Humans have been ingesting cows' milk (CM) and related products (i.e. cheese and butter) over the course of many millennia. In 2003, the worldwide consumption of CM and related products was estimated to be approximately half a million tons, which corresponds to approximately 81 kg per capita per year(1). Despite this massive consumption of CM, the physiological effects of this nutrient and energy resource have not been fully elucidated.
Over the previous decade, several studies have examined the effects of CM and its products on human obesity, diabetes and the risk of the metabolic syndrome. These studies revealed inverse relationships between dairy product consumption and body weight, blood glucose levels and blood pressure(Reference Mirmiran, Esmaillzadeh and Azizi2–Reference Pereira, Jacobs and Van Horn8). Studies using animal models have also demonstrated the beneficial effects of skimmed milk and whey protein against the metabolic syndrome(Reference Belobrajdic, McIntosh and Owens9, Reference Zemel and Sun10). In mice, a diet containing skimmed milk reduced body-weight gain in comparison with a normal diet(Reference Parra, Bruni and Palou11), and in rats a whey protein-based diet had positive effects on insulin resistance(Reference Pichon, Potier and Tome12). In these animal studies, however, very high amounts of milk and/or its components were included in the test diets. Approximately 5–10 litres CM/person per d should be ingested to be equivalent to the amount of milk and/or its components used in most of the animal studies. Additionally, skimmed milk and whey protein were replaced with alternative ingredients. These considerations raise questions regarding the results of previous studies and the true effects of CM on insulin resistance.
We made a hypothesis that such a high amount of milk and/or its components may not be necessary to have a beneficial effect on insulin resistance even in animal models. We also hypothesised that a supplementation of CM to the daily diet is presumably a more suitable animal experimental model than the replacement of the main ingredients of the diet with CM components, since CM is normally ingested not as an essential nutrient component but as a supplement in humans.
Our aim was to determine whether the beneficial effects of CM on reduced insulin sensitivity are experimentally reproducible using normal doses of CM in rats. In addition, we designed our experiments to mimic a human pattern of milk consumption (i.e. drinking milk before breakfast every day). Insulin resistance was assessed because this parameter is strongly associated with the metabolic syndrome(Reference Mokdad, Ford and Bowman13, Reference O'Keefe and Bell14). We examined the effects of CM on insulin sensitivity compared with artificial milk (AM), an emulsion with the same energetic and nutritional composition as CM. Rats fed a high-sucrose (S) diet to induce insulin resistance were compared with control rats fed a dextrin (D)-based diet(Reference Gomez Dumm15, Reference Oliart Ros, Torres-Márquez and Badillo16). To gain insight into the molecular mechanism(s) responsible for the beneficial effects of milk consumption, we also examined the mRNA expression of the Na+-dependent glucose transporter (SGLT1), glucose transporter type 2 (GLUT2) and proglucagon in the small intestine, and analysed changes in caecal microbiota and SCFA composition. It was previously reported that glucose absorption increased in patients with obesity and diabetes(Reference Thomson and Wild17, Reference Tandon, Srivastava and Pandey18). Furthermore, these changes were accompanied by the increased expression of GLUT, including SGLT1 and GLUT2, in the intestinal brush-border membrane(Reference Marks, Carvou and Debnam19–Reference Corpe, Basaleh and Affleck21). Diabetic therapy based on incretins such as proglucagon, which is processed to yield the insulin secretion-enhancing glucagon-like peptide-1, has been the focus of a number of recent studies(Reference Palnaes Hansen, Andreasen and Holst22, Reference Printz, Reiter and Samadi23). In addition, the role of gut microbial metabolism in the metabolic syndrome has been demonstrated in both human subjects and experimental animals(Reference Bäckhed, Ding and Wang24–Reference Anderson and Bridges27). In particular, SCFA, which are the endproducts of bacterial fermentation in the intestine, may have an impact on insulin resistance(Reference Robertson28).
Materials and methods
Animals and diets
Male F344 rats (Japan SLC, Shizuoka, Japan) aged 3 weeks and weighing approximately 50 g were individually housed in stainless-steel cages with wire-mesh bottoms. The cages were placed in a room with controlled temperature (22–24°C), relative humidity (40–60 %) and lighting (lights on from 05.00 to 17.00 hours). The rats were allowed ad libitum access to deionised water and a semi-purified diet based on the American Institute of Nutrition AIN-93G formulation(Reference Reeves, Nielsen and Fahey29), for an acclimatisation period of 3 d. The Hokkaido University Animal Committee approved the study and all animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals.
