Observational evidence linking particular dietary factors with a reduced incidence of cardiovascular morbidity and mortality has been used extensively to support various public health promotion strategies(Reference He, Nowson and MacGregor1, Reference Trichopoulou, Costacou and Bamia2). Increasingly, however, investigators are designing randomised controlled trials to confirm whether those diets or food groups may offer vascular protection. While hard clinical endpoints such as myocardial infarction, stroke and death are the ideal in such work, their use would necessitate prohibitively large, prolonged studies. The Dietary Approaches to Stop Hypertension (DASH) trial typifies much of the research in this field in that it recruited mildly hypertensive, but otherwise healthy, volunteers to an 8-week intervention(Reference Appel, Moore and Obarzanek3). While DASH was large enough to successfully employ a clinically relevant endpoint (brachial blood pressure), most dietary intervention studies rely on surrogate measures that will sensitively detect much earlier changes in arterial physiology. To underline this point, a recent meta-analysis considered randomised controlled trials which examined the link between flavonoids/flavonoid-rich foods and cardiovascular risk(Reference Hooper, Kroon and Rimm4). Of the 133 studies included, none had cardiovascular morbidity or mortality as endpoints.
A range of vascular function methodologies is available to clinical researchers, and the choice for any one study is usually governed by local expertise and experience. However, it is vital that nutrition researchers have a clear understanding of the metric which their chosen technique will generate, and how applicable this is to their overall research question.
Here we consider endothelial vasodilator function, pulse wave mechanic analysis and biomarker measurement in the evaluation of arterial health during nutrition intervention studies conducted among human volunteers.
Endothelial dysfunction
All blood vessels are lined by an active cellular monolayer, known as the endothelium, which is responsible for many vital aspects of vascular homeostasis(Reference Félétou and Vanhoutte5). Endothelial cells produce a wide range of paracrine mediators, with multiple beneficial actions including anti-thrombotic, anti-platelet, anti-inflammatory and vasodilatory effects(Reference Davignon and Ganz6). Established cardiovascular risk factors are known to encourage the evolution of an atherosclerotic plaque by unfavourably altering endothelial cell physiology(Reference Hansson7). Thus, assessing the status of endothelial cells in vivo through their ability to produce NO, and thus mediate arterial dilatation, is common in cardiovascular research(Reference Tousoulis, Antoniades and Stefanadis8). Provocation of NO production can be either mechanical (flow-mediated) or pharmacological.
Flow-mediated dilatation
Flow-mediated dilatation (FMD) describes arterial dilatation in response to increased intra-luminal shear stress. In humans, this phenomenon has been described using a forearm technique, in which reactive hyperaemia following release of an arm cuff at suprasystolic pressures mediates increased brachial artery diameter(Reference Celermajer, Sorensen and Gooch9). The vasodilatation can be quantified using B-mode ultrasound, and agreed protocols have been published to guide investigators using this procedure(Reference Corretti, Anderson and Benjamin10, Reference Deanfield, Donald and Ferri11). Since the technique does not involve needles, it has proven popular with researchers and less daunting for potential volunteers.
A growing body of research has not only demonstrated impaired brachial artery FMD among patients with recognised cardiovascular risk factors(Reference Lekakis, Papamichael and Vemmos12–Reference Evans, Anderson and Graham14), but also suggests that the measure may serve as an independent prognostic indicator in both high-risk populations(Reference Brevetti, Silvestro and Schiano15–Reference Patti, Pasceri and Melfi17) and healthy volunteers(Reference Yeboah, Crouse and Hsu18).
Brachial FMD has been employed by several groups as an endpoint during diet-related intervention trials. The effect of n-3 fatty acids on endothelial function has recently been reviewed(Reference Egert and Stehle19), as has the effect of fruit polyphenols(Reference Chong, Macdonald and Lovegrove20), berries(Reference Basu, Rhone and Lyons21) and green tea(Reference Moore, Jackson and Minihane22, Reference Wolfram23) on vascular health, and these reviews include studies using FMD endpoints. In Table 1 (Reference Balzer, Rassaf and Heiss24–Reference Hermann, Spieker and Ruschitzka34), the intervention studies that have examined the effect of chocolate or cocoa on FMD are shown. Consumption of dark chocolate has been shown to improve brachial FMD, both acutely (six out of seven studies), and chronically (five out of six studies).
