The composition of the diet is known to greatly influence digestibility and gut health in many animal species and in man(Reference Moore, Brant and Kunkle1, Reference Pellet2). This is particulary the case in broiler chickens, where it is well documented that different diets, all fulfilling dietary requirements for energy content, amino acids, vitamins and minerals, greatly differ in digestibility when wheat, rye and/or barley as opposed to maize are used as main components. Indeed, broiler chickens on a maize-based diet thrive better than broilers on wheat-, rye- or barley-based diets(Reference Meng, Slominski and Guenter3–Reference Lázaro, García and Medel5). The latter cereals contain considerable amounts of NSP, and it is generally accepted that these NSP have ‘anti-nutritive’ effects(Reference Rubio, Grant and Dewey6, Reference Choct and Annison7), which can be overcome by the use of low dietary dosages of antimicrobials (commonly called growth promoters)(Reference Choi and Ryu8). It is thought that the antimicrobial growth promoters act by suppressing certain gut microbiota populations, although other mechanisms of action also have been proposed(Reference Niewold9). The use of antimicrobial growth promoters in animal feed has been banned in the European Union since 1 January 2006. Alternatively, certain NSP-degrading enzymes such as xylanases have been shown to improve digestibility of cereal-based broiler diets(Reference Cowieson and Adeola10).
NSP are known to increase the viscosity of the gut contents and have negative effects on nutrient digestion and absorption(Reference Choct and Annison7). The underlying mechanisms of the so-called ‘anti-nutritive’ effects of cereals in general and NSP in particular have, however, not yet been investigated in detail. Nevertheless, a good understanding of the physiopathological changes not only may help to control these problems in broilers but also may shed light on the role of the diet in persistent gut health problems in other animal species and in man.
The purpose of the present study was to analyse the alterations in the gut ecosystem (immune cell infiltration, gut morphology and microbiota composition) in relation to the cereal type (wheat/rye v. maize) used in the diet of broiler chickens.
Materials and methods
Animals and diets
A total of 800 newly hatched male Ross 308 broiler chickens were used in the study. The animals were given four different diets (treatments), of which for each diet, five replicates (pens) of forty chickens were used in the experiment. Forty chickens were housed per pen of 2·1 m2 on a solid floor covered with wood shavings. Lighting in each room was controlled by time switch set to provide a 23 h light and 1 h dark photoperiod. Two treatment groups were fed a maize–soyabean-based diet of which one contained the antibiotic growth promoter Zn-bacitracin at 100 mg/kg. The two other treatment groups were fed a wheat/rye–soyabean-based diet. Again, one was supplemented with Zn-bacitracin at 100 mg/kg. Each diet consisted of a starter, a grower and a finisher meal, of which the composition is shown in Table 1.
Table 1 Composition and nutrient content of the wheat/rye–soyabean (W/R) and the maize–soyabean (M) starter, grower and finisher diets

HF, heat-treated full-fat; mEq, milli-equivalents.
At 2, 4 and 6 weeks of age, all the broilers were weighed, feed intake was determined and feed conversion ratios were calculated. At these time points also five chickens of each replicate were euthanised by intravenous embutramid (T61; Intervet, Mechelen, Belgium) injection. Samples of the small intestine and caecum were collected, rinsed in PBS and fixed in 4 % (v/v) buffered formalin. Samples of approximately 3 cm were taken from the second limb of the duodenum, the jejunum starting 1 cm after Meckel's diverticulum, the ileum immediately before the ileocaecal junction, and the middle part of one caecum. Sampling 1 cm from Meckel's diverticulum at a certain time interval will cause the point from which the sample is taken to shift with the growth of the bird relative to that of earlier samples. These samples were fixed in formalin and embedded in paraffin. Also the content of the jejunum, ileum and caeca was collected by rinsing according to Apajalahti et al. (Reference Apajalahti, Särkilahti and Mäki11). Necropsy was done immediately after euthanasia; the time lapse between T61 injection and necropsy was less then 30 min.
