Disturbances in one-carbon metabolism may potentially facilitate carcinogenesis by causing aberrant DNA methylation and DNA synthesis( Reference Davis and Uthus 1 ). Choline and its metabolite betaine are important methyl nutrients involved in one-carbon metabolism( Reference Ueland 2 ). A low status of choline and betaine could disturb the methyl pool and may thereby be related to carcinogenesis( Reference da Costa, Niculescu and Craciunescu 3 – Reference Zeisel 6 ). Some studies have assessed the relationship between choline and betaine intake and breast cancer risk, but the results remained inconsistent. The Nurses’ Health Study found no protective effect of choline or betaine intake on breast cancer risk( Reference Cho, Holmes and Hankinson 7 , Reference Cho, Holmes and Hankinson 8 ). However, two case–control studies, the Long Island Breast Cancer Study Project (LIBCSP)( Reference Xu, Gammon and Zeisel 9 ) and our previous study conducted in China( Reference Zhang, Pan and Li 10 ), indicated a significant reduction in breast cancer risk associated with a higher choline intake. Numerous factors could contribute to these inconsistent observations, including differences in study design and study populations, variations in the intake ranges and detail of dietary assessment. Genetic variations in the choline-related one-carbon metabolism enzymes may also partly contribute to the inconsistent results.
Choline metabolism in the one-carbon metabolic pathway includes three key enzymes. Phosphatidylethanolamine N-methyltransferase (PEMT) catalyses the de novo synthesis of phosphatidylcholine in the liver using the phosphatidylethanolamine as a substrate. Choline dehydrogenase (CHDH) catalyses the oxidation of choline to betaine. Betaine-homocysteine methyltransferase (BHMT) catalyses the methylation of homocysteine, with betaine serving as a substrate. A SNP of PEMT rs7946 is associated with a valine-to-methionine substitution, and it results in a 30 % loss of enzyme function( Reference Song, da Costa and Fischer 11 ). The SNP CHDH rs9001 has been shown to have a protective effect on susceptibility to choline deficiency( Reference da Costa, Kozyreva and Song 12 ). The BHMT rs3733890 is a non-synonymous SNP resulting in the substitution of an arginine-to-glutamine residue at amino acid position 239, which is associated with altered affinity of BHMT to homocysteine( Reference Li, Feng and Lee 13 ). The only report on the interactions between each of the three SNP and dietary choline/betaine intake with respect to breast cancer was from the LIBCSP( Reference Xu, Gammon and Zeisel 9 ), in which women with variant genotype of PEMT rs7946 and lower dietary betaine consumption had an elevated risk of breast cancer. However, dietary pattern and genetic background in Chinese women are different from their counterparts in Western countries. Furthermore, the associations between SNP of choline-metabolising genes and breast cancer risk, as well as gene–nutrient interactions, have not been explored among Chinese women.
This hospital-based case–control study, therefore, was conducted to investigate whether choline/betaine intake interact with polymorphisms in the PEMT, BHMT and CHDH gene in the development of breast cancer among Chinese women.
Methods
Study population
A detailed description of the ongoing case–control study has been published elsewhere( Reference Zhang, Pan and Li 10 ). In brief, potential cases were recruited in two hospitals in Guangzhou, China, between 1 December 2011 and 30 September 2014. Eligible cases were patients aged 25–70 years with histologically confirmed breast cancer diagnosed no more than 3 months before the interview, and who were Guangdong natives or those who have lived in Guangdong for at least 5 years. Women were excluded if they simultaneously had a history of any other cancers. All cases were confirmed by the physician and medical records. In total, 613 eligible cases were identified, of whom 570 were successfully interviewed (93·0 %).
Controls who were cancer-free were selected from the departments of Ear-Nose-Throat, Ophthalmology, Plastic and Reconstructive Surgery and Vascular Surgery in the same hospitals during the same period as the cases. They were frequency-matched to cases on the basis of age, with a 5-year interval. A total of 576 controls out of 613 eligible controls completed in-person interviews (94·0 %).
All participants were informed of the requirements of the study and signed a written consent form. The study was approved by the Ethical Committee of School of Public Health, Sun Yat-sen University.
