in BN and in BED [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30].
Depression, anxiety symptoms, and ED psychopathology seem to be related to a change in diversity of gut microbiota, especially bacterial species producing butyrate [Reference Kleiman, Carroll, Tarantino and Bulik12,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34,Reference Reigstad, Salmonson, Rainey, Szurszewski, Linden and Sonnenburg97]. In particular, a lower bacterial diversity is associated with more severe depression and anxiety [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29,Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30,Reference Mörkl, Lackner, Müller, Gorkiewicz, Kashofer and Oberascher33,Reference Borgo, Riva, Benetti, Casiraghi, Bertelli and Garbossa34]. Longitudinal studies on AN cohorts suggested that weight gain during renourishment leads to the improvement of psychological symptoms with specific changes in microbial composition that might participate in AN pathophysiology [Reference Kleiman, Watson, Bulik-Sullivan, Huh, Tarantino and Bulik29]. Serotonin secretion regulation and the role of gut microbiota during its synthesis in the intestine are well documented [Reference Gershon and Tack98,Reference Yano, Yu, Donaldson, Shastri, Ann and Ma99]. It could be speculated that variableness in gut microbiota in EDs may affect the expression of the tryptophan hydroxylase protein or serotonin transporters and result in consequential abnormalities in serotonergic activity and psychopathological symptoms [Reference Reigstad, Salmonson, Rainey, Szurszewski, Linden and Sonnenburg97]. The altered homeostasis in gut microbiota seems related to the altered secretion of serotonin [Reference Mandić, Woting, Jaenicke, Sander, Sabrowski and Rolle-Kampcyk100] and the cross-reactive mechanisms through the productions of autoantibodies against neuropeptides [Reference Fetissov, Legrand and Lucas80]. As above mentioned, the ClpB concentration in BN and BED stimulates the production of autoantibodies against α-MSH. The different binding of ClpB has been associated with psychological traits in BN and BED patients, especially with more anxiety, high MADRS, and EDI-2 total score [Reference Fetissov, Harro, Jaanisk, Järv, Podar and Allik77,Reference Fetissov and Hökfelt82], supporting a link between bacterial ClpB and ED pathophysiology [Reference Breton, Legrand, Akkermann, Järv, Harro and Déchelotte30]. In a recent case report, serotonin levels tended to decrease throughout after FMT [Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39], but mood and eating pattern of purgative AN remained unmodified, despite significant improvement in the microbiota post-FMT [Reference Prochazkova, Roubalova, Dvorak, Tlaskalova-Hogenova, Cermakova and Tomasova39] probably due to the long duration of illness or a short follow-up. On the other hand, patients with AN were treated with antidepressants, which are known to induce microbiota alterations due to their antimicrobial activity [Reference Munoz-Bellido, Munoz-Criado and Garcìa-Rodrìguez101] and could be responsible for contrasting results.
Future directions
New therapeutic options in the clinical management of EDs are currently being investigated as direct and/or adjunctive therapies [Reference Long-Smith, O’Riordan, Clarke, Stanton, Dinan and Cryan102]. Among them, the so-called psychobiotics have been studied more extensively. These are probiotic live organisms that, when ingested in adequate amounts, produce a health benefit in patients suffering from psychiatric illness [Reference Dinan, Stanton and Cryan103]. Lactobacilli, Bifidobacteria, Enterococci, and yeasts are used mostly in the formulation of probiotics [Reference Pandey, Naik and Vakil104] and are important for the production of SCFAs, the biosynthesis of vitamins B and K, the production of neuroactive substances such as gamma-aminobutyric acid and serotonin, the activation of the immune system and regulation of cytokine and immunoglobulin release, the reinforcement of intestinal barrier function through tight junctions, and the increase of mucin levels [Reference Dinan, Stanton and Cryan103,Reference Kho and Lal105]. Studies on mice [Reference Savignac, Kiely, Dinan and Cryan106,Reference Möhle, Mattei, Heimesaat, Bereswill, Fischer and Alutis107] were replicated in human volunteers [Reference McKean, Naug, Nikbakht, Amiet and Colson108] and demonstrated an increase in neuropsychiatric disorders after inducing dysbiosis that subsided after oral administration of probiotics. These results provided evidence of antidepressant and anxiolytic effects of probiotics probably due to a decrease in the levels of proinflammatory plasma cytokines [Reference Lew, Hor, Yusoff, Choi, Yusoff and Roslan109].
Enterococcus and Lactobacillus seem to regulate the Enterobacteriaceae responsible for the production of autoantibodies against α-MSH [Reference Fetissov, Legrand and Lucas80]. Probiotics such as Roseburia, a butyrate producer [Reference Mack, Penders, Cook, Dugmore, Mazurak and Enck16,Reference Mack, Cuntz, Grmer, Niedermaier, Pohl and Schwiertz31], are among the proposals for future therapeutic protocols in the management of AN.