Morphological examination
Formalin-fixed intestinal segments were dehydrated in xylene and embedded in paraffin. Sections of 4 μm were cut using a microtome (Microm; Prosan, Merelbeke, Belgium). Deparaffinisation was done in xylene (2 × 5 min). Then the sections were rehydrated in isopropylene (5 min), 95 % alcohol (5 min) and 50 % alcohol (5 min) and stained with haematoxylin and eosin. Sections were examined using light microscopy. Villus length in duodenum was measured for five chickens of all replicate groups, per treatment. This was done by random measurement of fifteen villi per section (of all intestinal segments) using an Olympus BX61 Digital Camera DP50 (Olympus NV, Aartselaar, Belgium) and a personal computer-based image analysis system (Analysis® J-2; P4 Technologies, Inc., Waldorf, MD, USA). Only intact villi were measured, meaning villi for which the tip as well as the base of the villus were in the plane of the section. Thickness of the tunica muscularis in the duodenum, jejunum, ileum and caeca was also measured using the Analysis® J-2 software (P4 Technologies, Inc.). For each section, nine measurements were performed on different locations. Measurements were done on cross-sections of ring-shaped intestinal segments which allow unbiased perpendicular measurements. The amount of fused villi was determined using a scoring system (0 = no villus fusion; 1 = occasional fusion of two villi in a gut section; 2 = 1/5 of the villi were fused per gut section; 3 = 1/4 of the villi were fused per gut section; 4 = 1/3 of the villi were fused per gut section; 5 = half of the villi were fused per gut section; 6 = 2/3 of the villi were fused per gut section, 7 = all villi in the gut section were fused). Detection of goblet cells was done using periodic acid–Schiff staining as described by Forder et al. (Reference Forder, Howarth and Tivey12).
Immunohistochemical examination
Deparaffinisation of paraffin-embedded tissue sections (4 μm) was performed as described above. The pressure cooker antigen retrieval method (Tender Cooker; Nordic Ware, Minneapolis, MN, USA) was applied to the samples. Immunohistochemical labelling of leucocytes was performed as described by Mast et al. (Reference Mast, Goddeeris and Peeters13). Briefly, endogenous peroxidase in the tissue sections was blocked with H2O2 (3 %) in methanol for 30 min (21°C). After rinsing with PBS, sections were incubated for 1 h (21°C) with monoclonal antibodies directed against T-lymphocytes (KUL05)(Reference Van Immerseel, De Buck and De Smet14). After rinsing thoroughly, a goat anti-mouse IgG1 conjugate, labelled with peroxidase (Dako, Heverlee, Belgium), was added for 30 min (21°C). After rinsing, tissue sections were incubated with avidin/biotin complex–horseradish peroxidase (ABC–HRP) complex (Dako, Heverlee, Belgium) for 30 min (21°C). After rinsing again, positive cells were stained brown after conversion of the substrate (3,3′ diaminobenzidine tetrahydrochloride; Sigma, St Louis, MO, USA) in the presence of H2O2. The number of T-lymphocytes in the mucosa was scored with an automatic image analysis system (Optimas 6.5; Media Cybernetics, Bethesda, MD, USA), measuring the area percentage occupied by the labelled cells. The mucosa is the part of the intestinal wall which is composed of villi, crypts and propria mucosae and is bounded by the inner border of the tunica muscularis. The measurements were done in the mucosa (detecting T-lymphocyte infiltration in the propria mucosae). For each section, eight randomly selected sites were analysed by the image analysis program. From all five replicates of each dietary treatment, five chickens were analysed in the duodenum, jejunum, ileum and caeca. Immune cell aggregates were analysed using the sections stained for T-lymphocytes.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labelling
Rehydrated, deparaffinised tissue sections (4 μm) were used for the detection of apoptosis in the crypts and at the tips of the villi. The In Situ Cell Death Detection Kit, POD (peroxidase) (Roche Diagnostics, Vilvoorde, Belgium) was used with some modifications. For apoptosis at the tips of the villi, sections were incubated with 0·05 % Triton X-100 for 8 min at room temperature. Rinsing was done twice for 2 min with PBS, followed by incubation with 5 % bovine serum albumin (25 min; room temperature). After rinsing with PBS, the tissue sections were incubated with the kit enzyme solution (1 h; 37°C), followed by rinsing and incubation with kit converter POD (30 min; 37°C). After rinsing again, positive cells were stained brown after conversion of the substrate (3,3′ diaminobenzidine tetrahydrochloride, DAB; Sigma-Aldrich, St Louis, MO, USA) in the presence of H2O2 (1 min; room temperature). To measure apoptosis in the crypts, the same method was used, with the exception that no Triton X-100 was used and staining was done using incubations with DAB for 5 min.