Preparation of test milk emulsion
Commercially available CM was obtained from Meiji Dairies Co. Ltd (Tokyo, Japan). AM emulsions were prepared using a high-pressure homogeniser (Gaulin 15MR-8TBA; APV Gaulin Inc., Wilmington, MA, USA)(Reference Yajima, Kanno and Yajima30). The AM formula had the same energetic and nutritional composition as CM (Table 1) (31–Reference Christie and Fox34). The AM was composed of general non-milk-related food materials to replace the components of milk. Lactose, milk protein and milk lipid were replaced by maltose, egg white protein and lard, respectively. Chemically synthesised reagents were used as a mineral source in AM.
Table 1 Composition of artificial milk (AM) and cows' milk (CM) (g/kg)

* See Walstra(Reference Walstra32).
† Processed Egg (Taiyo Kagaku Co. Ltd, Mie, Japan).
‡ Wako Pure Chemical Industries, Ltd, Osaka, Japan.
§ See Gopal & Gill(Reference Gopal and Gill33).
∥ See Christie(Reference Christie and Fox34).
¶ Snow Brand Milk Products Co., Ltd, Sapporo, Japan.
** Potassium sulfate (Wako Pure Chemical Industries, Ltd, Osaka, Japan).
†† Calcium carbonate (Wako Pure Chemical Industries, Ltd, Osaka, Japan).
‡‡ Magnesium oxide (Wako Pure Chemical Industries, Ltd, Osaka, Japan).
§§ Sodium dihydrogen phosphate dehydrate (Nacalai Tesque Inc., Kyoto, Japan).
∥∥ Content of Na in AM was that estimated from the source of P (sodium dihydrogen phosphate dehydrate).
Study design
Following the acclimatisation period, the rats were divided into four groups, each consisting of six rats. Two groups were fed the D diet and two groups were fed the S diet for 7 weeks. The diet compositions are listed in Table 2. On each day throughout the experimental period, access to the diet was restricted from 17.00 until 09.00 hours the following morning; thus, 8 h food deprivation was imposed per d. Within each diet assignment, one of the two groups was orally administered 25 ml (approximately 8 % of total energy intake) of CM (D-CM and S-CM groups) per kg body weight; the remaining two groups were administered the same amount of AM (D-AM and S-AM groups) via a feeding tube (35 cm; TERUMO, Tokyo, Japan) at 17.00 hours. The test diet was made available immediately after the test milk administration. Body weight and food intake were measured daily. Blood samples were collected at days 42 and 45.
Table 2 Experimental diet composition (g/kg diet)

* ALACID (New Zealand Daily Board, Wellington, New Zealand).
† TK-16 (Matsutani Chemical Industry Co., Ltd, Hyogo, Japan).
‡ Sucrose (Nippon Beet Sugar Manufacturing Co., Ltd, Obihiro, Japan).
§ Lard (Snow Brand Milk Products Co., Ltd, Sapporo, Japan).
∥ Mineral and vitamin mixtures were prepared according to the AIN-93G formulation(Reference Reeves, Nielsen and Fahey29) (CLEA Japan, Inc., Tokyo, Japan).
¶ Wako Pure Chemical Industries, Ltd, Osaka, Japan.
** Cellulose powder (JustFiber; Morimura Bros., Inc., Tokyo, Japan).
On the last day of the 7-week experimental period, all rats were anaesthetised with sodium pentobarbital between 09.00 and 11.00 hours. The abdominal cavity was opened, abdominal aortic blood was collected, and the rats were euthanised. Segments were taken from the duodenum (below the stomach), jejunum (5 cm distal to the Trietz ligament) and ileum (5 cm proximal to the ileocaecal junction). The segments were cut open along the mesenteric border to form flat sheets, rinsed with ice-cold saline (154 mm-NaCl) and immediately immersed into RNAlater (Sigma, Tokyo, Japan). After 4 h, the serosa and muscle layers of the segments were removed and re-immersed in RNAlater. The samples were then stored at − 80°C for subsequent analyses. The caecal tissues and contents were weighed and the contents were stored at − 80°C for subsequent analyses.