Data collection
Trained interviewers administered face-to-face interviews to all participants via a standardised questionnaire, which included questions on socio-demographic factors, anthropometric factors, lifestyle behaviours, reproductive information and family history of cancer. We also recorded relevant medical information and medical diagnosis by reviewing hospital medical records. More specifically, in the present study, regular smoking was defined as smoking at least 1 cigarette/d for >6 consecutive months. Passive smoking was defined as non-smokers who reported being exposed to the smoke exhaled by smokers at least 15 min/d in a week. Regular drinking was defined as drinking alcohol at least once per week over the past year. Menopausal status was defined as having cessation of menstrual period for at least 12 months since the last menstruation. Women were considered as premenopausal if they were currently menstruating, or if they had ceased menstruation because of hysterectomy and were younger than 50 years. Women who reported menstrual cessation or underwent bilateral oophorectomy and who were older than 50 years were defined as postmenopausal. BMI was calculated by dividing weight (kg) by height squared (m2). Body weight and height were directly measured by nurses on the 1st day after admission. Data on current occupational activity were obtained by asking each participant on their employment status and the level of physical activity done at work (non-working, sedentary, standing, manual, heavy manual). Information on frequency and duration of household activities (mopping, cooking and so on) and recreational activities (walking, jogging, climbing, running, playing table tennis and so on) during the past year were collected. A metabolic equivalent (MET) value was assigned to each reported activity based on the Compendium( Reference Ainsworth, Haskell and Whitt 14 , Reference Ainsworth, Haskell and Herrmann 15 ). MET hours per week (how many days per week×how many hours per day×MET for a specific activity) were calculated for household and recreational activities.
Dietary assessment
A validated 81-item FFQ was used for assessing dietary information during the previous year before diagnosis for cases or before the time of interview for controls. Each subject was asked how frequently, on average, they consumed each type of food over the previous year, and detailed alterations in diets were further asked for those who changed their diet habits during the preceding 5 years. Pictures about different portion size of foods were used to help participants quantify the amount of food intake. Choline and betaine intake per day was calculated from FFQ based on frequency of food consumption, food items and serving sizes. Nutrient values in foods were obtained from the Chinese Food Composition Table( Reference Yang, Wang and Pan 16 ). The validity and reliability of FFQ have been described elsewhere( Reference Zhang and Ho 17 ). The correlation coefficients between FFQ and 18-d dietary records were 0·34 for choline and 0·26 for betaine. The correlation coefficients between the two FFQ of choline and betaine were 0·59 and 0·44 correspondingly.
Genotype of polymorphisms
Approximately 5 ml of fasting blood sample of each participant was obtained on the 2nd day after patients were admitted to the hospital. Blood samples were fractioned into plasma, buffy coat and red cells, and were stored at −80°C in a continuously alarmed and monitored refrigerator until needed for the analysis. Blood samples were available for all participants. Genomic DNA samples were extracted from buffy coat using a TIANamp Genomic DNA Kit (TianGen Biotech Co., Ltd) according to the manufacturer’s instruction. Genotyping of PEMT rs7946, BHMT rs3733890 and CHDH rs9001 was performed by Genesky Bio-Tech Co., Ltd using an improved multiplex ligation detection reaction technique. The alleles of each SNP were discriminated by different fluorescent labels of allele-specific oligonucleotide probe pairs. Different SNP were further distinguished by extended lengths at the 3' end. Genotyping of each sample was analysed by the GeneMapper 4.1 software (Applied Biosystems). The laboratory staff was blinded to the case–control status of the samples. We interspersed fifty-five random duplicate samples (4·8 % of the total) as quality control samples. The concordance rate was 100, 100 and 98 % for PEMT rs7946, CHDH rs9001 and BHMT rs3733890, respectively. All of three selected SNP in this study had possible functional significance located in exons and with a minor allele frequency (MAF) >5 % among Chinese population.
Statistical analysis
Differences in baseline characteristics of breast cancer cases and controls were examined using Student’s t test for continuous variables and χ 2 test for discrete variables. The χ 2 test was used to detect whether genotype distribution was in agreement with Hardy–Weinberg equilibrium among controls. Dietary choline and betaine intake were adjusted for total energy intake by the regression residual method( Reference Willett, Howe and Kushi 18 ) and then categorised into quartiles based on the distribution among the control subjects. OR and 95 % CI for breast cancer risk in relation to dietary nutrition intake and gene polymorphisms were determined using unconditional logistic regression. OR were adjusted for several potential confounders, which were selected based on comparison of baseline characteristics between cases and controls. The following variables were adjusted in multivariate models: education (primary school or below, junior high school, senior high school/secondary technical school, college or above), income (<2000, 2001–5000, 5001–8000, >8000 yuan/month), age at menopause (≤46, >46–49, >49–52, >52–55, >55 years), first-degree relative with cancer (yes/no), regular drinking (yes/no) and passive smoking (yes/no). Tests for trend were undertaken by entering categorised variable as continuous in the regression model.