Table 1 Effect of cocoa or chocolate interventions on flow-mediated dilatation (FMD): evidence from randomised controlled trials

↑ , Significant increase; CAD, coronary artery disease; ↔ , no significant change.
While it provides a minimally invasive option for vascular function assessment, the measurement of brachial FMD does rely on considerable skill in ultrasound image acquisition and concerns about this technique's reproducibility have been expressed(Reference Sanderson, Sattar and Olthof35). De Roos et al. report substantial within-subject variability for FMD among healthy volunteers, with a CV estimated at approximately 50 %(Reference De Roos, Bots and Schouten36). Although much better CV figures (7–10 %) have been published by Deanfield's group(Reference Donald, Charakida and Cole37, Reference Donald, Halcox and Charakida38), there remains a concern that, when employed in smaller, less experienced centres, the technique's inherent variability precludes adequate study power. In addition, the NO dependency of FMD has been questioned(Reference Tschakovsky and Pyke39), such that this response is better considered a consequence of interplay between competing dilator and constrictor influences(Reference Mullen, Kharbanda and Cross40).
Another potential concern is that not all prospective studies have identified brachial FMD as an independent predictor of cardiovascular morbidity. Fathi et al. report no relationship between FMD and the risk of death, acute coronary syndrome or stroke among patients with established risk factors(Reference Fathi, Haluska and Isbel41). Austrian investigators note similar findings among patients undergoing coronary angiography(Reference Frick, Suessenbacher and Alber42) while in almost 3000 older adults, brachial artery diameter and FMD proved equally effective predictors of vascular outcome(Reference Yeboah, Crouse and Hsu18). However, although not always shown to be an independent predictor of cardiovascular events, this does not preclude its use as an endpoint in dietary intervention studies, as long as interpretation of the result is appropriate.
Despite these reservations, brachial FMD is widely regarded as the ‘gold standard’ technique by which to assess conduit vessel function in cardiovascular research(Reference Deanfield, Halcox and Rabelink43). However, before adopting FMD as the endpoint of choice, trial investigators should consider whether conduit vessel function is the best metric with which to detect any intervention-related effect. The response quantified in standard FMD protocols depends upon forearm ischaemia-induced hyperaemic flow, which is itself a function of reduced vascular resistance. The latter is determined largely by endothelium-dependent microvascular tone and thus measures of reactive hyperaemia have been suggested as novel arterial descriptors(Reference Widlansky44). Analysis of brachial ultrasound recordings from over 2000 Framingham volunteers showed that Doppler-derived indices of hyperaemic shear stress correlated better with established cardiovascular risk factors than did FMD(Reference Mitchell, Parise and Vita45). Huang et al. have shown that lower hyperaemic flow velocities in the brachial artery following a standard period of forearm ischaemia independently predicted postoperative morbidity and mortality among patients with peripheral arterial disease undergoing elective vascular surgery(Reference Huang, Silver and Shvenke46).
It has been speculated that the microvascular dysfunction implied by reduced hyperaemic flow responses may be more sensitive to early atherosclerotic change than are disturbances in conduit artery vasomotion as quantified by FMD(Reference Philpott and Anderson47). This has particular relevance for the design of dietary intervention trials which often seek to detect subtle changes in healthy volunteers. It would therefore be considered best practice to record FMD and reactive hyperaemia concurrently, as reactive hyperaemia can be measured simultaneously with FMD, using the same equipment.
Pharmacological provocation of endothelium-dependent vasomotion
Where FMD uses mechanical shear stress to provoke arterial endothelial vasodilator production, the local infusion of appropriate agonists can produce a similar effect. While based on well-established physiological and pharmacological principles, this approach does rely on successful arterial puncture.
Furchgott & Zawadzki's description of acetylcholine-mediated endothelium-dependent vasodilatation using isolated arterial segments in vitro was soon extended to in vivo human work(Reference Furchgott and Zawadzki48). Within the coronary circulation, direct intra-arterial injection of acetylcholine mediated vasoconstriction among patients with significant atherosclerotic lesions but dilated angiographically normal vessels(Reference Ludmer, Selwyn and Shook49). Several prospective studies among patients with clinical indications for cardiac catheterisation have established an abnormal coronary response to endothelium-dependent agonists as a powerful independent predictor of cardiovascular morbidity(Reference Al Suwaidi, Higano and Holmes50–Reference Targonski, Bonetti and Pumper53). The potential risks associated with invasive cardiac assessment limit this technique's applicability to trials conducted in healthy volunteers. However, the forearm is an accessible and relatively safe vascular bed, in which pharmacological challenges analogous to those described for epicardial vessels can be performed.
