Introduction
Cashew (Anacardium occidentale L.), belonging to the family Anacardiaceae, is a fruit tree native to South America, is grown commercially, and has been naturalized throughout the tropics (Adiga et al., Reference Adiga, Muralidhara, Preethi and Savadi2019; Santos et al., Reference Santos, Mayo, Bittencourt and de Andrade2019). Being the second most important tree nut crop in world, it plays a significant agronomic role (Oliveira et al., Reference Oliveira, Mothé, Mothé and Oliveira2020). In addition, it is cultivated in over six million hectares in more than 30 countries worldwide (Savadi et al., Reference Savadi, Muralidhara and Preethi2020). Global cashew consumption is growing due to increasing awareness of associated health benefits as well as increased income in developing countries (Kahlmann and Kohn, Reference Kahlmann and Kohn2018). According to data from the FAO (2018), the Vietnam, India and Cote d'Ivoire were the major producers of cashew nut in 2018 with production of 4 million tons. Moreover, the value of the global cashew nut market is worth US$ 9.9 million (Savadi et al., Reference Savadi, Muralidhara and Preethi2020).
Despite the increase in global consumption of cashews, supply is unable to keep pace with the growing demand (Kahlmann and Kohn, Reference Kahlmann and Kohn2018). It is reckoned that by 2030, about 6.0–6.3 million tons of raw cashew nut production is required to meet the globally growing demands for cashews (Savadi et al., Reference Savadi, Sowmya, Megha, Muralidhara and Mohana2021). The main underlying limitations for cashew production are the widespread use of low-yielding varieties and losses due to diseases, pests, abiotic stresses and poor crop management (Saroj et al., Reference Saroj, Bhat and Srikumar2016; Wonni et al., Reference Wonni, Sereme, Ouedraogo, Kassankagno, Dao, Ouedraogo and Nacro2017).
Cashew genetic improvement aims to meet the demands of the production chain, through the development and availability of cultivars with high productive potential, superior agronomic traits (Nair, Reference Nair and Nair2021). Therefore, efficient strategies for the sustainable production of cashews with high yield, resistance to pests and disease and abiotic stress tolerance need to be developed to meet the growing demand in the consumer market (Rossetti et al., Reference Rossetti, Vidal and Barros2019). To achieve this, it is necessary to select and develop plants with greater yield potential and ability to combat damage induced by the biotic and abiotic components (Paiva et al., Reference Paiva, Barros, Cavalcanti, Jain and Priyadarshan2009).
Brazil is one of the largest producers of cashew nuts, with 10,968 thousand tons of cashew production; it also stands out in the production of the pseudofruit, with 1805 thousand tons production (Savadi et al., Reference Savadi, Muralidhara and Preethi2020). Due to its geographical location, the northeast of Brazil is one of the most favourable regions for cashew cultivation. This is of great importance for small farmers as it provides an alternative source of crop production in the dry season in the region and also generates income with different products such as cashew nuts, pseudofruits (cashew apple) and cashew nut shell liquid (Carneiro et al., Reference Carneiro, Silva, Gomes, Santos, Valente, Gomes and Costa2019).
Although cashew plays an important role in the daily life of subsistence farmers and as a component of biodiversity, the crop has not been given the attention it deserves in terms of research that aims to improve its production and yield (Palei et al., Reference Palei, Dasmohapatra, Samal, Rout, Al-Khayri, Jain and Johnson2019). Breeding cashew is limited due to the lack of knowledge pertaining to the phenotypic diversity of the germplasm and this situation is made worse by the narrow genetic base of recommended cashew varieties and the change in production environment (Adu-Gyamfi et al., Reference Adu-Gyamfi, Barnnor, Akpertey and Padi2022).
Cashew tree orchards originating from seeds have high variability and, probably, adaptability that can be used for selection. Therefore, these areas have notable strategic value for plant breeding for the purpose of identifying superior genotypes (Matos et al., Reference Matos, Nunes, Lopes and Gomes2019). In this sense, the objective of this study was to estimate the phenotypic diversity of cashew trees grown by seeds in Piauí, in the northeastern region of Brazil, based on agro-morphological and physicochemical traits, in order to select genotypes with potential for use in crop improvement programmes.