Stratified analyses by menopausal status (premenopausal or postmenopausal) were performed. Potential gene–environment interactions were evaluated by adding the multiplicative interaction terms (genotype×dietary intake) to the final models as indicator variables. Median intake of choline and betaine were calculated based on controls. Women with homozygous wild-type genotype and dietary nutrient intake above the medians were selected as the reference. Because the socio-economic factors were not well comparable between cases and controls, stratified analyses by income level (≤5000 yuan/month and >5000 yuan/month) and educational level (junior high school or below, senior high school or secondary technical school or above) were also performed. In this study, significance was defined as P<0·05, and all statistical tests were two-tailed. Statistical analyses were conducted using the SPSS software (version 20.0).
Results
A comparison of baseline characteristics between cases and controls is displayed in Table 1. Compared with controls, cases were, in general, older at menopause and less highly educated. They were also more likely to have a lower household income, to be exposed to passive smoking, to be regular drinkers and to have a history of a first-degree relative with cancer. Case and control groups were similar in marital status, occupation, BMI, occupational activity, smoking status, age at menarche, parity, age at first live birth, percentage of adopting breast-feeding, menopausal status and use of oral contraceptive.
Table 1 Socio-demographic characteristics and selected risk factors of breast cancer in the study population (Numbers and percentages; mean values and standard deviations)
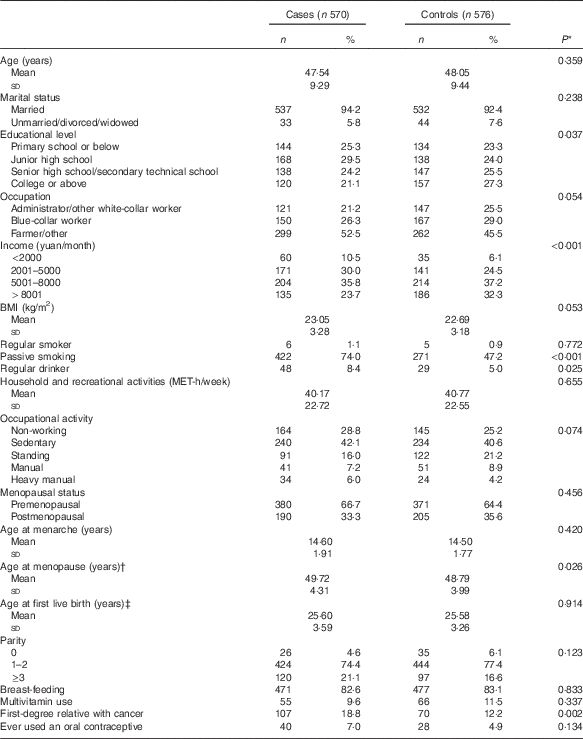
MET, metabolic equivalent.
* Continuous variables were analysed by t tests. Categorical variables were analysed by χ 2 tests.
† Among menopausal women.
‡ Among women who have had a live birth.
As shown in Table 2, lower choline intake was associated with an increased risk of breast cancer after controlling for the potential confounders. The multivariate OR of breast cancer comparing women in the lowest quartile of choline intake with those in the highest quartile was 2·01 (95 % CI 1·43, 2·82). Dietary betaine showed no significant association with breast cancer risk, and OR for bottom v. top quartile of betaine was 1·00 (95 % CI 0·72, 1·39). The association between choline intake and breast cancer risk was not significantly different between premenopausal and postmenopausal women (data not shown).
Table 2 Risks for breast cancer according to quartiles of the energy-adjusted daily nutrient intake (Odds ratios and 95 % confidence intervals)
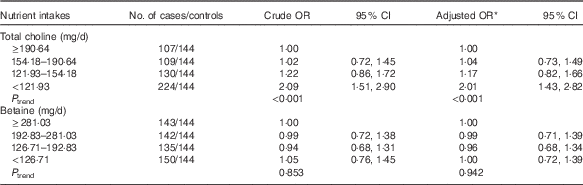
* OR adjusted for education, income, age at menopause, first-degree relative with cancer, regular drinking and passive smoking.
