Cognitive decline is worthy of study, both as an important outcome in its own right, and because of what it may tell us about future risk for dementia. It is associated with a significant public health burden, as shown by strong associations with dependency (Reference Gill, Richardson and TinettiGill et al, 1995), morbidity (Reference Weiler, Lubben and ChiWeiler et al, 1991) and mortality (Reference Liu, Lacroix and WhiteLiu et al, 1990). It is a core component of the dementia syndrome, although dementia cannot be diagnosed without additional functional impairment (DSM-IV) (American Psychiatric Association, 1994), and the level of cognitive function and the rate of cognitive decline at a given age are indicators of risk for later dementia (Reference Prince, Lewis and BirdPrince et al, 1996a ). Research into factors which influence an individual's position on the continuum of cognitive decline in the ageing population is therefore seen as a useful adjunct to work on dementia (Reference Elias, Wolf and D'AgostinoElias et al, 1993; Reference Feskens, Havekes and KalmijnFeskens et al, 1994). Earlier recognition of the onset of dementia by ascertaining cognitive decline could offer possibilities for early treatment and delay of disability.
This article presents our study of factors predicting change in cognitive function in a cohort of subjects with mild hypertension who volunteered for a hypertension treatment trial. Comprehensive measures of cognitive function, vascular risk factors and vasculopathy were obtained on entry to the trial. We revisited the cohort 9-12 years after baseline, which gave us a unique opportunity to study factors influencing cognitive change over a relatively long period, as survivors aged from their late sixties to late seventies or early eighties.
METHOD
Study design and sample
This analysis examines factors predicting the cognitive outcome in 1994-1995 of 387 survivors from 1083 subjects recruited between 1983 and 1985 into the cognitive sub-study of the Medical Research Council (MRC) Treatment Trial of Hypertension in Older Adults (1982-1989).
The designs of both the Medical Research Council treatment trial (MRC Working Party, 1992) and its cognitive sub-study are described in detail elsewhere (Reference Bird, Blizard and MannBird et al, 1990; Reference Prince, Lewis and BirdPrince et al, 1996a ). The MRC trial compared mortality and morbidity among 4396 subjects randomised to receive beta-blocker, a thiazide diuretic or placebo. Inclusion criteria were age 65-74 years and systolic blood pressure 160-209 mmHg. Exclusion criteria were current anti-hypertensive medication, and serious cardiovascular, cerebrovascular or other intercurrent illnesses, including dementia. Subjects were recruited in 226 UK MRC General Practice Research Framework (MRC GPRF) practices after invitations had been sent to all registered patients within the eligible age range. In 1991 we reviewed a subsample of 1545 participants from 71 of the 226 practices in an attempt to ascertain all cases of dementia and Alzheimer's disease incident since the beginning of the MRC Trial (Reference Prince, Cullen and MannPrince et al, 1994). In 1994, 54 of these practices were still included in the MRC GPRF and were willing to take part in a further follow-up. They had recruited 1083 subjects into the MRC trial. The MRC GPRF had been notified of 431 deaths (39.8%), leaving 652 persons potentially available (60.2%) for the re-survey. Survivors were invited to attend the practice or to be interviewed in their own homes by an MRC GPRF research nurse, for an assessment of cognitive function, a blood test, and a risk factor interview.
Measures
Baseline (Time I) cognitive function
Upon entry to the MRC trial (1983-1985, henceforward referred to as Time 1) cognitive tests had been administered to all subjects. Fluid intelligence was measured using : (a) The Paired Associate Learning Test (PALT) (Reference InglisInglis, 1959); (b) the Trial-Making Test (TMT) (Reference ReltanReltan, 1959); and (c) Raven's Progressive Matrices (RPM) (Reference RavenRaven, 1940). The TMT and PALT were repeated 1 month later. For these two tests, the mean of the scores at entry and after 1 month was used, to minimise random error. Principal component analysis with varimax rotation was used to extract a single factor from the three Time 1 measures of cognitive function accounting for 51% of the variance, with adequate loading values for the three Time 1 tests (RPM 0.79, TMT 0.60 and PALT 0.64). Premorbid IQ (crystallised intelligence) was measured at Time 1 using the National Adult Reading Test (NART) (Reference Nelson and O'ConnellNelson & O'Connell, 1978).