While coronary procedures have relied upon quantitative angiography, forearm studies mainly employ venous occlusion plethysmography to measure the efficacy of intra-brachial vasodilators. Concordant, proportionate responses to acetylcholine have been described in synchronously infused coronary and brachial arteries(Reference Hirooka, Imaizumi and Tagawa54, Reference Tagawa, Mohri and Tagawa55). Poor forearm dilator responses to acetylcholine have been shown to predict increased rates of cardiovascular morbidity among hypertensive volunteers(Reference Perticone, Ceravolo and Pujia56) and patients with coronary artery disease(Reference Heitzer, Schlinzig and Krohn57, Reference Fichtlscherer, Breuer and Zeiher58).
It is important to appreciate that, when infused into the brachial artery, vasoactive agents exert their influence on forearm blood flow by modulating small vessel tone and this technique is thus an assessment of microvascular function(Reference Wilkinson and Webb59).
Forearm blood flow response to locally infused endothelium-dependent vasodilators has been used as an endpoint in several dietary intervention trials. Healthy adults who consumed a Mediterranean-style diet for 6 weeks showed significant improvements in endothelium-dependent forearm hyperaemia(Reference Singh, Graves and Taylor60, Reference Ambring, Friberg and Axelsen61); however, Ambring et al. subsequently reported negative findings for a similar 4-week intervention conducted among younger volunteers using the same endpoint(Reference Ambring, Friberg and Axelsen61). Investigators have also employed this technique to document the deleterious effects of increasing salt consumption. It was shown that 5 d of salt loading significantly reduced forearm blood flow responses to intra-brachial acetylcholine(Reference Tzemos, Lim and Wong62). Our group recently reported a significant dose-dependent relationship between increased dietary fruit and vegetable consumption and improved microvascular endothelial function, as quantified using this method(Reference McCall, McGartland and McKinley63).
However, the applicability of forearm blood flow manipulation through local vasodilator infusion to large, multi-centre clinical trials is limited by its reliance on arterial puncture, and the prospect of needle insertion is likely to prove inherently unattractive to many.
The most serious risks of brachial artery cannulation include occlusion of the artery leading to limb ischaemia, and median nerve damage due to direct trauma or compression by haematoma or infection. In reality, complications are rare, and usually involve minor bruising or local discomfort that resolve quickly, without intervention. It is, however, essential that this procedure is performed by an experienced researcher (with a background in vascular access and aseptic techniques) such as an intensivist, cardiologist or surgeon. This therefore restricts the use of this methodology to research centres with access to the aforementioned skilled operators, and also limits its use to smaller studies. The requirement for needle insertion may also deter participants, and those on oral anticoagulation or with significantly increased BMI must be excluded(Reference Wilkinson and Webb59, Reference Benjamin, Calver and Collier64, Reference Kennedy, Grocott and Schwartz65).
An alternative, less invasive, approach to pharmacological manipulation of the microvascular endothelium involves transdermal drug delivery by iontophoresis(Reference Morris and Shore66). A small electrical current is applied to the forearm and mediates movement of polar drugs such as acetylcholine into cutaneous vessels. Changes in skin blood flow are then quantified using laser Doppler flowmetry(Reference Kvandal, Stefanovska and Veber67). In a recent study, a strong correlation between this measure of skin microvasculature and FMD of the brachial artery was reported(Reference Debbabi, Bonnin and Ducluzeau68). Therefore, this may offer an alternative endpoint for future intervention trials, although the clinical relevance is, as yet, less established than for other methods.
A small number of trials have already used this endpoint, including a trial of fruit and vegetable purée-based drinks (a trend towards an effect shown on this endpoint in both acute and chronic settings)(Reference George, Niwat and Waroonphan69), a trial of a green tea polyphenol extract (no effect in the tested chronic setting)(Reference Frank, George and Lodge70), a study of acute fish oil consumption (effect on endpoint demonstrated)(Reference Armah, Jackson and Doman71) and a chronic study of weight reduction and exercise (no effect demonstrated)(Reference Hamdy, Ledbury and Mullooly72). A recent study of orange juice demonstrated an acute postprandial effect of orange juice or hesperidin consumption on microvascular reactivity, but no effect on fasting reactivity after 4 weeks of consumption(Reference Morand, Dubray and Milenkovic73).