Terminal-restriction fragment length polymorphism
DNA extraction
DNA was extracted from the jejunal, ileal and caecal content of broilers. The content from the five chickens of one replicate euthanised at the same time point was pooled. From these samples an amount of 1·0 g of intestinal or caecal material was taken. From these samples the DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen GmbH, Hilden, Germany). The extraction was carried out in accordance with the instructions of the manufacturer, with an additional step of lysozyme treatment, which was added to the procedure before the use of InhibitEX tablets provided in the QIAamp DNA Stool Mini Kit. An amount of 140 μl of a 10 mg/ml solution of lysozyme (Sigma-Aldrich) in 2-amino-2-hydroxymethyl-propane-1,3-diol-EDTA buffer (10:1 mm), pH 8, was added to each extraction tube and the samples were incubated at 37°C for 30 min. The DNA was eluted in 200 μl buffer AE (Qiagen GmbH) and stabilised by adding 4 μl of a 40 mg/ml bovine serum albumin (Ambion, Huntingdon, Cambs, UK) and 2 μl of ribonuclease-A. All DNA samples were stored at − 20°C until further processing.
The DNA concentrations were measured in a spectrophotometer (Pharmacia LKB Biochrom Ltd, Cambridge, Cambs, UK) and samples were diluted to a concentration of 5 μg DNA per ml before PCR.
Polymerase chain reaction conditions
PCR was run in a PTC-200 thermal cycler (MJ Research, Watertown, MA, USA) in three replicate 50 μl PCR mixtures for each sample. The PCR mixture was as follows: 5 μl PCR buffer (HT Biotechnology Ltd, Cambridge, Cambs, UK); 200 μm (each) deoxynucleoside triphosphates; 0·1 μm-FAM (carboxyfluorescein-N-hydroxysuccinimide ester-dimethyl sulfoxide)-labelled forward primer S-D-Bact-0008-a-S-20 (5′-AGAGTTTGATCMTGGCTCAG-3′); 0·1 μm-reverse primer S-D-Bact-0926-a-A-20 (5′-CCGTCAATTCCTTTRAGTTT-3′); 1·25 U of DNA polymerase (SuperTaq; HT Biotechnology Ltd) in a 50 μl reaction. The primers amplified 16S rDNA. In each PCR reaction, 2 μl DNA extract (5 μg/ml) was amplified. The running conditions were as follows: initial denaturation at 94°C for 6 min; followed by thirty cycles of denaturation at 94°C for 30 s, annealing at 57°C for 45 s, and extension at 72°C for 2 min, and a final extension at 72°C for 3 min. The amplicons were verified by electrophoresis on a 2 % agarose gel.
Restriction digest
The PCR products from the three replicates were pooled and purified with a QIAquick PCR purification kit (Qiagen GmbH) and eluted in a final volume of 30 μl double-distilled water. The purified PCR products were digested overnight at 37°C with 20 U of HhaI (Boehringer, Mannheim, Germany) in 20 μl reaction mixtures.
Terminal-restriction fragment length polymorphism
The fluorescently labelled terminal-restriction fragment (T-RF) mixture was denatured at 95°C for 5 min and transferred to ice. T-RF, 1 μl, were mixed with 10 μl Hi-Di™ formamide standard mix (35 μl 6-carboxyl-X-rhodamine (ROX) per ml formamide). Analysis on fragment size was performed on an automatic sequence analyser (ABI 3130xl Genetic Analyser, 16 Cap.; Applied Biosystems, Foster City, CA, USA). The internal standard was Geneflo625 (EURx, Gdansk, Poland).