Materials and methods
Plant material
Individual plants were selected in a cashew population cultivated in the Ipiranga do Piauí region, Brazil, (Fig. 1). The cashew trees of these areas were derived from planting of seeds, which makes genetic variability possible. The plants were georeferenced with Global Positioning System (GPS) and identified by numbered plates, with each plant being considered a genotype. Forty-three cashew trees (genotypes) were randomly selected based on phenotype (phenotypic selection) with the assistance of farmer's, using the following traits: colour of the pseudofruit epidermis (cashew apple), plant size, precocity and phytosanitary condition of the tree.

Fig. 1. Location of the cashew population in Ipiranga do Piauí, Piauí, Brazil.
The cultivation area had no definite spacing between plants, and crop treatment was limited to weed control by hand and mowing around the crop. The sampled plants had no known planting date. The soil in this area has a sandy clay loam texture in the 0 to 40 cm deep layer, and the climate, according to Köppen's classification, is Aw (Medeiros et al., Reference Medeiros, Cavalcanti and Medeiros2020).
Morphological and physicochemical characteristics
The characterization of genetic diversity in cashew trees was based on the guidelines published by IBPGR (1986). Ten fruits with pseudofruits were evaluated from each sample. We selected the following 16 phenotypic characteristics that represent well-defined traits and are important for the characterization of cashew: total weight referring to fruit and pseudofruit weight (TW)/(g), pseudofruit weight (PW)/(g), fruit weight (FW)/(g), fruit length (FL)/(mm), fruit width (FWI)/(mm), fruit thickness (FT)/(mm), kernel weight (KW)/(g), kernel length (KL)/(mm), kernel width (KWI)/(mm), kernel thickness (KT)/(mm), pseudofruit length (PFL)/(mm), basal diameter of pseudofruit (BDPF)/(mm) and apical diameter of pseudofruit (ADPF)/(mm), titratable acidity (TA)/(%), total soluble solids (TSS)/(°Brix) and total soluble solids/titratable acidity ratio (TSS/TA) (Fig. 2).
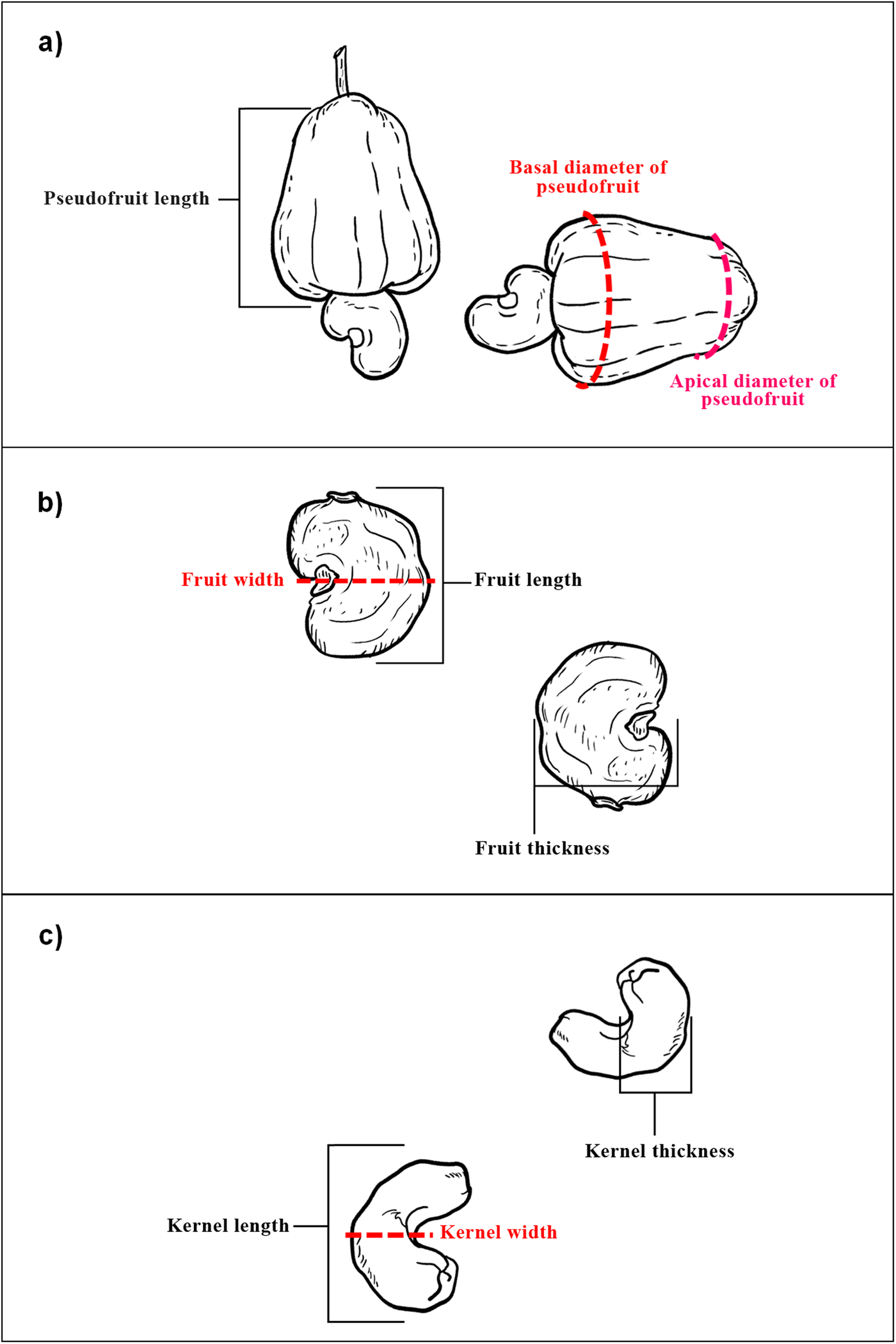