Measurement of postprandial serum and insulin responses
On day 42, 100 μl of tail blood was collected before the administration of either CM or AM (0 min), and 2 g of the test diet were made available 30 min after milk administration. All rats consumed the available food within 10 min. Blood samples were collected 30, 60, 90, 150, 210 and 270 min after milk administration. The blood samples were immediately centrifuged (1300 g for 15 min at 4°C) and sera were stored at − 80°C. Serum glucose and insulin concentrations were analysed using a glucose test (Wako Pure Chemical Industries, Tokyo, Japan) and a rat insulin ELISA kit (Shibayagi, Gunma, Japan), respectively, according to the manufacturers' instructions.
Oral glucose tolerance test
On day 45, tail blood samples were collected at 17.00 hours without test milk administration or test diet feeding. A glucose solution (200 g/l) was administered via a feeding tube at a dose of 2 g/kg body weight. Tail blood was collected again at 15, 30, 60 and 120 min after glucose administration. Serum collection and serum glucose and insulin analysis were carried out as described above.
Serum TAG and fructosamine analyses
TAG concentrations were determined in serum samples collected at 0 min during the oral glucose tolerance test (OGTT) using a TAG test (Wako, Tokyo, Japan). Serum samples collected from the abdominal aorta at the end of the experiment were used to determine fructosamine levels. Fructosamine was measuring using a colorimetric assay in which glycated proteins reduce nitroblue tetrazolium (BML, Tokyo, Japan).
Real-time quantitative polymerase chain reaction
Total RNA extraction and reverse transcription (cDNA synthesis) was performed as described by Inoue et al. (Reference Inoue, Otsuka and Nishio35). Real-time PCR was performed using a 7300 Real-Time PCR System (Applied Biosystems, Tokyo, Japan). The primers and probes (TaqMan Assays-on-Demand Gene Expression Products) were designed at Biosearch Technologies, Inc. (Tokyo, Japan) from gene sequences obtained from GenBank (proglucagon, NM-017077; glucoamylase, XM-231714; isomaltase–sucrase complex, NM-013061; lactase, XM-341115; SGLT1, NM-013033; GLUT2, NM-012879). Amplification was carried out in a 10 μl reaction volume containing 5 μl of Premix Ex Taq™ (Perfect Real Time; TaKaRa Bio Inc., Shiga, Japan), 0·4 μl cDNA, 0·9 mmol/l of each primer, and 0·25 mmol/l of Taqman probe. The thermal cycling profile was 10 s at 95°C followed by forty cycles of 5 s at 95°C and 34 s at 60°C. The β-actin gene was amplified as a housekeeping gene; however, SYBR Premix Ex Taq™ (Perfect Real Time; TaKaRa Bio Inc.) was used instead of Premix Ex Taq™. The arbitrary gene expression unit for each sample was calculated as described by Inoue et al. (Reference Inoue, Tsuruta and Nojima36).
Terminal-restriction fragment length polymorphism analysis
Whole bacterial DNA was extracted from caecal contents using the QuickGene DNA Tissue kit SII (Fuji Film, Tokyo, Japan), which is a DNA extraction kit for use with a semi-automated nucleic acid extraction machine (QuickGene810; Fuji Film). PCR was performed using primers homologous for conserved regions on the bacterial 16S ribosomal RNA (rRNA) gene: forward primer (27F), 5′-AGA GTT TGA TCC TGG CTC AG-3′; reverse primer (1492R), 5′-GGC TAC CTT GTT ACG ACT T-3′. The forward primer was fluorescently labelled with Beckman D4 dye (Sigma Genosys, Hokkaido, Japan). Amplification was carried out in a 50 μl reaction mixture containing 1 μl of DNA template, 5 μl of 10 × Ex Taq buffer (TaKaRa Bio Inc.), 0·2 mmol/l of each deoxynucleoside triphosphate (dNTP), 0·4 μmol/l of each primer and 1·5 units of Ex Taq DNA polymerase (TaKaRa Bio Inc.). The thermal cycling profile was 94°C for 10 min, followed by thirty cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min 30 s, and a final extension step at 72°C for 5 min. All PCR products were purified using a NucleoFast 96 PCR clean-up plate (Machery-Nagel, Düren, Germany) according to the manufacturer's instructions. Single-stranded DNA sections of the 16S rRNA gene amplicon were digested using mung bean nuclease (Promega, Osaka, Japan). The purified PCR product was incubated for 30 min at 37°C with 4 units of mung bean nuclease and the appropriate buffer in a total volume of 100 μl. After digestion, PCR products were purified using a NucleoFast 96 PCR clean-up plate. The purified products were digested using a restriction enzyme overnight at 37°C in a total volume of 20 μl. Each digest consisted of 17 μl of purified PCR product, 10 units of either restriction endonuclease HhaI or MspI (TaKaRa Bio Inc.) and the appropriate 10 × buffer (M or T buffer; TaKaRa Bio Inc.). The digested products were precipitated in ethanol and re-suspended in 24·7 μl of sample loading solution (SLS; Beckman Coulter, Tokyo, Japan) and 0·3 μl of CEQ 600 bp standard (Beckman Coulter). Terminal-restriction fragment length polymorphism (T-RFLP) profiles were obtained using a Beckman Coulter CEQ8000 DNA analysis system, which was equipped with Beckman Coulter CEQ 8000 Genetic Analysis System software (version 9.0 series, product number A16650)(Reference Countway, Gast and Savai37).