Non-invasive assessment using pulse wave mechanics and pulse contour analysis
A range of commercially available devices offers clinical investigators the opportunity to rapidly acquire arterial descriptors, usually by non-invasively recording pulse pressure tracings through a device, which then computes one or more output variables. While user friendly and therefore popular, these techniques rely on important biomechanical assumptions which often complicate their applicability and interpretation.
Translating intermittent ejection of blood from the heart to smooth end-organ perfusion is a complex biomechanical process that relies on optimal ventricular–vascular coupling. A variety of mechanical arterial descriptors has been used to quantify unfavourable disease-related changes in this interaction(Reference Hamilton, Lockhart and Quinn74). Since these parameters are derived largely from non-invasive techniques, they have proved popular surrogate endpoints during intervention trials in cardiovascular medicine(Reference Deanfield, Halcox and Rabelink43). Popular methods include measuring pressure pulse wave velocity (PWV) across a particular arterial segment and calculating indices of vascular compliance by mathematical pulse contour analysis. The effects of dietary and nutrient interventions on these endpoints have recently been systematically reviewed(Reference Pase, Grima and Sarris75).
Pulse wave velocity
The velocity with which pressure pulse waves travel along an arterial segment can be mathematically related to that vessel's mechanical properties, by either the Moens–Kortweg or Bramwell–Hill equations(Reference Oliver and Webb76). These formulae predict that pressure pulse waves will travel faster in less distensible arteries and thus PWV is a commonly cited descriptor of ‘arterial stiffness’(Reference Hughes, Dixon and McVeigh77). A pressure transducer or tonometer is used to detect passage of the pulse wave between two anatomical locations. This can be done sequentially(Reference Schillaci, Pirro and Vaudo78) or simultaneously(Reference Laurent, Katsahian and Fassot79), with gating to a contemporaneously recorded electrocardiogram. The carotid and femoral arteries are commonly chosen tonometry sites, as this allows estimation of aortic PWV.
Prospective data are now available to suggest that carotid-femoral PWV (CFPWV) is an independent predictor of cardiovascular morbidity among healthy individuals(Reference Mattace-Raso, van der Cammen and Hofman80, Reference Willum-Hansen, Staessen and Torp-Pedersen81). While tonometry at the radial site is much more convenient for both volunteer and investigator, carotid-radial PWV did not predict coronary events or strokes among a group of patients with end-stage renal failure in whom CFPWV did have independent prognostic value(Reference Pannier, Guerin and Marchais82). This finding reflects structural distinctions between muscular forearm arteries and larger elastic vessels such as the aorta where medial cells have ectodermal rather than mesodermal origins(Reference Safar and Laurent83).
As the intra-luminal pressure within an artery increases, progressively more collagen fibres are recruited and thus its mechanical characteristics change(Reference Payne and Webb84). An intervention which reduces blood pressure is, therefore, also likely to reduce PWV independent of any pleiotropic effect on the arterial wall(Reference O'Rourke and Nichols85). This is an important caveat to the interpretation of any trial which employs PWV as an endpoint. Several recent trials have reported significant reductions in CFPWV following interventions including weight reduction(Reference Dengo, Dennis and Orr86), DASH(Reference Blumenthal, Babyak and Hinderliter87), low carbohydrate(Reference Keogh, Brinkworth and Noakes88) and low-glycaemic index(Reference Philippou, Bovill-Taylor and Rajkumar89) diets. However, in each case, significant blood pressure reductions are also recorded. Similarly, in a Na-loading study among hypertensive volunteers, changes in CFPWV were positively correlated with changes in brachial blood pressure(Reference Todd, Macginley and Schollum90), whilst 6 months' supplementation with conjugated linoleic acid(Reference Sluijs, Plantinga and de Roos91) or 2 weeks of dietary salt restriction(Reference Dickinson, Keogh and Clifton92) failed to alter either blood pressure or CFPWV. However, an isoflavone intervention over 6 weeks did mediate significantly slower CFPWV in healthy volunteers without 24 h ambulatory blood pressure reduction(Reference Teede, McGrath and DeSilva93).
In summary, if an intervention mediates a significant effect on arterial blood pressure, a concordant effect on PWV will be observed. This does not imply an alteration of vascular structure or function and can be predicted from arterial physiology.