Time 2 cognitive outcome
In the 1994 follow-up, MRC GPRF research nurses in each of the participating practices administered the Mini-Mental State Examination (MMSE) (Reference Folstein, Folstein and McHughFolstein et al, 1975). The MMSE was transformed by : (a) reversing the scoring, so that high scores indicated poor function (scores were subtracted from 31, giving a range of 1-31); (b) log-transforming the positively skewed data distribution; (c) reversing the sign of (b), so that once again high scores indicated good function. The score of the Time 2 cognitive outcome measure ranges from 0 to 1.49, with a mean of 0.92 and a standard deviation of 0.32. Our analytical strategy was to use this transformed MMSE as the cognitive outcome measure, adjusting in all analyses for the principal component factor derived from the Time 1 cognitive measures. Factors associated with cognitive outcome while controlling for cognitive function 9-12 years previously are in effect predicting cognitive change.
Independent variables (explanatory measures)
Risk factors for vascular disease.
Information was recorded at Time 1 regarding the following risk factors for vascular disease : systolic and diastolic blood pressure, serum cholesterol, body mass index and smoking behaviour at entry to the trial (ex-smokers were not distinguished from those who had never smoked). Repeated measures of blood pressure over the 5-year trial period allowed the decline in systolic blood pressure from baseline to be calculated. Thus, we calculated the mean of successive measures and subtracted it from the entry value.
Evidence of vascular disease.
Signs of arrhythmia or ischaemia on electrocardiogram (ECG).
Additional (retrospective) exposure data.
At Time 2 we administered an expanded risk factor questionnaire to both the subject and an informant, collecting information on years of education, diet, alcohol use, lifetime smoking (pack-years), social class, and current area of residence. A loading for family history of dementia was derived by estimating the family person-years' risk for the disease (the sum of the years lived by parents and siblings after the age of 60) and then calculating the probability (according to the Poisson distribution), given the number of years at risk, that the same or fewer number of dementia cases are observed in the family pedigree. The same approach was used to estimate the loading for a family history of heart disease and stroke.
APOE genotyping and fibrinogen.
Blood samples were taken at Time 2. One blood sample was centrifuged immediately upon collection and serum fibrinogen concentrations assayed. The other was stored until DNA was extracted. Apolipoprotein-E (APOE) allelic status was determined using a PCR assay as detailed elsewhere (Reference Prince, Lovestone and CervillaPrince et al, 2000).
Statistical analysis
In a univariate analysis we tested for crude associations between the independent variables and cognitive outcome using, as appropriate, one-way ANOVA or simple linear regression. In a general factorial MANOVA analysis these crude associations were first adjusted for Time 1 cognitive function. We then explored the relationship between education, social class, NART and cognitive outcome, and finally identified a parsimonious model for cognitive outcome. The effect size of associations was measured, estimated by η2. We also tested for all second-order interactions. We repeated the multivariate analysis, excluding all cases of dementia.
RESULTS
Response
Out of a cohort of 1083 subjects, we collected full data from 387 (35.3% of the initial cohort; 58.6% of the survivors); 431 (39.8%) had died prior to Time 2 interviews, 112 (10.3%) had moved away from their original practice's catchment area, and 158 (14.6%) refused to take part in this phase of the study. Those for whom full data were available in 1994/5 differed systematically from other groups; in particular, those who refused interview in 1994-1995 tended to have lower premorbid IQ (NART), and lower baseline cognitive test scores (Raven's Matrices and Trail Making Test) than those who were followed up (see Table 1). Male gender and smoking at entry to the MRC trial were associated with death before follow-up.