T-RF length polymorphism (T-RFLP) fingerprint profiles of the microbial communities were collected by the software GeneMapper v. 3.7 (Applied Biosystems). DNA from different organisms will give rise to T-RF of different size. A fingerprint of the community is visualised as peaks in an electropherogram. These fingerprints were transformed to band patterns by the use of BioNumerics (Applied Maths, St Martens Latem, Belgium). The lengths of the fluorescently labelled fragments (each represented by one band) were determined by comparing them with the internal standard. Two fingerprint replicates of the bacterial community of each sample were compared. All bands only present in one of the two replicates and also those of which the intensity was too low were rejected to exclude false T-RF from the fingerprint of a microbial community. After this the band patterns (presence or absence of bands) of the different samples were compared using BioNumerics. The comparisons were based on the Dice similarity coefficient and the unweighted pair group method using arithmetic averages for clustering. Dendrograms reflect the grouping and relatedness of samples. The relative similarity between samples can be depicted from the coefficient bar above each diagram in Fig. 1.

Fig. 1 Dendrogram showing the relationship between terminal-restriction fragment length polymorphism patterns of caecal contents of groups of broiler chickens fed a wheat/rye-based (W/R) or a maize-based (M) diet, either or not supplemented with Zn-bacitracin (B), at 15 d of age. For the maize-based diet only four replicates are shown because DNA extraction of one of the replicates failed. The comparisons were based on the Dice similarity coefficient and the unweighted pair group method using arithmetic averages (UPGMA) for clustering. Dendrograms reflect the grouping and relatedness of samples. The relative similarity between samples can be depicted from the coefficient bar above the diagram.
Statistical analysis
The effect of the diet and sampling location (duodenum, jejunum, ileum, caecum) on villus length, villus fusion, thickness of the tunica muscularis, T-cell infiltration and the presence of T-lymphocyte aggregates was evaluated by means of a linear mixed effect model with ‘chicken’ as the random effect to correct for the relatedness of repeated measurements on the same animal. Model assumptions, such as normality of the outcome variable, homosedasticity and linearity, were checked by evaluating the residuals. When the standardised residuals were not randomly distributed about 0 throughout the range of the model a transformation of the outcome variable was performed so that the model assumptions were met. In all models pairwise comparisons of the different levels of the fixed factors (diet, location) were done by means of the least square difference test. Statistical analysis was done in S-PLUS 8 (TIBCO Software Inc., Palo Alto, CA, USA).
Animal ethics
The experiments reported were approved by the local ethical committee of the Faculty of Veterinary Medicine of Ghent University. All husbandry practices and euthanasia were performed with full consideration of animal welfare.
Results
Body weight, feed intake and feed conversion ratio
No significant differences were detected in body weight, feed intake and feed conversion between animals given a wheat/rye-based diet without Zn-bacitracin and the animals given a maize-based diet without Zn-bacitracin. Zn-bacitracin addition resulted in a significant increase in body weight at 15 and 29 d, but not at the age of 42 d, in both the wheat/rye- and maize-based diet groups. Zn-bacitracin addition also caused a significant decrease of the feed conversion in the first period (day 1 to day 15) in the animals fed a wheat/rye-based diet (Table 2).
Table 2 Feed conversion ratio (FCR), body weight (BW) and feed intake (FI), measured during different time intervals, of animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)
(Mean values and standard deviations)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
Intestinal morphology
Villus length
Adding Zn-bacitracin to the broiler diets increased the duodenal villus length significantly at days 15 and 29, regardless of the cereal type. The cereal type did not significantly affect villus length in the duodenum. At day 42, neither cereal type nor bacitracin affected villus length (Table 3). Analysis showed a statistically significant overall effect of Zn-bacitracin (P < 0·0001), but not the cereal type (P = 0·1283), on villus length.
Table 3 Length of villi (μm) in duodenal sections on days 15, 29 and 42 in animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)*
(Mean values and standard deviations of fifteen villi measured from five chickens from five different replicates)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Random measurement of fifteen villi for fifteen gut sections was performed by a personal computer-based analysis system.
Villus fusion
In general the wheat/rye diet induced more fusion of villi compared with the maize diet (Table 4). When both diets were supplemented with Zn-bacitracin, the effect of the cereal type was no longer statistically significant except in the ileum at day 15, duodenum at day 29 and jejunum at day 42. Adding Zn-bacitracin to the wheat/rye diet generally decreased fusion of villi, while this was not the case with the maize-based diet. The statistically significant differences between groups can be seen in Table 4. Both Zn-bacitracin (P < 0·0001) and cereal type (P < 0·0001) had a statistically significant overall effect on villus fusion.