Before choosing it as endpoint, investigators should postulate a mechanism by which their proposed intervention could change CFPWV. Through an in vitro model, impairment of local endothelial NO production has been shown to increase PWV across predefined arterial segments(Reference Wilkinson, MacCallum and Cockcroft94, Reference Schmitt, Avolio and Qasem95). Furthermore, brachial artery FMD has been independently associated with CFPWV in cross-sectional technique comparison studies among healthy volunteers(Reference McEniery, Wallace and Mackenzie96) and patients with isolated systolic hypertension(Reference Wallace, Yasmin and McEniery97). A significant, positive correlation between carotid-radial PWV and microvascular function in the forearm has also been described(Reference McCall, McGartland and Woodside98).
However, during the relatively brief trials that typify much human dietary intervention work it is questionable whether subtle alterations in levels of an endothelium-derived paracrine mediator could significantly change CFPWV which principally reflects aortic medial elastin:collagen ratios. Thus, this parameter is not ideal for use in short-term studies which aim to evaluate the possible endothelial effects of an intervention.
Pulse contour analysis
Palpation and analysis of the radial pulse as a means by which to assess systemic arterial health is a long-established practice in cardiovascular medicine(Reference Oparil and Izzo99). Algorithm-based reconstruction of the aortic pressure pulse from tonometer-derived radial waveforms has fuelled renewed interest in this approach(Reference O'Rourke and Nichols85). A commercially available device has been widely used in cardiovascular research to estimate the aortic augmentation index, a measure of arterial wave reflection(Reference Karamanoglu, O'Rourke and Avolio100, Reference Chen, Nevo and Fetics101). Since it is non-invasive, requires minimal training and generates an easily interpretable numeric output, this technique has proved popular in cardiovascular research.
Among patients undergoing cardiac catheterisation, those in the highest quartile of augmentation index had significantly more coronary disease(Reference Weber, Auer and O'Rourke102). Similarly, patients with end-stage renal failure who had higher augmentation indices were more likely to die during an 8-year follow-up period(Reference London, Blacher and Pannier103). However, a recent review of arterial stiffness by The Framingham Heart Study found that whilst a higher aortic PWV was associated with a 48 % increase in cardiovascular events, the aortic augmentation index, central pulse pressure and pulse pressure amplification showed no such correlation(Reference Mitchell, Hwang and Ramachandran104). Like PWV, the aortic augmentation index is dependent on distending arterial blood pressure(Reference Booth, Wallace and McEniery105, Reference Dart, Gatzka and Cameron106), but additional variables, including height(Reference Yasmin and Brown107) and heart rate(Reference Wilkinson, MacCallum and Flint108), must be factored in to its interpretation.
A number of nutrition intervention studies have used pulse contour analysis as an intermediate measure of vascular function. In an acute feeding study, food and water, but not water alone, reduced the aortic augmentation index 2 h after consumption(Reference Ahuja, Robertson and Ball109). This finding is confounded by concomitant decreases in arterial blood pressure and could be argued to reflect mean blood pressure change and therefore altered vessel haemodynamics, rather than an intrinsic alteration of vascular function. After 8 weeks, both low-fat and low-carbohydrate hypoenergetic diets significantly decreased brachial blood pressure among overweight volunteers, but only the former mediated a significant reduction in aortic augmentation(Reference Bradley, Spence and Courtney110).
Again, investigators should have a clear hypothesis about the mechanism through which their intervention is likely to alter pulse wave morphology before choosing aortic augmentation as the endpoint. It has been shown that pharmacological manipulation of systemic endothelial NO production significantly changes aortic pressure pulse wave morphology and thus the augmentation index(Reference McEniery, Wallace and Mackenzie96). In a situation analogous to the intra-brachial infusion of vasodilators and subsequent forearm blood flow measurement, systemic salbutamol (endothelium-dependent) and nitroglycerine (endothelium-independent) are administered with resulting changes in tonometry-derived aortic augmentation quantified to arrive at a ‘global endothelial function index’(Reference McEniery, Wallace and Mackenzie96). Such an approach may prove more sensitive to altered vascular health than resting measurements of aortic augmentation.
Biomarkers of vascular function
Biomarker measurement remains a popular endpoint in clinical research, as it is minimally invasive and samples can be stored for future analysis. A wide variety of biochemical species has been employed to quantify inflammation, oxidative stress, endothelial activation and arterial injury. Since no single ‘gold standard’ measure has emerged, it is common practice during dietary research trials to employ a panel of such markers.