The frequencies of PEMT rs7946, CHDH rs9001 and BHMT rs3733890 and the associations of these SNP with breast cancer risk are presented in Table 3. All genotype distributions were in accordance with Hardy–Weinberg equilibrium among controls, except for the PEMT rs7946 polymorphism (P=0·036). The frequencies of the minor alleles were 16·7, 34·8 and 36·0 % for PEMT rs7946, CHDH rs9001 and BHMT rs3733890 among controls and 17·4, 33·9 and 34·8 % among cases. None of the three SNP was associated with breast cancer risk in our study, but a significant reduced risk was observed among postmenopausal women who carry homozygous variant AA genotype of BHMT rs3733890 (OR 0·49; 95 % CI 0·25, 0·98) (Table 4).
Table 3 Association between genetic polymorphisms in choline-metabolising genes and breast cancer risk (Numbers and percentages; odds ratios and 95 % confidence intervals)

PEMT, phosphatidylethanolamine N-methyltransferase; CHDH, choline dehydrogenase; BHMT, betaine-homocysteine methyltransferase.
* OR adjusted for education, income, age at menopause, first-degree relative with cancer, regular drinking and passive smoking.
Table 4 Association between genetic polymorphisms in choline-metabolising genes and breast cancer risk according to menopausal status (Odds ratios and 95 % confidence intervals)
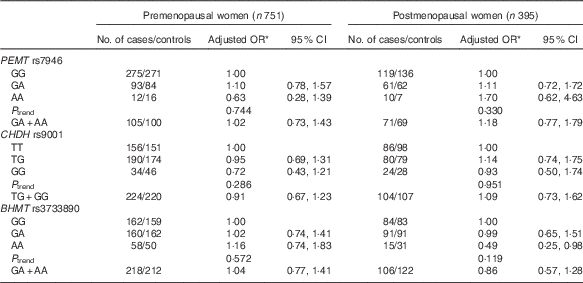
PEMT, phosphatidylethanolamine N-methyltransferase; CHDH, choline dehydrogenase; BHMT, betaine-homocysteine methyltransferase.
* OR adjusted for education, income, age at menopause, first-degree relative with cancer, regular drinking and passive smoking.
Table 5 presents the joint effect of the genetic polymorphisms and dietary choline and betaine on breast cancer risk. Notably, significant interactions were observed for choline intake with SNP PEMT rs7946 (P interaction=0·029) and BHMT rs3733890 (P interaction=0·006). Compared with women with the PEMT rs7946 GG genotype and choline intake >154 mg/d, those having the PEMT rs7946 GG genotype and choline intake <154 mg/d had the greatest increased risk of breast cancer (OR 1·83; 95 % CI 1·36, 2·45). Similarly, a substantial increased risk of breast cancer was seen for women with BHMT rs3733890 GG genotype in the presence of lower choline intake in comparison with those with GG genotype in the presence of higher choline intake (OR 2·48; 95 % CI 1·70, 3·63). Betaine intake did not significantly interact with any of the three SNP. The associations of choline intake, betaine intake and the three studied SNP in relation to breast cancer risk did not differ significantly stratified by socio-economic status (income level and education level) (data not shown).
Table 5 Interactions of the genetic polymorphisms in choline-metabolising genes and energy-adjusted nutrient intakes in relation to breast cancer risk (Odds ratios and 95 % confidence intervals)

PEMT, phosphatidylethanolamine N-methyltransferase; CHDH, choline dehydrogenase; BHMT, betaine-homocysteine methyltransferase.
* Median intake among controls.
† OR adjusted for education, income, age at menopause, first-degree relative with cancer, regular drinking and passive smoking.
Discussion
To our knowledge, this is the first study to examine associations of choline-related one-carbon metabolism genes with breast cancer in Chinese populations. The present study confirmed that dietary choline intake was inversely associated with breast cancer risk. None of the three SNP of choline-metabolising gene was associated with breast cancer risk among Chinese women. However, women with low choline intake and wild genotype of PEMT rs7946 or BHMT rs3733890 had a substantially increased risk of breast cancer.