Table 4 Scores for villus fusion for duodenal, jejunal and ileal sections on day 15, day 29 and day 42 of animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)*
(Mean values and standard deviations of five chickens from five different replicates)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Villus fusion was scored for twenty-five chickens per diet per time point.
Thickness of the tunica muscularis
In all intestinal segments at all time points except in samples of the jejunum and caecum that were taken at day 42, the tunica muscularis was significantly thicker in the group fed a maize-based diet compared with the animals fed a wheat/rye-based diet (Table 5). Zn-bacitracin significantly increased the tunica muscularis thickness in all intestinal segments of the animals fed a wheat/rye-based diet at days 15 and 29. Both Zn-bacitracin (P < 0·0001) and cereal type (P < 0·0001) had a statistically significant overall effect on the thickness of the tunica muscularis.
Table 5 Thickness of the tunica muscularis (μm) for duodenal, jejunal, ileal and caecal sections on day 15, day 29 and day 42 in animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)*
(Mean values and standard deviations of nine measurements from five chickens from five different replicates)

a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Nine random measurements of the tunica muscularis per three gut sections were done by a personal computer-based analysis system.
Goblet cells
Although not quantitatively analysed, periodic acid–Schiff staining appeared to show more and larger goblet cells both on the villi and in the crypts at the age of 29 d in broilers given a wheat/rye diet than in those given a maize diet (Fig. 2). This was especially the case in the ileum and caecum.
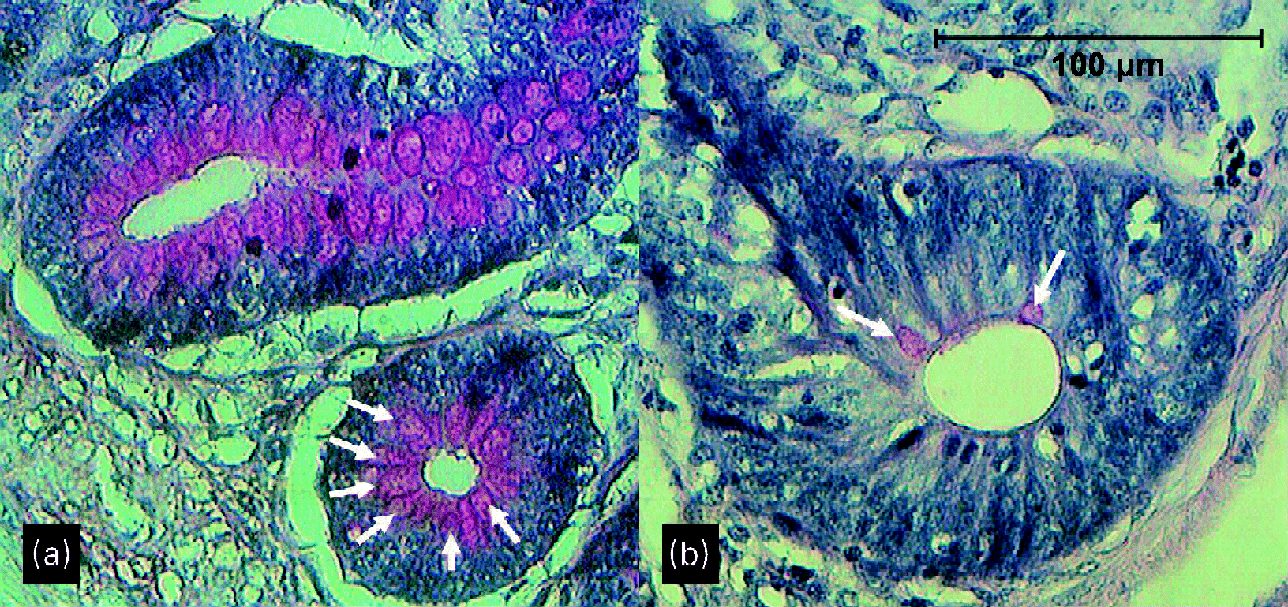
Fig. 2 Crypts of ileum of 29-d-old broilers (a) fed a wheat/rye diet and (b) fed a maize diet. Arrows point to goblet cells.