Biochemical measures
Since atherosclerosis is characterised by ongoing vascular inflammation, the acute-phase reactant C-reactive protein (CRP) has been proposed as a useful tool for improving disease prediction models(Reference Albert and Ridker111). A high-sensitivity assay is used to accurately measure the lower CRP concentrations encountered among healthy individuals. Recent data suggest that statin-mediated reductions in CRP are associated with lower rates of cardiovascular morbidity(Reference Ridker, Danielson and Fonseca112).
The evidence regarding dietary interventions and CRP is equivocal. For example, as summarised in Table 2 (Reference Berry, Mulla and Chowienczyk113–Reference Watzl, Kulling and Moseneder128), only five out of sixteen fruit and vegetable randomised controlled trials (including Mediterranean diet and DASH trials, as fruit and vegetables are key components of these diets) have demonstrated a lowering in CRP levels. The duration of these five studies varied from 4 weeks in three studies, to 3 months in one study and 2 years in another. Of the studies, two were juice-based, one was a carotenoid-rich FV intervention and two were Mediterranean diet interventions. We refer the reader to a review by Giugliano et al. (Reference Giugliano, Ceriello and Esposito129) for a broader discussion of the association between diet and inflammation.
Table 2 Effect of fruit and vegetable (FV) interventions on C-reactive protein (CRP): evidence from randomised controlled trials

↔ , No significant change; ↓ , significant decrease; DASH, Dietary Approaches to Stop Hypertension; VBA, vegetables, berries and apples; CAD, coronary artery disease.
* Orange and blackcurrant juice reduced CRP relative to sugar drink.
† CRP decreased only after Mediterranean diet with olive oil.
In mediating leucocytic infiltration of the arterial intima, glycoprotein membrane components such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) promote oxidative stress and ongoing arterial inflammation(Reference Ferrario and Strawn130). Activated endothelial cells are thought to shed these molecules into the circulation, allowing levels of their soluble form to be quantified as an estimate of ongoing arterial injury(Reference Hwang, Ballantyne and Sharrett131). Adipocytes also express ICAM-1, and the soluble form of ICAM-1 is elevated in obese patients, being expressed in the stromal-vascular fraction of adipose tissue(Reference Brake, O'Brian Smith and Mersmann132). This is likely to contribute to the link between obesity and inflammatory complications such as atherosclerosis.
Supplementation with isoflavones for 6 weeks has been reported to significantly reduce serum levels of VCAM-1 in healthy subjects(Reference Teede, McGrath and DeSilva93), while additional dietary α-linolenic acid had a similar effect among dyslipidaemic male patients(Reference Rallidis, Paschos and Papaioannou133). Over a 24-month period, daily consumption of the phyto-oestrogen genistein significantly reduced circulating levels of both ICAM-1 and VCAM-1(Reference Atteritano, Marini and Minutoli134). While additional cherry consumption for 4 weeks lowered CRP levels among healthy volunteers, it had no effect on ICAM-1 concentrations(Reference Kelley, Rasooly and Jacob135) and similar negative results have been noted for brief fruit and vegetable interventions(Reference Paterson, Gordon and Niwat125).
Oxidation both contributes to and follows on from the continuous cycle of low-grade vascular inflammation which characterises atherosclerotic arterial degeneration(Reference Chisolm and Steinberg136). Markers of systemic oxidative stress in biological fluids have long been suggested as surrogates for vascular injury, and the isoprostanes, derived from non-enzymic arachidonic acid peroxidation, are commonly measured(Reference Roberts and Morrow137). A dietary intervention study has reported that urinary levels of 8-iso-PGF2α were significantly reduced among healthy women consuming nine or ten portions of fruit and vegetables daily for 8 weeks(Reference Thompson, Heimendinger and Sedlacek138). However, investigators have largely reported negative findings when this endpoint has been employed to study the effects of black tea(Reference O'Reilly, Mallet and McAnlis139), a Mediterranean diet(Reference Ambring, Friberg and Axelsen61), five or six portions of fruit and vegetables daily(Reference McCall, McGartland and Woodside98, Reference Thompson, Heimendinger and Sedlacek138) and pure dietary flavonoids(Reference Loke, Hodgson and Proudfoot140).