Fig. 2. Schematic drawings of cashew indicating the traits analysed in the morpho-agronomic characterization, established by IBPGR (1986) (a) morphological traits of pseudofruit, (b) morphological traits of fruit, (c) morphological traits of kernel.
Fruit (cashew nut), pseudofruit (cashew apple) and kernel (cashew nut kernel) measurements were performed using analytical scales and digital calipers, with weights expressed in g and the lengths, widths and thicknesses expressed in mm. Total soluble solids were determined with a benchtop refractometer and expressed in degrees Brix scale; titratable acidity was determined according to the methodologies described by Instituto Adolfo Lutz (IAL, 2008).
Statistical analysis
Descriptive statistical parameters (mean, maximum value, minimum value, standard deviation and coefficient of variation) were calculated for the standardized quantitative data. The variation coefficient (CV) for each trait was estimated. For each parameter, Pearson's correlation analysis was used to estimate the relationships between the evaluated variables. In order to classify the genotypes based on the similarities of the parameters used and to identify the most important traits, principal component analysis (PCA) was performed. A scatter plot was created based on the first two components of the PCA.
Furthermore, the samples were clustered using Unweighted Pair-Group Method with Arithmetic averages (UPGMA) (Sneath and Sokal, Reference Sneath and Sokal1973), based on Euclidean distance with standardized values. A cophenetic value matrix of the UPGMA clustering was also used to test the consistency of the dendrogram. These cluster analyses were conducted using the factoextra R package (Kassambara and Mundt, Reference Kassambara and Mundt2017). To measure the capacity of the plant to express a character, spatial repeatability coefficient and number of measures necessary to estimate that character were assessed, with a coefficient of determination of 95%. Genes software (Cruz, Reference Cruz2013) was used for these statistical-genetic analyses.