Hierarchical clustering analysis of terminal-restriction fragment length polymorphism profiles
T-RFLP fragment profiles were analysed using the above-mentioned fragment-analysis software and all fragment sizes for each profile were exported in comma-separated values (CSV) file format. The data were further analysed using hierarchical clustering analysis with Euclidean square distances using an Excel macro program as described by Inoue et al. (Reference Inoue and Ushida38).
Organic acid analysis
The concentrations of organic acids (acetic, propionic, butyric, succinic and lactic acid) in homogenates of caecal contents were measured according to the methods described by Shiga et al. (Reference Shiga, Hara and Okano39). Briefly, caecal contents were diluted in four volumes of deionised water and homogenised using a Teflon homogeniser (Iuchi-Seleido, Osaka, Japan). The homogenate was mixed with a NaOH solution (final concentration of 20·8 mmol/l) containing crotonic acid at a final concentration of 4·17 mmol/l. The mixture was then centrifuged and passed through a filter disk with a pore size of 0·2 μm. Filtered samples were analysed via HPLC using a solvent-delivery system (SLC-10 AVP; Shimadzu, Co. Ltd), a double ion-exchange column (Shim-Pack SCR-102 h, 8 × 300 mm; Shimadzu) and an electroconductivity detector (CDD-6A; Shimadzu).
Calculations and statistics
All values are expressed as the mean values with their standard errors. The effects of CM administration on the area under the curve (AUC) for glucose and insulin levels, blood fructosamine concentrations, and caecal organic acids were analysed using two-way ANOVA. Differences in postprandial and OGTT glucose and insulin levels between the CM- and AM-treated groups during the evaluation period were analysed via two-way ANOVA. A Tukey–Kramer test was used for comparisons among groups. A difference of P < 0·05 was considered statistically significant. All data were analysed using STATCEL2 (OMS, Saitama, Japan), which is an add-in application for Microsoft Excel (Redmond, WA, USA).
Results
Initial and final body weights, food intake and energy efficiencies did not differ among groups (data not shown).
Postprandial serum glucose and insulin levels
Postprandial serum glucose concentrations increased slowly until 60 min after feeding and then increased rapidly from 60–90 min in all four groups (Fig. 1(a)). The rats fed the D diet showed significantly higher peak serum glucose concentrations at 90 min compared with the S-fed rats in both the CM-treated (D-CM, 11·31 (sem 0·29) mmol/l v. S-CM, 10·21 (sem 0·20) mmol/l) and AM-treated groups (D-AM, 13·30 (sem 0·72) mmol/l v. S-AM, 11·36 (sem 0·30) mmol/l). Thereafter, serum glucose concentrations decreased gradually until 270 min. Postprandial serum insulin concentrations peaked before postprandial glucose concentrations at 60 min (Fig. 1(b)). Peak values were significantly higher in the AM-treated groups than in the CM-treated groups for both the D- and S-fed rats.

Fig. 1 Changes in postprandial serum glucose (a) and insulin (b) concentrations after oral milk administration (5 ml/rat) and subsequent test diet administration (2 g/rat) in rats. (- -○- -), Dextrin-fed, artificial milk administered; (- -△- -), dextrin-fed, cows' milk administered; (–●–), sucrose-fed, artificial milk administered; (–▲–), sucrose-fed, cows' milk administered. Postprandial serum glucose and insulin tolerance tests were performed on day 43 after the start of experimental diet administration. Values are means for six rats per group, with standard errors represented by vertical bars. P values were estimated for peak values via two-way ANOVA: (a) 90 min blood glucose: diet, P = 0·002; milk, P = 0·001; diet × milk, P = 0·335; (b) 30 min blood insulin (milk administration after 30 min): diet, P < 0·001; milk, P < 0·001; diet × milk, P = 0·027; 60 min (diet fed after 30 min): diet, P = 0·620; milk, P = 0·001; diet × milk, P = 0·97.