Other studies have confirmed how these supplements have been able to rehabilitate malnourished and hungry rodents, confirming the usefulness of this supplement in the treatment of malnutrition [110–112].
Strengths and limitations
This represent a comprehensive systematic review on the role of microbiota in the pathogenesis and treatment in individuals with EDs. It is also the first to include studies on AN, BN, and BED in an attempt to explain the possible role of dysbiosis in the pathogenesis of these EDs. Nevertheless, some limitations should be noted. First, the heterogeneity among the included studies may be a confounding variable in interpreting results. Few studies have a longitudinal design, and the majority of studies are cross-sectional observational or case report studies that preclude conclusions about causality. We found only two studies evaluating FMT in AN to illustrate the therapeutic potential of this innovative treatment. Second, the study samples were mainly made up of female participants, which limits generalizability to males, and the variable sample size reduces the power to detect differences between patients and controls over the course of renourishment. Third, interpretation of the results of the included studies is hindered by limitations inherent in the confounding variables affecting dysbiosis: self-reported information on dietary intake, impact of starvation, short timeline of longitudinal studies, antidepressant treatment, and indirect analysis of microbiota through the fecal sample. However, the strongest limitation concerns the composition of a “normal” microbiota, which changes according to geographical area of origin, eating habits, food choice, gender, age, and type of birth delivery. Moreover, current scientific research focused mainly on bacteria and less on other microbial species. Randomized, placebo-controlled studies designed to unravel the gut–microbiota cross-talk mechanisms are therefore needed.
Conclusions
Understanding the composition and functions of microbiota and dysfunctional mechanisms could be important in preserving and improving its balance. However, this is only the tip of an iceberg of complex interactions between the microbiota and the host. A healthy gut microbiota profile improves health and prevents several disorders. The importance of the diet in regulating the physiological balance between Firmicutes and Bacteroides is well known, showing how diets rich in fibers and biotic supplements can restore the normal commensal flora even in EDs. Sufficiently strong and solid scientific evidence has not yet been produced, but the activity of SCFA-producing bacteria, especially butyrate, is known to be fundamental in preserving the epithelial integrity of the gut. The depletion of these bacteria and the consequent increase in the number of mucin-degrading bacteria promote intestinal inflammation, permeability of the enteric epithelium, and therefore the passage of lipopolysaccharide and ClpB protein into the circulation. These can trigger immune reactions in the hunger/satiety regulation center by losing the ability to control food intake. The reduction of butyrate-producing bacteria and the increase of pathobiont bacteria, such as Clostridium, are related to cross-reactive mechanisms involving the HPA axis in patients with EDs, anxiety, and depression.
This systematic review highlights the extreme and delicate communication network between gut, endocrine system, and brain. A better and more in-depth characterization of gut bacterial species in the near future may provide useful indications to improve not only the therapeutic but also the preventive approach in EDs. Future research may be able to distinguish between changes of intestinal microbiota that reflect weight gain versus recovery from EDs and identify microbial biomarkers of renourishment versus recovery from psychopathology used as therapeutic targets. In this light, it may be possible to identify the patients who will benefit most from these new therapies and those who may not have any benefit at all. Just think of the latest proposed procedure, the FMT, where a desired phenotype could induce other disorders. A new promising therapeutic strategy may be the administration of probiotics or prebiotics for restoring the gut microbiota in patients with AN, BN, and BED. It is also increasingly evident that the gut microbiota represents a real “organ” with specific and fundamental functions for the protection and prevention of many disorders, as well as playing a possible role in their pathogenesis.
Further research may investigate in-depth microbiota changes with particular regard for BN and BED comparing to HCs. Future studies should also disentangle if there are differences in gut microbiota composition between obese patients after weight loss and AN and finally elucidate the possible role of new therapeutic strategies.
Conflict of Interest
The authors declare no conflicts of interest.
Authorship Contributions
E.A.C. and P.D.A. independently screened titles and abstracts of the identified articles, and they read the full texts of articles complying eligibility criteria, supervised by C.S.G. who made the final decision in cases of disagreement. E.A.C. wrote the initial draft of the manuscript. G.V. participated in the literature search and writing of the final manuscript. C.S.G. and P.D.F. critically reviewed the manuscript. E.A.C., P.D.A., G.V., P.D.F., and C.S.G. approved the final version of the manuscript.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1192/j.eurpsy.2020.109.
Acknowledgment
Authors are grateful to Felicia Sestito for her collaboration.






Comments
No Comments have been published for this article.