Results
Morphological and physicochemical characterization
The evaluation of the cashew genotypes revealed significant variation in TW, FW, KW, PL, TA and TSS/TA (Table 1). Genotypes 10 and 28 stood out with respect to TW and BDPF. The fruit lengths, widths and thicknesses found in the 43 genotypes were greater than those of nine genetic materials of early dwarf cashew trees evaluated by Melo-Filho et al. (Reference Melo-Filho, Costa, Cavalcante Junior, Bezerra and Mesquita2006), who observed maximum values of length, width and thickness of 35.26, 28.39 and 20.15 mm, respectively.
Table 1. Morphological and physicochemical evaluation of fruits and pseudofruits of 43 cashew genotypes in Ipiranga do Piauí – PI, northeastern region of Brazil

CV, coefficient of variation; r, estimates of the repeatability coefficient; R 2, estimates of the coefficient of determination; no, number of measurements; TW, total weight; PW, pseudofruit weight; FW, fruit weight; FL, fruit length; FWI, fruit width; FT, fruit thickness; KW, kernel weight; KL, kernel length; KWI, kernel width; KT, kernel Thickness; PFL, pseudofruit length; BDPF, basal diameter of pseudofruit; ADPF, apical diameter of pseudofruit; TA, titratable acidity; TSS, total soluble solid; TSS/TA, total soluble solids/titratable acidity ratio.
The average weight of the fruits was 6.95 g, with highlight to the genotypes 7 and 24, which was a promising observation considering that fruits weighing from 6.07 to 8.29 g are commercially indicated for extraction of nuts (Oliveira et al., Reference Oliveira, Silva, Resende, Pereira, Silva and Egea2019). Regarding the coefficient of variation (CV), the values of BDPF, KWI and AL showed limited variation. In contrast, the physicochemical variables TA and TSS/TA showed high CV, indicating high variability.
Genotypes have high potential for utilization based on their physicochemical characteristics. The acidity values were lower for genotypes 4 and 10, while the highest levels were observed in genotypes 35 and 36. Based on the reference standards established by the Ministry of Agriculture, Livestock, and Supply (MAPA) for the processing of cashew pulp (Brasil, 2013), almost all genotypes of the population met the standards, except for genotypes 3 (8.17 °Brix) and 4 (6.47 °Brix). Among the accessions with the best use potential, the following stood out: genotypes 17, 26, 28 and 37, with values for total soluble solid recorded to be 16.25, 14.67, 14.50 and 14.80 °Brix, respectively.
For selection with maximum efficiency and lower cost, the magnitude of the repeatability should be considered in order to define the number of measurements required to assess the individual's performance. If the repeatability is low, a greater number of measurements increases accuracy considerably if the repeatability is high, a greater number of measurements only slightly increases the accuracy, whereas when the coefficient is medium, it is rarely advantageous to make further measurements to increase the accuracy (Cruz et al., Reference Cruz, Ferreira and Pessoni2012). In this population, the estimated repeatability values ranged from medium to high (Table 1).
The increase in the number of measurements would have little influence on the elevation of accuracy, as confirmed by the determination coefficient (R 2) values, which were above 90%. Thus, adoption of ten measurements for measuring fruits, pseudofruits and kernel was deemed satisfactory. For total soluble solids and acidity, only one measurement was necessary, but for the expression of diversity, three measurements were made. Moreover, the high coefficient of genetic determination (R 2), particularly in most yield-contributing traits, also indicated a favourable condition to identify superior genotypes in relation to the traits.
Correlation coefficients were estimated between the characters to verify the relationship between phenotypic descriptors (Table 2). These estimates were mostly significant, positive and medium to high in magnitude (>0.40). However, TSS did not correlate with other characteristics, except for TA, whose coefficient was positive and of low magnitude (<0.40); acidity showed negative correlation with all other characteristics.