This study found no association between PEMT rs7946, CHDH rs9001 and BHMT rs3733890 and breast cancer risk. This result was consistent with the LIBCSP study, the only study examining the genetic polymorphisms in choline-metabolising genes and breast cancer risk( Reference Xu, Gammon and Zeisel 9 ). Some studies examined the relationship between SNP BHMT rs3733890 and cancer risk at other sites, and controversial results have been reported. Variant A allele of BHMT rs3733890 has been observed to be associated with a reduced risk of uterine cervical carcinoma( Reference Mostowska, Myka and Lianeri 19 ) and colorectal adenoma among persons with high methyl status( Reference Hazra, Wu and Kraft 20 ), whereas AA genotype was associated with an increased risk of colorectal cancer( Reference Koushik, Kraft and Fuchs 21 ). No association was observed for variant AA genotype with either colorectal cancer( Reference Martinelli, Scapoli and Mattei 22 ) or ovarian cancer( Reference Pawlik, Mostowska and Lianeri 23 ), which is in line with our results.
Our results revealed that BHMT rs3733890 SNP was associated with a 51 % decreased risk of breast cancer only among postmenopausal women. BHMT, a zinc metalloenzyme that is expressed in the kidney cortex and in liver hepatocytes, catalyses the conversion of homocysteine to methionine. Although methylation of homocysteine catalysed by BHMT only occurs in certain organs, animal studies have shown that this pathway is equally important as another parallel pathway that uses 5-methyltetrahydrofolate as the methyl donor( Reference Finkelstein and Martin 24 ). BHMT rs3733890 SNP has been found to produce an enzyme with higher affinity to homocysteine than the wild genotype( Reference Li, Feng and Lee 13 ), and thus mutant enzyme promotes a higher rate of the homocysteine remethylation. Supporting this finding, the variant AA genotype has been found to be associated with lower plasma homocysteine concentrations than wild GG carrier in two previous studies( Reference Morin, Platt and Weisberg 25 , Reference Biselli, Zampieri and Goloni-Bertollo 26 ). The disturbance of homocysteine removal may contribute to DNA hypomethylation( Reference James, Melnyk and Pogribna 27 ), which is considered important in carcinogenesis. In addition, carriers of the variant alleles of BHMT rs3733890 have been reported to have favourable health profiles. Follow-up study of LIBCSP found that individuals with BHMT rs3733890 AA genotype have a 36 % lower risk of dying from breast cancer than the wild GG genotype( Reference Xu, Gammon and Zeisel 28 ). The minor A allele of the BHMT rs3733890 has also been shown to protect against CVD( Reference Weisberg, Park and Ballman 29 ), spina bifida( Reference Shaw, Lu and Zhu 30 ), orofacial clefts( Reference Mostowska, Hozyasz and Wojcicki 31 ), uterine cervical carcinoma( Reference Mostowska, Myka and Lianeri 19 ) and maternal Down syndrome risk( Reference Zampieri, Biselli and Goloni-Bertollo 32 – Reference Amorim, Moura and Gomes 34 ). There is some evidence that cloned human BHMT gene contains several consensus sites for steroid hormone receptors, including oestrogens( Reference Park and Garrow 35 ). The null association between the BHMT rs3733890 and breast cancer risk among premenopausal women may be explained by the confounding effect from oestrogen.
The present study showed that women with low choline intake and wild genotype of BHMT rs3733890 had a substantially increased risk of breast cancer. Choline, similar to folate, is essential in the formation of S-adenosylmethionine, the chief methyl donor for DNA methylation reactions. Aberrant DNA methylation and impaired DNA repair because of choline deficiency were thought to be involved in carcinogenesis( Reference Zeisel 4 ). Animal studies have shown that a choline-deficient diet itself can induce liver cancer without using any carcinogens( Reference Henning and Swendseid 36 ). Results from LIBSCP and our previous two-stage case–control study also support the protective role of higher choline intake in breast cancer( Reference Xu, Gammon and Zeisel 9 , Reference Zhang, Pan and Li 10 ). Furthermore, our result suggested that wild GG genotype is particularly deleterious when intake of choline is comparatively low, with a 2·48-fold increased risk of breast cancer observed. This result is biologically plausible. In agreement with our finding that variant AA genotype of BHMT rs3733890 protected against breast cancer among postmenopausal women, the increased homocysteine remethylation efficacy of mutant enzyme may mitigate the adverse effect of low choline intake, whereas such an effect is unavailable for the enzyme produced by wild GG genotype. Besides, gene–environment interaction has been found in an animal study in which dietary choline induced BHMT expression when choline was added to a methionine-deficient diet( Reference Park and Garrow 35 ).