Table 1 Cohort groups and baseline measures
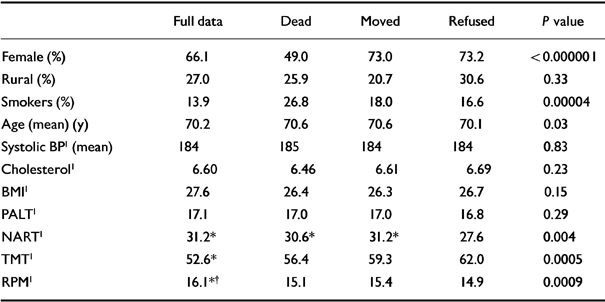
| Full data | Dead | Moved | Refused | P value | |
|---|---|---|---|---|---|
| Female (%) | 66.1 | 49.0 | 73.0 | 73.2 | <0.000001 |
| Rural (%) | 27.0 | 25.9 | 20.7 | 30.6 | 0.33 |
| Smokers (%) | 13.9 | 26.8 | 18.0 | 16.6 | 0.00004 |
| Age (mean) (y) | 70.2 | 70.6 | 70.6 | 70.1 | 0.03 |
| Systolic 1. (mean) | 184 | 185 | 184 | 184 | 0.83 |
| Cholesterol1 | 6.60 | 6.46 | 6.61 | 6.69 | 0.23 |
| BMI1 | 27.6 | 26.4 | 26.3 | 26.7 | 0.15 |
| PALT1 | 17.1 | 17.0 | 17.0 | 16.8 | 0.29 |
| NART1 | 31.2 * | 30.6 * | 31.2 * | 27.6 | 0.004 |
| TMT1 | 52.6 * | 56.4 | 59.3 | 62.0 | 0.0005 |
| RPM1 | 16.1 * † | 15.1 | 15.4 | 14.9 | 0.0009 |
Cognitive outcome
The crude associations with cognitive outcome at Time 2 are summarised in Table 2. After adjusting for baseline cognitive function, poorer cognitive outcome at Time 2 was associated with increasing age, less decline in systolic blood pressure over the trial period, a greater loading for family history of dementia, never having smoked, abstinence from alcohol before the age of 60 and over the past 3 months, lower social class, lower education and lower NART score. We also found a significant trend in the association between cognitive outcome and APOE group, in that those with an APOE ϵ2 allele had a better cognitive outcome than did those with APOE ϵ3/3 alleles who, in turn, did better than those with any APOE ϵ4 allele. There were near significant trends for associations between poorer cognitive outcome and both arrhythmia, recorded on ECG at baseline, and a higher loading for family history of heart disease. We found no association between cognitive decline and ECG ischaemia, body mass index, serum fibrinogen, serum cholesterol, diastolic or systolic blood pressure at entry, use of non-steroidal anti-inflammatory drugs (NSAIDs), loading for family history of stroke, alcohol consumption over the past 3 months, rural residence, vegetarian diet and gender.
Table 2 Univariate associations with cognitive outcome 1
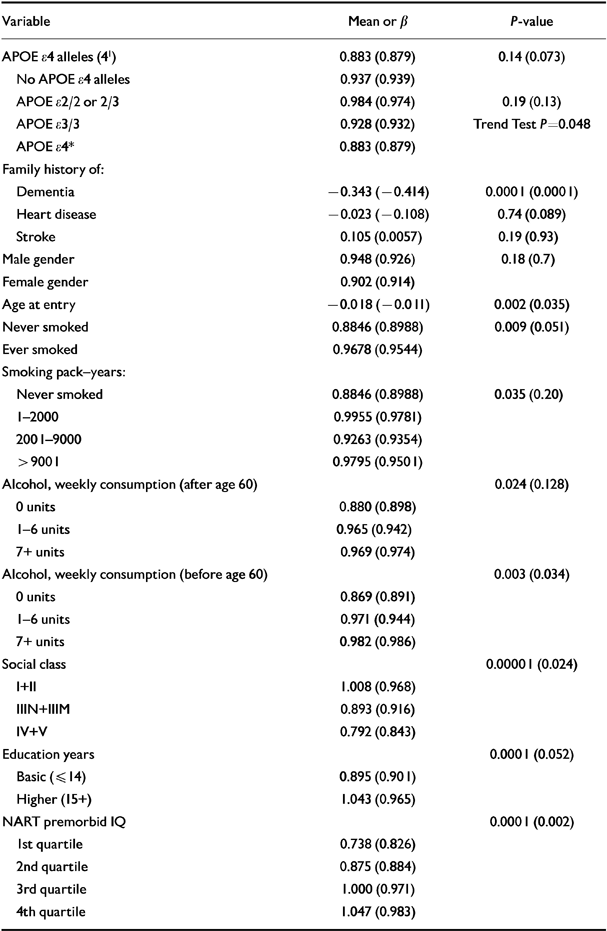
| Variable | Mean or β | P-value |
|---|---|---|
| APOE ϵ4 alleles (41) | 0.883 (0.879) | 0.14 (0.073) |
| No APOE ϵ4 alleles | 0.937 (0.939) | |
| APOE ϵ2/2 or 2/3 | 0.984 (0.974) | 0.19 (0.13) |
| APOE ϵ3/3 | 0.928 (0.932) | Trend Test P=0.048 |
| APOE ϵ4* | 0.883 (0.879) | |
| Family history of : | ||
| Dementia | -0.343 (-0.414) | 0.0001 (0.0001) |
| Heart disease | -0.023 (-0.108) | 0.74 (0.089) |
| Stroke | 0.105 (0.0057) | 0.19 (0.93) |