Table 2. Estimates of the correlations between morpho-agronomic and physicochemical characteristics in 43 genotypes cashew in Ipiranga do Piauí, Brazil

n.s, not significant; TW, total weight; PW, pseudofruit weight; FW, fruit weight; FL, fruit length; FWI, fruit width; FT, fruit thickness; KW, kernel weight; KL, kernel length; KWI, kernel width; KT, kernel Thickness; PFL, pseudofruit length; BDPF, basal diameter of pseudofruit; ADPF, apical diameter of pseudofruit; TA, titratable acidity; TSS, total soluble solid; TSS/TA, total soluble solids/titratable acidity ratio.
** and * significant at 1 and 5% respectively by the t-test.
Phenotypic diversity
To analyse the variation pattern of the population genotypes, the UPGMA method was adopted using the Euclidean distance coefficient. A dendrogram showing the phenotypic relationships between the accessions is shown in Fig. 3. When establishing the cutoff point in the dendrogram, based on the average genetic distances, it was possible to observe the formation of six distinct clusters. Group I consisted of 14 genotypes, group II had 10 genotypes, groups III and V had only one representative, group IV contained 14 genotypes and group VI contained three genotypes. Thus, the results of the analysis of clusters by the UPGMA method supported the scatter plot distribution of the first two principal components, which separated accessions into six clusters.

Fig. 3. Dendrogram obtained with UPGMA method using the Euclidean distance matrix for 43 cashew trees of the population of Ipiranga do Piauí, Brazil (ccc = 0.85).
Group I was composed mainly of genotypes with average values for fruit, pseudofruit and kernel characters; group II was formed by cashews with high values of total and pseudofruit weights and a TSS/TA ratio above the average. Group III, with a single individual, was separated owing to a high TSS/TA ratio and titratable acidity. The genotypes of the fourth group were gathered due to below the average values for most of the characteristics. Group V consisted of genotype 10, and was separated by measurements well above the average, in particular those for acidity and TSS/TA ratio. Group VI was formed by individuals with high average total weight and medium TSS/TA ratios. The results of cluster analysis suggested that there was sufficient variation among cashew genotypes in terms of different agro-morphological and physicochemical traits.
The accuracy of the identified groups in the dendrogram was confirmed using the cophenetic correlation coefficient (ccc). According to Rohlf (Reference Rohlf1970), cophenetic correlation values below 0.7 indicate the inadequacy of the method. In this study, the dendrogram showed a ccc of 0.85, which implied that the method was successful in discriminating between different groups.
PCA analysis using 16 phenotypic traits showed that the first two main axes represented 79.3% of the total variations observed (online Supplementary Table S1). The first principal component (PC1), which is the most important component, explained 70.1% of the total variance and had high contributing factor loadings from TW (0.287), PW (0.284), FW (0.272) and FL (0.279). Therefore, they were considered as highly contributive characters for the estimation of diversity, which can be used as a selection criterion. The second axis represented 9.2% of the total variation and differentiated the genotypes based on TA (0.474) and TSS/TA (−0.442).
A scatter plot was constructed by plotting the first two principal components in a two-dimensional space (Fig. 4), revealing the distances between the genotypes and showing their grouping as well as demonstrating the correlations between the characteristics. Therefore, the graphic analysis in relation of these two PCs allowed for the characterization of the genotypes. According to the graph, there was a wide dispersion of accessions, indicating high intrapopulation diversity. The population was clustered into six overlapping groups based on the first two axes of PCA. Genotypes 4, 28 and 43, which were the most divergent genotypes, were observed to be important sources for hybridization. Genotypes 4 and 43 were influenced by the acidity vector, while genotypes 10, 24 and 28 were influenced by fruit, kernel and pseudofruit vectors. Genotype 9 had values close to the overall average for most of the studied characteristics.

Fig. 4. Two-dimensional plot of PCA using all analysed traits for all cashew genotypes of the 43 evaluated population.