Apoptosis
More apoptotic cells were observed at the tips of the villi in the gut sections of the animals given a wheat/rye-based diet, compared with animals given a maize-based diet. Zn-bacitracin reduced the number of apoptotic cells at the tips of the villi. Although not quantitatively analysed, there appeared to be greater numbers of apoptotic cells in the crypts and along the villi in the animals given a wheat/rye-based diet as compared with the animals given a maize-based diet. Regardless of the cereal type, after Zn-bacitracin addition no apoptosis was observed in the crypts.
Intestinal immunology
T-lymphocyte infiltration
In all four intestinal segments T-lymphocyte infiltration in the intestinal mucosa was generally higher in the group fed a wheat/rye-based diet in comparison with the animals fed a maize-based diet (Table 6). Zn-bacitracin generally decreased T-cell infiltration. Both Zn-bacitracin (P < 0·0001) and cereal type (P < 0·0001) had a statistically significant overall effect on the T-lymphocyte infiltration in the mucosa. These data were log transformed to make them normally distributed.
Table 6 T-lymphocyte infiltration (surface %) for duodenal, jejunal, ileal and caecal sections on day 15, day 29 and day 42 in animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)*
(Mean values and standard deviations of eight measurements from five chickens from five different replicates)

a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Eight random measurements of T-lymphocyte infiltration in the mucosa per three gut sections were done by a personal computer-based analysis system.
Lymphoid aggregates
The number of T-lymphocyte aggregates was influenced by the cereal type, especially at day 42 where all four intestinal sections showed statistically significantly higher numbers of lymphoid aggregates in the group of animals fed a wheat/rye-based diet compared with the group of animals fed a maize-based diet (Table 7). Adding Zn-bacitracin generally had little effect on the amount of lymphoid aggregates in the mucosa. Analysis showed a statistically significant overall effect of the cereal type (P < 0·0001), but not Zn-bacitracin (P = 0·2719), on the number of T-lymphocyte aggregates.
Table 7 Number of T-lymphocyte aggregates for duodenal, jejunal, ileal and caecal sections on day 15, day 29 and day 42 in animals fed a wheat/rye-based (W/R) and a maize-based (M) diet, either or not supplemented with zinc-bacitracin (B)*
(Mean values and standard deviations for five chickens of each of the five replicates)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Counting of the amount of T-lymphocyte aggregates in the mucosa was per three sections.
Microbiota composition
Clustering of the T-RFLP patterns of caecal samples of replicates of broilers given the same treatment was generally observed (Fig. 1). All samples of the animals given a wheat/rye diet, either supplemented with Zn-bacitracin or not, clustered and showed a minimal similarity of 57 %. The maize group replicates showed a minimum similarity of approximately 81 %, the replicates of the Zn-bacitracin-supplemented maize group showed a minimum similarity of 73 %. The cereal type had more impact on the composition of the microbiota, compared with supplementation of Zn-bacitracin to the feed (Fig. 1). The differences were more explicit on day 15 (Fig. 1) than on days 29 and 42 (data not shown). Samples taken from the jejunum and ileum gave only a few bands, not allowing comparison.
Discussion
Feeding cereals high in NSP leads to increased feed conversion ratios and lower body-weight gain(Reference Choct and Annison15–Reference Choct, Hughes and Wang18). The soluble NSP content of wheat and rye is rather high compared with maize, what is generally believed to be the main reason for the effects on performance(Reference Choct, McNab and Boorman19). Adding NSP-degrading enzymes improves nutritient digestibility and thus also body-weight gain and feed conversion(Reference Meng, Slominski and Guenter3, Reference Choct, Hughes and Trimble20, Reference Olukosi, Cowieson and Adeola21).
The mechanism of action of NSP on nutrient absorption can be explained by different mechanisms. First, NSP lead to increased digesta viscosity. This is not always accompanied by increases in intestinal motility, and thus the efficiency of mixing in the small intestine can be decreased(Reference Lentle, Janssen and Asvarujanon22, Reference Lentle and Janssen23). Second, NSP are shown to result in a thickening of the mucous layer on the intestinal mucosa(Reference Choct, McNab and Boorman19). Both mechanisms can potentially lead to reduced digestive efficiency and nutrient absorption. In the present study, however, several additional factors have been detected that may contribute to the negative effect of NSP on performance.