| Male gender | 0.948 (0.926) | 0.18 (0.7) |
| Female gender | 0.902 (0.914) | |
| Age at entry | -0.018 (-0.011) | 0.002 (0.035) |
| Never smoked | 0.8846 (0.8988) | 0.009 (0.051) |
| Ever smoked | 0.9678 (0.9544) | |
| Smoking pack-years : | ||
| Never smoked | 0.8846 (0.8988) | 0.035 (0.20) |
| 1-2000 | 0.9955 (0.9781) | |
| 2001-9000 | 0.9263 (0.9354) | |
| > 9001 | 0.9795 (0.9501) | |
| Alcohol, weekly consumption (after age 60) | 0.024 (0.128) | |
| O units | 0.880 (0.898) | |
| 1-6 units | 0.965 (0.942) | |
| 7+ units | 0.969 (0.974) | |
| Alcohol, weekly consumption (before age 60) | 0.003 (0.034) | |
| 0 units | 0.869 (0.891) | |
| 1-6 units | 0.971 (0.944) | |
| 7+ units | 0.982 (0.986) | |
| Social class | 0.00001 (0.024) | |
| I+II | 1.008 (0.968) | |
| IIIN+IIIM | 0.893 (0.916) | |
| IV+V | 0.792 (0.843) | |
| Education years | 0.0001 (0.052) | |
| Basic (≤14) | 0.895 (0.901) | |
| Higher (15+) | 1.043 (0.965) | |
| NART premorbid IQ | 0.0001 (0.002) | |
| 1st quartile | 0.738 (0.826) | |
| 2nd quartile | 0.875 (0.884) | |
| 3rd quartile | 1.000 (0.971) | |
| 4th quartile | 1.047 (0.983) |
We compared the independent effects of social class, education and NART scores on cognitive outcome. After adjusting for Time 1 cognitive function, NART predicts cognitive decline, independently of the effects of a higher number of years of education and social class. A higher number of years of education was not independently associated with cognitive outcome. Social class predicted cognitive outcome independently of education, but not after adjusting by NART.
The most parsimonious multivariate model accounted for 29% of the variance (Table 3). Poorer cognitive outcome, after adjusting for Time 1 cognitive function, was independently associated with increasing loading for family history of dementia, older age at entry, abstinence from alcohol prior to the age of 60 and less decline in systolic blood pressure over the trial period. APOE ϵ4 alleles did not add any additional explanatory power to the model (F=1.28, P=0.258 for all cases; F=0.3, P=0.86 excluding dementia cases) and did not modify the effect of any of the independently associated factors. APOE was therefore excluded from the final model. We finally applied the same parsimonious model excluding those 41 cases that had a DSM-IV diagnosis of dementia. The parameters changed very little, although the effect of decline in systolic blood pressure fell just beyond the traditionally accepted values of statistical significance (P=0.06).