Discussion
The cashew genotypes exhibited considerable diversity for the morphological traits analysed; all genotypes could be distinguished by at least one trait. The presence of variation among the genotypes for the morphological and physicochemical traits is indicative of the presence of genetic diversity that may be of value for future breeding programmes (Paiva et al., Reference Paiva, Barros, Cavalcanti, Jain and Priyadarshan2009; Palei et al., Reference Palei, Dasmohapatra, Samal, Rout, Al-Khayri, Jain and Johnson2019).
In the current study, the average values found for weight and length of the pseudofruit were close to those reported by Das and Arora (Reference Das and Arora2017) for A. occidentale (40–75 mm in length and 50–140 g in weight). Overall mean values for length, width, thickness and weight of cashew nuts were similar to those from Côte d'Ivoire, Beninin and Burkina Faso (Wallis et al., Reference Wallis, Bagnan, Akossou and Kanlindogbe2016; Stéphane et al., Reference Stéphane, Halbin and Charlemagne2020; Semporé et al., Reference Semporé, Songré-Ouattara, Tarpaga, Bationo and Dicko2021). The allogamous nature of the species could explain this variability (Ehoniyotan and Udo, Reference Ehoniyotan and Udo2019). Probably trees were freely pollinated and this contributed to the observed high variability. Furthermore, the morphological variations observed in the cashew may be related to human selection, factor having a strong influence on the dispersion of the fruit (Chipojola et al., Reference Chipojola, Mwase, Kwapata, Bokosi, Njoloma and Maliro2009; Borges et al., Reference Borges, Lopes, Britto, Vasconcelos and Lima2018).
Among the 43 cashew, 51.16% (genotypes 5, 7, 10, 11, 12, 14, 15, 16, 17, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 33 and 39) of nuts presented a high weight according to an international classification (Semporé et al., Reference Semporé, Songré-Ouattara, Tarpaga, Bationo and Dicko2021). Thus, most of the nuts have a weight higher than 6 g and therefore acceptable on the international market. These findings are important, considering that in cashew it is interesting to improve traits such as weight, size and nutritional quality. This is because from larger cashew nuts, the industry offers good products to obtain higher consumption patterns (Oliveira et al., Reference Oliveira, Mothé, Mothé and Oliveira2020).
Similarly, physicochemical analysis confirmed the presence of considerable diversity between cashew. For example, a high concentration of total soluble solids observed in some genotypes is desirable for both fresh consumption of fruits and food industry involved in the processing of juices and jellies. High levels of total soluble solids in fruit results in the addition of less sugar as well as less time spent during the processing, thus increasing profits for the industry (Brasil, 2013; Oliveira et al., Reference Oliveira, Silva, Resende, Pereira, Silva and Egea2019).
Damasceno-Júnior and Bezerra (Reference Damasceno-Júnior and Bezerra2002), evaluating a cashew population, obtained lower values of total soluble solids than those shown by some genotypes in this study, whose range of variation was from 6.47 to 16.25 °Brix. Likewise, Pereira et al. (Reference Pereira, Silva, Souza, Pereira, Assunção and Costa2019) when assessed the genetic variability among genotypes of bushy cashew, also found °Brix values lower than those found in this study, confirming the potential of the evaluated genotypes for the industry. Although some accessions have low values of total soluble solids, they can still be used, as fruits with high acidity are quite appreciated, especially in terms of food security, because low pH and high acidity values are results that favour product preservation and prevent the growth of yeasts (Borges, Reference Borges2015; Freitas et al., Reference Freitas, Dantas, Araújo and Garruti2020).
Overall, the 16 phenotypic traits analysed in this study showed high correlations. Correlation analysis is highly important in extending the association between traits in such a way that selection of a character, particularly in complex economically important traits such as yield, results in progress (Akinwale et al., Reference Akinwale, Gregorio, Nwilene, Akinyele, Ogunbayo and Odiyi2011).
The lack of correlation between TSS (°Brix) and fruit, pseudofruit and kernel characteristics shows the potential for selecting superior genotypes for these characteristics. On the other hand, the selection of genotypes with desirable fruit and pseudofruit characters is facilitated by the presence of positive and significant correlations between them, and indirect selection can be performed using easily measurable descriptors (Gadissa et al., Reference Gadissa, Tesfaye, Dagne and Geleta2020).