We also noted a significant interaction between choline intake and PEMT rs7946 polymorphism in the present study. Women who had the wild GG genotype with low choline intake have a substantially increased breast cancer risk. Choline is derived not only from the diet but also from de novo synthesis of phosphatidylcholine using S-adenosylmethionine catalysed by PEMT. PEMT rs7946, a missense mutation in exon 8 of the gene, has been reported to result in diminished enzyme activity( Reference Song, da Costa and Fischer 11 ) but was not associated with susceptibility to choline deficiency( Reference da Costa, Kozyreva and Song 12 ). However, this interaction should be interpreted with caution because the genotype distribution of PEMT rs7946 was not in accordance with Hardy–Weinberg equilibrium among controls (P=0·036). It is worth noting that de novo synthesis of choline through S-adenosylmethionine-dependent transmethylation would be a futile cycle in the presence of low dietary choline( Reference Park and Garrow 35 ). The PEMT gene expression is induced by oestrogen, which is mainly present in premenopausal women, and these women make some of their own needed choline. It was reported that premenopausal women are relatively resistant to choline deficiency compared with postmenopausal women and men( Reference Resseguie, Song and Niculescu 37 ). However, in the present study, the effects of diet and PEMT rs7946 on breast cancer risk are not different in premenopausal and postmenopausal women. In addition, a study indicated that neural tube defect cases were more likely to have the wild GG genotype of PEMT rs7946 than controls( Reference Mills, Fan and Brody 38 ). All of the above explanations on the gene–environment associations are highly putative, and further studies with larger sample size are needed to clarify the mechanisms of these interactions.
We did not observe a significant interaction between CHDH rs9001 and choline, although CHDH rs9001 had a protective effect on susceptibility to choline deficiency( Reference da Costa, Kozyreva and Song 12 ). The SNP CHDH rs9001 is less studied, and its biological function needs to be determined in future studies.
The strengths of our study include standardised specimen and data collection, and extensive collections of multiple risk factors. Some limitations of the present study warrant consideration. This study was a hospital-based case–control study, and thus selection bias may have affected the results. Hospital-based controls may not be representative of the general population, and the diseases they suffered may potentially be related to diet. To minimise the bias, we ensured that controls were recruited from several disease conditions with no apparent relation to dietary causes. In addition, in the present study, allele frequencies were similar to previous studies in Chinese population( Reference Bi, Zhao and Zhang 39 , Reference Jin, Chen and Hou 40 ), which demonstrated that selection bias may not be a serious problem. The homogeneous ethnic background (99·6 % Han Chinese) of the study population also decreased the potential confounding effect from ethnicity. Second, diet information was collected after breast cancer diagnosis, and recall bias may occur in the present study. We tried to interview cases as soon as the diagnosis was made, and 77·6 % of cases were interviewed within 3 d of the cases admitted to hospitals. Moreover, pictures about different portion size of foods were provided to assistant participants with quantification of food intake. Third, in the present study, only potentially functional SNP located in exons and SNP with reported MAF >5 % in Chinese were selected. Further studies using whole-genome sequencing including all SNP in exons and introns are needed to examine associations between these SNP and breast cancer risk.
In summary, the present study showed that PEMT rs7946 and BHMT rs3733890 polymorphisms may interact with choline intake on breast cancer risk. Women with low choline intake and wild genotype of PEMT rs7946 or BHMT rs3733890 had a substantially increased risk of breast cancer. Additional large-scale epidemiological studies are required to prove the present findings.
Acknowledgements
The authors gratefully acknowledge the cooperation of the study participants and the assistance of the student helpers Bisara mardan and Luo Xin.
This study was supported by Science and Technology Program of Guangzhou, China (no. 201510010151), the National Natural Science Foundation of China (no. 81102188) and National Undergraduate Innovation Training Program.
The authors’ responsibilities were as follows: Y.-F. D. conducted the data collection, analysed the data and wrote this paper. W.-P. L., B. Y., M. X., W.-Q. H. and J. H. participated in the data collection. F.-Y. L., Z.-Q. L. and X.-F. M. were responsible for connecting and coordinating the field work. C.-X. Z. constructed the project design, supervised and contributed to manuscript writing.
None of the authors has any conflicts of interest to declare.