The cereal type in feed had a major impact on the histological parameters of the intestine. Feeding wheat and rye led to epithelial cell apoptosis along the villi and the crypts, fusion of villi and more and larger goblet cells. Increased numbers and activity of goblet cells may explain the thickening of the mucus layer on the epithelial lining described by Lentle et al. (Reference Lentle, Janssen and Asvarujanon22). Also a significant T-cell infiltration in the mucosa was seen in broilers given a wheat/rye diet, forming aggregates in the mucosa, pointing to an excessive stimulation of the immune system in the intestinal tract. The increased epithelial cell apoptosis could be explained by increased levels of cytokines, such as interferon-γ, that are known to suppress anti-apoptotic signals in the epithelium(Reference Edelblum, Yan and Yamaoka24). It has been shown that the nutritional cost of mounting an immune response in broilers is high and is inversely related to body-weight gain(Reference Humphrey and Klasing25). Different mechanisms may account for the observed histological changes. First, a greater shear damage of cells due to the hydrodynamic flow conditions induced by NSP and undigested fragments could be an explanation of the inflammatory state of the mucosa(Reference Lentle and Janssen23). Second, it is well known in laboratory animal models that changes in diet may alter leucocyte recruitment by regulating enterocyte gene expression(Reference Sanderson26). Third, colonisation by pathogens that induce epithelial cell damage and trigger inflammation can cause the observed histological changes. As an example, NSP are known to stimulate proliferation of Clostridium perfringens in the chicken gut(Reference Riddell and Kong27, Reference Annett, Viste and Chirino-Trejo28). This bacterium can induce epithelial cell damage and fusion of villi, due to toxin production, and induce inflammation(Reference Gholamiandehkordi, Timbermont and Lanckriet29). In the present study, however, no detailed analysis of specific bacterial populations was carried out. A fourth possible reason for the observed histological changes when given a wheat/rye-based diet compared with maize is that the commensal microbial population is increased in concentration(Reference Hübener, Vahjen and Simon30), leading to an increased fermentation in the gut(Reference Choct, Hughes and Wang18). Also a shift in the microbial composition in the gut towards one that induces gut damage and immune cell infiltration is a possibility. The hypothesis of a microbial origin of the histological effects is further supported by the fact that addition of bacitracin reversed the adverse effects on gut health of the wheat/rye-based diet. The exact nature of the microbiota components that are repressed or induced in the treatments are currently not known. T-RFLP results in the present study do not allow direct identification of bacterial groups involved in the observed effects. Nevertheless, there is considerable interest in the literature in trying to identify disease-associated compositional shifts in bowel bacterial communities, especially in the light of inflammatory bowel diseases in man(Reference Tannock31), which are also characterised by massive T-cell infiltration. Further research in this area should focus on tools identifying and quantifying specific subsets or even individual species of bacterial communities. Special attention should be paid to bifidobacteria and lactate-utilising butyrate-producing bacteria, since these groups' beneficial effects have already been documented(Reference Turroni, Ribbera and Foroni32, Reference Sato, Matsumoto and Okumura33).
For the time being, we can conclude that a wheat/rye-based diet, high in NSP, compared with a maize-based diet, induces villus fusion, a thinner tunica muscularis, T-lymphocyte infiltration, more and larger goblet cells, more apoptosis of epithelial cells in the mucosa and a shift in microbiota.
Acknowledgements
We would like to thank Sofie Callens, Delphine Ameye and Christian Puttevils for their skilful technical assistance. The present study was funded by grant S6169 of the FOD (Federal Public Service Health, Food Chain Safety and Environment).
The contributions of the authors were: E. T., setting up and carrying out the experiment, sample analysis and writing; L. B., T-RFLP profiling; V. E., setting up and carrying out the experiment; G. H., in vivo trial supervision; F. P., setting up and carrying out the experiment; F. H., department head, overall supervision; J. D., statistical analysis; R. D., promoter of the project, setting up and carrying out the experiment; F. v. I., promoter of the project, setting up and carrying out the experiment.
The authors state that there are no conflicts of interest.