The correlation coefficient between ADPF and PFL was the only non-significant relationship between the morphological characteristics evaluated. Thus, the selection of genotypes with desirable characteristics for pseudofruit and kernel in a single plant is possible. Hence, genotypes with greater weight, length and width should receive special attention in efforts toward the improvement of cashew yield.
The results of the PCA showed that parameters considered in this study can constitute two major synthetic axes that abridge 79.3% of the variability within the population. Andrade et al. (Reference Andrade, Nascimento, Sousa, Santos and Mayo2019) reported an accumulated variation of 76.9% for the first five principal component axes in a morphometric study of ten wild coastal cashew populations from Piauí, northeast Brazil. Kouakou et al. (Reference Kouakou, Konan, N'Da Adopo, N'Da, Djaha, Minhibo, Djidji, Dosso and N'Guessan2018) reported a cumulative variance of 82.21% for the first four axes in the evaluation of the morphological diversity of 48 cashew trees cultivated in the regions of Poro and Bagoué, North-Central Côte d'Ivoire. Likewise, Carneiro et al. (Reference Carneiro, Silva, Gomes, Santos, Valente, Gomes and Costa2019) showed that the first three principal components explained 81.72% of the diversity shown by the genotypes of cashew trees in northeast Brazil; the variables related to the pseudofruit had higher scores and were the main constituents of the first principal component.
The pseudofruit length vector was the least expressed in the morpho-agronomic descriptors used. This result is in agreement with a study by Santos and Santos-Junior (Reference Santos and Santos-Junior2015), who, working with A. humile, described length as the least important character. Classification of species of the genus Anacardium based on the analysis of principal components was performed by Dasmohapatra et al. (Reference Dasmohapatra, Rath, Pradhan and Rout2014), Vieira et al. (Reference Vieira, Mayo and Andrade2014) and Borges (Reference Borges2015).
The method of clustering using phenotypic descriptors was adequate to verify the existence of genetic diversity and similarity among the accessions. Genotypes 32 and 35 (0.352) were the closest, and genotypes 28 and 43 (3.053) were the most distant according to the Euclidean distance matrix. According to Carneiro et al. (Reference Carneiro, Silva, Gomes, Santos, Valente, Gomes and Costa2019), the selection of genotypes based on a given cluster depends on the extent of inter-cluster distance, cluster mean and per cent performance, as they make it possible to describe and differentiate accessions and identify contrasting genotypes in order to perform promising crosses. This is interesting from the point of view of breeding, as it may indicate the existence of distinct gene pools that can be explored by selection (Hawerroth et al., Reference Hawerroth, Bordallo, Oliveira, Vale, Vidal and Melo2019; Kouakou et al., Reference Kouakou, Konan, Doga and Kouadio2022). Thus, based on pairwise cluster distance, genotypes 28 and 43 may be further explored for their potential use in plant breeding for crop improvement. The cluster pattern set by UPGMA was in agreement with PCA, with few inconsistencies in relation to the number of genotypes in the formation of clusters. The results showed that the distribution of genotypes of the first two principal components was in agreement with the cluster analysis. Moreover, the interpretation of these analyses demonstrated high phenotypic diversity among the genotypes of the study population. This genetic diversity may be of use for future breeding programmes to develop high-yielding cashew varieties with improved quality attributes.
Conclusion
Northeast Brazil, with its characteristic climatic conditions, has a rich diversity of cashew (A. occidentale) which could constitute a suitable genetic resource for breeding cashew. Herein, we have reported the existence of a wide diversity among the cashew genotypes of the evaluated population as well as confirmed the usefulness and importance of agro-morphological and physicochemical traits to study phenotypic diversity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262123000102.
Acknowledgements
The authors are grateful to Federal University of Piauí (UFPI) for the support and encouragement in conducting this research. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.