AUC analysis (0–270 min) for both postprandial serum glucose and insulin showed significant differences between milk treatments (P = 0·020 for glucose and P = 0·014 for insulin; Table 3). The AUC for insulin was lower in the CM-treated groups than in the AM-treated groups.
Table 3 Area under the curve analysis for postprandial serum glucose and insulin concentrations in rats in response to oral milk administration (5 ml/rat) and subsequent feeding of each test diet (2 g/rat)†
(Mean values with their standard errors for six rats per group)

AM, artificial milk; CM, cows' milk.
* P < 0·05.
† The postprandial blood glucose and insulin tolerance tests were performed on day 43 of the experimental diet administration.
Oral glucose tolerance test
No significant differences were observed in serum glucose concentrations among groups at 0 min (Fig. 2(a)). However, significant differences in serum insulin concentrations were detected at 0 min between the CM- and AM-treated groups (two-way ANOVA; Fig. 2(b)). For all groups, serum glucose and insulin concentrations peaked 15 min after glucose administration and returned to basal levels within 120 min. Two-way ANOVA revealed that peak glucose and insulin values at 15 min were influenced by both diet carbohydrate source and milk type (P < 0·05; Fig. 2(a) and (b)). Peak serum glucose and insulin values were higher in the S-fed rats than in the D-fed rats. In addition, peak serum glucose and insulin values were lower in the CM-treated groups than in the AM-treated groups.

Fig. 2 Changes in serum glucose (a) and insulin (b) concentrations as determined by oral glucose tolerance tests (OGTT) in rats. (- -○- -), Dextrin-fed, artificial milk administered; (- -△- -), dextrin-fed, cows' milk administered; (–●–), sucrose-fed, artificial milk administered; (–▲–), sucrose-fed, cows' milk administered. OGTT were performed on day 45 of the experimental diet. Values are means for six rats per group, with standard errors represented by vertical bars. P values were estimated for peak values via two-way ANOVA: (a) 0 min serum glucose: diet, P = 0·071; milk, P = 0·815; diet × milk, P = 0·401; 15 min serum glucose: diet, P = 0·003; milk, P = 0·004; diet × milk, P = 0·501; (b) 0 min serum insulin: diet, P = 0·108; milk, P = 0·034; diet × milk, P = 0·150; 15 min serum insulin: diet, P < 0·001; milk, P = 0·021; diet × milk, P = 0·137.
In the OGTT, the AUC for serum glucose and insulin concentrations was calculated at 0–30 min, 0–60 min and 0–120 min (Table 4). Two-way ANOVA revealed that the AUC for insulin was higher in the S-fed rats than in the D-fed rats, and lower in the CM-treated groups than in the AM-treated groups. Changes in serum glucose AUC were small but significant at 0–30 min and 0–60 min, and the trends were similar to those observed for insulin. No interactions between diet and milk administration were observed for either insulin or glucose.
Table 4 Area under the curve analysis for serum glucose and insulin concentrations in rats in response to oral glucose administration (2 g/kg)
(Mean values with their standard errors for six rats per group)

AM, artificial milk; CM, cows' milk.
*P < 0·05.
Serum TAG and fructosamine concentrations
Serum TAG concentrations were higher in the S-fed rats than in the D-fed rats (Table 5). CM administration did not significantly affect serum TAG concentrations compared with AM administration. Serum fructosamine concentrations were lower in the CM-treated groups than in the AM-treated groups, regardless of diet type (Table 5). Two-way ANOVA revealed a significant difference in serum fructosamine concentrations between the CM- and AM-treated rats (P = 0·015).
Table 5 Fasted serum TAG and fructosamine concentrations in rats†
(Mean values with their standard errors for six rats per group)

AM, artificial milk; CM, cows' milk.
*P < 0·05.
† Tail blood from 8 h fasted rats was used to determine TAG concentrations on day 45. Abdominal aorta blood was used to determine fructosamine concentrations on day 49.