Other potential biomarkers of the atherosclerotic process include the enzyme lipoprotein-associated phospholipase A2(Reference Zalewski and Macphee141, Reference Packard, O'Reilly and Caslake142), tissue plasminogen activator(Reference Thogerson, Jansson and Boman143, Reference Thompson, Kienast and Pyke144) and plasminogen activator inhibitor-1(Reference Hamsten, de Faire and Walldius145).
Novel biochemical approaches
The array of biomarkers available and lack of an agreed ‘gold standard’ often prohibits rational study design. To quantify the biological effects of an ‘anti-inflammatory mix’ dietary supplement among obese volunteers, Bakker et al. employed a novel ‘nutrigenomics’ approach(Reference Bakker, van Erk and Pellis146). This involved measurement and integrated analysis of 120 plasma proteins, 274 plasma metabolites and peripheral blood cell transcriptomes. While such a comprehensive approach is labour intensive and statistically complex, it may represent a valuable means by which to define more subtle intervention-related changes.
More recently, circulating endothelial microparticles (EMP), endothelial progenitor cells and endothelial cells have also been used as indices of vascular health.
Endothelial microparticles
The endothelium is responsible for a diverse range of functions, including regulation of vascular vasomotor activity, coagulation activity, anti-inflammatory status, and therefore a comprehensive assessment of endothelial function or dysfunction is difficult, with available methods usually only providing information on one separate aspect of endothelial function. It has been proposed recently that EMP may fulfil the role of a more universal marker of vascular health(Reference Shantsila147). EMP are small non-nucleated phospholipid vesicles shed from injured endothelial cells in response to pro-inflammatory stimuli and vascular injury. They affect endothelial NO synthesis(Reference Amabile, Guerin and Leroyer148, Reference Boulanger, Scoazec and Ebrahimian149), diminishing acetylcholine-induced vasorelaxation and NO production by endothelial cells in vitro (Reference Brodsky, Malinowski and Golightly150), correlate with markers of inflammation(Reference Shantsila147), and have pro-coagulant potential(Reference Shantsila147, Reference Mallat, Benamer and Hugel151–Reference Jimenez, Jy and Mauro153).
A number of studies have examined EMP in relation to vascular damage and CVD risk. A significant increase in EMP has been shown in patients with CHD, the metabolic syndrome, diabetes and heart failure(Reference Mallat, Hugel and Ohan154–Reference Garcia, Chirinos and Jiminez158). A recent study by Wang et al. has shown that EMP count is associated with systolic blood pressure, being elevated even in patients with mild hypertension, and was also associated with arterial stiffness(Reference Wang, Su and Wang159). Wang et al. suggest that EMP may be a useful parameter for monitoring the process of vascular repair in hypertensive subjects, whilst the accompanying Editorial calls for further studies to determine whether EMP quantification might be a useful marker of endothelial dysfunction(Reference Shantsila147). Their use in dietary intervention research has been limited to date, but circulating microparticle concentrations are known to increase after ingestion of a fatty meal(Reference Harrison, Murphy and O'Connor160), whilst a recent paper has shown that following a Mediterranean diet for 4 weeks in older subjects significantly decreased total microparticle, EMP and apoptotic EMP concentrations when compared with a SFA-rich diet or a low-fat, high-carbohydrate diet(Reference Marin, Ramirez and Delgado-Lista161). They therefore potentially offer a novel and informative endpoint.
Endothelial progenitor cells
Asahara et al. isolated and characterised a circulating angioblast which had the potential to form endothelial cells in vitro, thus allowing subsequent quantitative flow cytometry in samples of peripheral blood(Reference Asahara, Murohara and Sullivan162). Endothelial progenitor cells (EPC) have a constitutive vasoreparative function, but after acute vascular damage, such as stroke or myocardial infarction, these cells are mobilised into peripheral blood where they participate in endothelial repair, regenerative processes and neovascularisation(Reference Shintani, Murohara and Ikeda163, Reference Wojakowski, Tendera and Michalowska164). It has been proposed that assessment of endothelial progenitor cells offers a dynamic, integrated index of systemic vascular damage and, as such, will offer more insight than any currently available biochemical markers(Reference Werner and Nickenig165). While a wide range of EPC subsets have been identified, most clinical studies have concentrated on CD34+ populations isolated from peripheral blood. An inverse relationship between circulating CD34+ EPC and cardiovascular risk factors has been demonstrated in both healthy subjects and patients with CVD(Reference Vasa, Fichtlscherer and Aicher166, Reference Hill, Zalos and Halcox167), whilst circulating EPC count may also act as a prognostic biomarker, being associated with worse outcome in patients with suspected or confirmed coronary artery disease(Reference Werner, Kosiol and Schiegl168). A recent study has shown that circulating CD34+ cells are inversely associated with obesity, and that weight loss results in an increase in circulating progenitor cells, including EPC(Reference Müller-Ehmsen, Braun and Schneider169).