Weight of caecal contents and concentration of caecal organic acids
Caecal content weights were higher in the CM-treated groups than in the AM-treated groups for both the D- and S-fed rats (Table 6). Total propionate per caecal content was higher in the CM-treated rats than in the AM-treated rats (Table 6). No significant differences were observed among groups in terms of the total molar weights of other organic acids.
Table 6 Pools of organic acids in caecal contents from rats†
(Mean values with their standard errors for six rats per group)

AM, artificial milk; CM, cows' milk.
*P < 0·05.
† Total SCFA = sum of acetate, propionate and n-butyrate concentrations. Caecal contents were used to determine pools of organic acids on day 49.
Caecal microbiota
T-RFLP profiles generated by HhaI digestion are shown in Fig. 3(a)–(d). Peak X (209 bp) was observed more frequently in the D-fed rats than in the S-fed rats, whereas peak Y (364 bp) appeared frequently in the S-fed rats, but not in the D-fed rats. Peak Z (641 bp) was almost uniquely observed in the CM-treated rats, regardless of diet type. After MspI digestion, a unique peak (163 bp) was also observed in the CM-treated rats (data not shown). According to a database generated using the MICA query function (http://mica.ibest.uidaho.edu/digest.php) and a BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi), 16S rRNA sequences from Phascolarctobacterium faecium (GenBank accession, X72865) showed restriction patterns of 641 and 163 bp following HhaI and MspI digestion, respectively. This bacterium is currently the only species known to show this restriction pattern when uncultured bacteria are excluded from the analysis.

Fig. 3 Terminal-restriction fragment length polymorphism profiles representing caecal bacterial diversity in rats administered cows' milk (CM) or artificial milk (AM) while on a dextrin (D)- (a and b) or a sucrose (S)-based diet (c and d). Peak X (209 bp) was observed more frequently in the D- v. S-fed rats, whereas peak Y (364 bp) appeared frequently in the S-fed rats, but not in the D-fed rats. Peak Z (641 bp) was almost uniquely observed in the CM-treated rats, regardless of diet type. Each profile is for a sample from an individual rat (six rats per group).
After constructing a dendrogram based on T-RFLP patterns generated by HhaI digestion, two distinct clusters were observed (Fig. 4). All samples from the D-fed rats, with the exception of D-AM5, clustered into one area; the remaining samples (mostly from S-fed rats) clustered into another area. Within these distinct clusters, samples from the CM-treated rats grouped within the same cluster, whereas samples from the AM-treated rats grouped within a second cluster. The only exception was S-AM6, which grouped with samples from the CM-treated rats. The dendrogram constructed from T-RFLP patterns generated by MspI digestion showed very similar clustering (data not shown).

Fig. 4 Dendrogram based on the terminal-restriction fragment length polymorphism profiles generated using a hierarchical clustering analysis with Euclidean square distances. D-AM, dextrin-fed, artificial milk administered; D-CM, dextrin-fed, cows' milk administered; S-AM, sucrose-fed, artificial milk administered; S-CM, sucrose-fed, cows' milk administered. Each line relates to a sample from an individual rat (six rats per group).
Expression of Na+-dependent glucose transporter-1, glucose transporter-2 and proglucagon in the small intestine
No significant differences were observed among groups in terms of SGLT1 or proglucagon mRNA expression in the duodenum, jejunum or ileum (data not shown). However, a significant difference among groups was detected for GLUT2 mRNA expression in the duodenum, although no significant differences were detected regarding jejunal or ileal GLUT2 mRNA levels. Regardless of diet type, the CM-treated rats showed lower GLUT2 mRNA expression in the duodenum compared with the AM-treated rats. The CM-treated rats showed a 20 % decrease in GLUT2 transcription compared with the diet-matched AM-treated rats (Fig. 5).