Among healthy volunteers, short-term dietary interventions with green tea(Reference Kim, Jeong and Cho170), vegetables(Reference Mano, Ishida and Ohya171), red wine(Reference Huang, Chen and Tsai172) and the Mediterranean diet(Reference Marin, Ramirez and Delgado-Lista161) have all been shown to significantly increase circulating concentrations of endothelial progenitor cells. Assessing EPC numbers and function may also be informative, as increasing red wine consumption in healthy volunteers has recently been shown to increase endothelial progenitor cell migration and proliferation and to decrease the extent of apoptosis(Reference Hamed, Alshiek and Aharon173), whilst a high-flavanol intervention has been shown to increase EPC number, but had no effect on their function (measured as ability to survive, differentiate, proliferate and to migrate)(Reference Heiss, Jahn and Taylor33).
Circulating endothelial cells
Circulating endothelial cells are also recognised as markers of endothelial damage, and have been shown to be increased in acute coronary syndromes, heart failure, stroke and diabetes mellitus(Reference Boof, Lip and Blann174). Although no dietary intervention studies have examined effects on numbers of circulating endothelial cells, they may be an informative target in future studies.
Considerations when choosing a method
Investigators should first consider the mechanism through which they propose that the intervention will act, alongside the locally available resources and skills. A decision tree outlining the main considerations and choices is shown in Fig. 1. A panel of approaches, assessing both conduit vessels and the microvasculature, would be most appropriate where possible. Recent studies using different vascular function methodologies, although also with variation in study designs and populations, have demonstrated contrasting results, despite similar interventions(Reference McCall, McGartland and McKinley63, Reference Berry, Mulla and Chowienczyk113), and such a panel of endpoints may well have been informative in explaining these differing results. The choice of method may depend on whether investigators propose and are testing an acute or chronic effect of the dietary intervention. Investigators should also consider whether effects of the intervention are likely to be demonstrated in the fasting or postprandial state, as a recent study of n-3 fatty acids has demonstrated effects on postprandial macro- and microvascular function, but no effect on fasting measures(Reference Stirban, Nandrean and Gotting175).
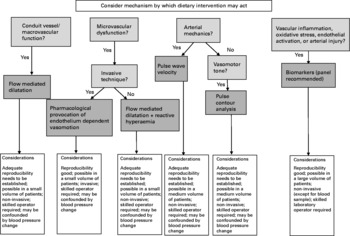
Fig. 1 Decision tree when considering method of vascular function assessment.
The confounding effects of blood pressure must be considered when interpreting study results. Changes in blood pressure, and therefore blood flow, will also cause changes in the FMD of a conduit artery, and which do not necessarily reflect a change in the endothelial function of that vessel(Reference Corretti, Anderson and Benjamin10). Forearm blood flow and PWV will also be affected by changes in blood pressure. In summary, if an intervention mediates a significant change in arterial blood pressure, a concordant effect on other vascular assessments will be observed. This does not imply an alteration of vascular structure or function and can be predicted from arterial physiology.
Conclusions
A wide variety of techniques are employed to provide surrogate vascular endpoints for short-term dietary trials in human subjects. FMD of the brachial artery and aortic PWV examine conduit and large elastic vessel properties respectively, and therefore do not estimate arterial function at a microvascular level where the bulk of endothelial cells are found.
The mechanism through which an investigator believes their intervention will act, combined with the resources and skill set of the investigating team, will most probably influence the choice of assessment method. At present no single, all-encompassing vascular function test exists, and perhaps a more useful approach would be to combine several methods to comprehensively assess arterial function and mechanics at multiple sites.
Potentially useful emerging techniques include the analysis of post-ischaemic arterial waveforms during FMD determination, quantifying pulse contour changes in response to vasodilator challenges and measuring circulating endothelial progenitor cell and microparticle concentrations.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
D. O. M. produced the first draft of the review. All other authors contributed substantially in terms of drafting, critical reviewing and editing subsequent versions of the manuscript.
None of the authors has any conflicts of interest to declare.