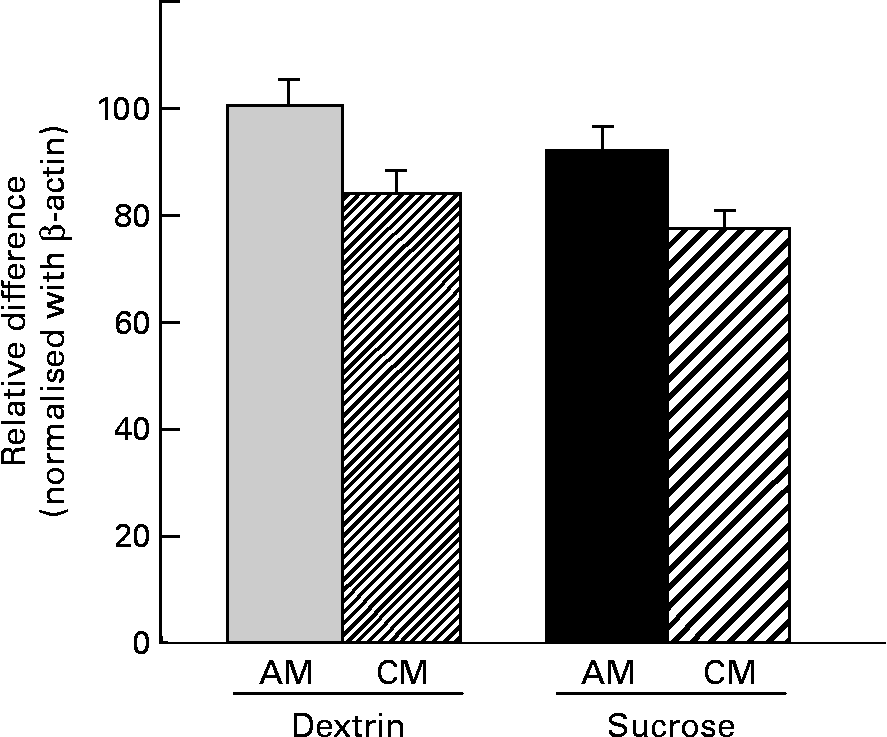
Fig. 5 Duodenal GLUT2 mRNA expression in rats administered cows' milk (CM) or artificial milk (AM) while on a dextrin (D)- or a sucrose-based diet. The arbitrary units for GLUT2 expression were normalised against β-actin expression and subjected to statistical analysis. Data are presented as relative differences when the value of D-AM = 100 %. Values are means for six rats per group, with standard errors represented by vertical bars. Results of the statistical analyses (two-way ANOVA) are: diet, P = 0·262; milk, P = 0·027; diet × milk, P = 0·876.
Discussion
No previous animal study has demonstrated the effects of long-term daily CM consumption on insulin sensitivity using reasonable doses of CM. We conducted these studies using rats, which allowed for the strict control of diet composition and feeding time. We demonstrated that CM improves insulin sensitivity, as determined via the OGTT, in rats fed either a D- or S-based diet. As we hypothesised, improvement of insulin sensitivity by CM was achieved with a reasonable dose of CM and without any replacement of the main ingredients in the diet by CM in a rat model. Rats fed the high-S diet showed insulin resistance; however, CM treatment improved insulin sensitivity, as evidenced by lower fasting and postprandial insulin levels and lower serum fructosamine concentrations compared with the AM-treated rats. This reduction in serum fructosamine suggests that decreased insulin resistance within the CM groups may have been observed at least a few weeks before dissection(Reference Woerle, Neumann and Zschau40, Reference Monnier, Sell and Dai41).
According to the FAO(1), the yearly per capita consumption of CM was 81 kg in 2003, which corresponds to approximately 220 ml/d. In addition, humans obtain approximately 628 kJ per d from CM and related products, which accounts for approximately 6–8 % of total daily energy consumption (8360–10 460 kJ/d). In a preliminary experiment, Fisher 344 rats weighing about 200 g consumed approximately 209 kJ/d on a voluntary basis. For this reason, 5 ml/200 g body weight (14·2 kJ/200 g body weight) was selected as a ‘normal’ daily dose of CM.
For the present study, we prepared AM to have a nutritional composition close to that of CM and to have no or fewer effects on insulin resistance. The egg white protein was used because its amino acid score is equal to that of CM. Although both maltose and sucrose are disaccharides, as is lactose, it is known that sucrose clearly affects insulin sensitivity(Reference Häberer, Thibault and Langhans42). Thus the former was used. The main fatty composition of lard, such as oleic acid, is close to that CM(31). Indeed, the AM-treated rats in the D-fed group showed almost the same concentrations of blood insulin and glucose as rats fed only a D diet in other reports(Reference Damiano, Rosón and Armando43, Reference Suzuki and Hara44). Thus it is suggested that the AM used in the present study might have almost no effects on insulin sensitivity.
No significant interactions were observed between the test diet and test milk in the OGTT and other evaluations, demonstrating that the effects of CM do not depend upon dietary carbohydrate source. These results suggest that CM not only improves insulin resistance in animals with the metabolic syndrome but also suppresses the progression of the metabolic syndrome with glucose intolerance.
Regarding the mechanism of reduced insulin sensitivity, two interesting results were obtained in the present study. The first is that duodenal GLUT2 mRNA expression decreased significantly in the CM-treated rats compared with the AM-treated rats. Second, caecal microbiota SCFA composition profiles differed markedly between the CM- and AM-treated rats.
GLUT2 is normally expressed in both the apical and basolateral membranes of intestinal epithelial cells and is responsible for approximately 60 % of small-intestinal glucose uptake(Reference Gouyon, Caillaud and Carriere45). Decreased GLUT2 expression after CM administration was observed only in the duodenum, not in the jejunum or ileum. Therefore, the impact of CM administration on small-intestinal glucose absorption may not be drastic. However, a 20 % reduction in duodenal GLUT2 mRNA expression after CM consumption may slow glucose absorption within the small intestine, which may underlie the observed improvement in insulin sensitivity. Because CM administration showed no effect on SGLT1 or proglucagon mRNA expression in the small intestine, SGLT1 and glucagon-like peptide-1 may not be involved in CM-reduced insulin sensitivity.
The characteristic T-RFLP peaks observed in the CM-administered rats matched the restriction patterns for 16S rRNA genes in P. faecium, which is reported to produce propionate(Reference Janssen and O'Farrell46, Reference van Gylswyk, Hippe and Rainey47). Indeed, propionate concentrations within caecal contents increased significantly in the CM-administered rats. It has been demonstrated in experimental animals that alterations in intestinal microbiota have a marked impact on insulin resistance(Reference Dumas, Barton and Toye25, Reference Cani, Amar and Iglesias26). Furthermore, in a double-blind human study, it was suggested that the oral administration of a propionate-containing capsule for 7 weeks significantly improved insulin resistance(Reference Venter, Vorster and Cummings48). Therefore, alterations in intestinal microbiota and SCFA profiles may represent a second explanation for the regulatory effects of long-term daily CM administration on insulin resistance.
T-RFLP was used in the present study to reveal the compositional difference in caecal microbiota among the experimental groups. However, T-RFLP is less suitable for the quantification of a specific bacterial population compared with fluorescent in situ hybridisation- and real-time PCR-based analyses of intestinal microbiota(Reference Zoetendal, Collier and Koike49). Thus investigation of the effects of CM on intestinal microbiota using these techniques should be further conducted in the future.
CM is an emulsion that consists of lactose, milk-fat, casein, whey protein, minerals (for example, K and Ca) and other components (for example, peptides and oligosaccharides)(31–Reference Christie and Fox34). It remains unclear which components of CM are responsible for the observed improvement in insulin resistance. Because the AM formula used here contained the same amount of Ca as CM, it is unlikely that Ca is involved in regulating insulin resistance. Instead, lactose and oligosaccharides may be responsible for the observed alterations in the caecal environment and improved insulin sensitivity in the CM-treated rats. Whey protein may also be an important factor in improved insulin resistance, including the reduction of the duodenal GLUT2 expression. Considering these results, the beneficial effects of CM on insulin resistance may be the result of multiple factors.
In conclusion, the present results demonstrate that long-term daily CM administration improves insulin resistance induced by a high-S diet. In addition, CM improves insulin sensitivity in normal rats, lowers small-intestinal glucose uptake via the suppression of GLUT2 expression and alters the large-intestinal environment. These factors may contribute to the observed beneficial effects of daily CM consumption. To our knowledge, the present study is the first to demonstrate improved insulin sensitivity after long-term daily CM ingestion in an animal model. The present results suggest that CM is effective in counteracting the metabolic syndrome, even at reasonable concentrations.
Acknowledgements
A part of the present study was supported by Japan Dairy Association. The authors would like to thank Mr T. Mochida, Mr I. Nojima, Ms A. Shinoki, Mr M. Hagio and Ms N. Matsukawa at the Graduate School of Agriculture (Hokkaido University) for their assistance with the animal experiments. We are also grateful to Ms Y. Kuzuma and Mr S. Hioki (Field Science Centre for Northern Biosphere, Hokkaido University), Dr K. Shimazaki (Graduate School of Agriculture, Hokkaido University) and Dr M. Yajima (Creative Research Initiative Sousei, Hokkaido University) for their assistance with the preparation of the AM.
All authors contributed to the preparation of the paper and agreed with the submitted content.
There are no conflicts of interest.