Table 3 Multivariate model independent associations with cognitive outcome over the 9-12-year period 1
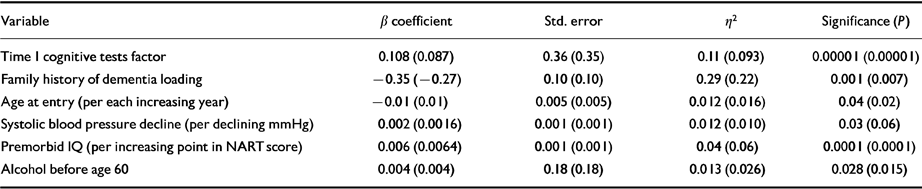
| Variable | β coefficient | Std. error | η2 | Significance (P) |
|---|---|---|---|---|
| Time I cognitive tests factor | 0.108 (0.087) | 0.36 (0.35) | 0.11 (0.093) | 0.00001 (0.00001) |
| Family history of dementia loading | — 0.35 (—0.27) | 0.10 (0.10) | 0.29 (0.22) | 0.001 (0.007) |
| Age at entry (per each increasing year) | — 0.01 (0.01) | 0.005 (0.005) | 0.012 (0.016) | 0.04 (0.02) |
| Systolic blood pressure decline (per declining mmHg) | 0.002 (0.0016) | 0.001 (0.001) | 0.012 (0.010) | 0.03 (0.06) |
| Premorbid IQ (per increasing point in NART score) | 0.006 (0.0064) | 0.001 (0.001) | 0.04 (0.06) | 0.0001 (0.0001) |
| Alcohol before age 60 | 0.004 (0.004) | 0.18 (0.18) | 0.013 (0.026) | 0.028 (0.015) |
DISCUSSION
Cognitive outcome
Prospective associations with deterioration in cognitive function should be less prone to bias than estimates of associations derived from cross-sectional studies. Longitudinally measured cognitive decline will also tend to reduce random measurement error and reduce measurement bias (Reference Prince, Lewis and BirdPrince et al, 1996a ). In the present study, we reassessed the cognitive function of hypertensive trial participants once only after a baseline initial assessment carried out 9-12 years earlier. Both more frequently repeated observations and longer intervals between tests increase the reliability with which cognitive decline can be ascertained, although the temporal perspective has the greater impact (Reference van Belle, Uhlmann and Hughesvan Belle et al, 1990).
Study limitations
Our results need however to be interpreted with caution, as there are limitations to our study. The cohort is based on the primary care network but consists of mildly hypertensive trial volunteers, suggesting limits to generalisability and potential selection bias. The response rate was only 58.6% of those cohort subjects who had survived until the follow-up survey, reflecting their age and frailty, the 9-12 year time interval and the logistic difficulties of tracing those who had moved far away from their original MRC research practice. Response bias cannot be excluded, even though no particular characteristics of subjects (other than their cognitive function) recorded at entry to the MRC trial tended not to be associated with their response status at follow-up.
Study advantages
The main strength of the study design is the complete ascertainment of prospectively recorded exposure data, including smoking status, serum total cholesterol and body mass index, and repeated measures of blood pressure level and ECG recordings, made before the onset of dementia and significant cognitive decline. Additionally, the long (9-12 years) follow-up period provides us with a rare opportunity to explore long-term effects of potential risk factors for failing cognition.
Age
We found, as expected, an association between older age at entry and lower cognitive outcome, which has been repeatedly reported previously (Reference Prince, Bird and BlizardPrince et al, 1996b ). Age may be understood as a proxy for an accumulation of age-related impairments that may themselves be feasible targets for intervention (Reference Prince, Lewis and BirdPrince et al, 1996a ).
Education, social class and premorbid IQ
Although better education was associated with better cognitive outcome in this study, its effect was weaker than, and not independent of, that of social class. Similarly, lower social class was only associated with cognitive decline before adjusting for the effect of premorbid crystallised IQ. Thus, of these three strongly correlated variables, premorbid IQ was the best independent predictor of cognitive outcome (η2=0.26; P=0.023), confounding or mediating the effects of education and social class. Our findings replicate those of a previous report and give further support to the ‘brain reserve’ hypothesis (Reference Schmand, Smit and GeerlingsSchmand et al, 1997).
Vascular factors
Several cardiovascular risk factors have been reported to predict cognitive decline (Reference StewartStewart, 1999). Atrial fibrillation was associated with cognitive impairment in one cross-sectional study (Reference Kilander, Nyman and BobergKilander et al, 1998) of a sample of men after adjusting for education, age, occupation, stroke and other vascular factors. A longitudinal study (Reference Alewijn, Monique and BretelerAlewijn et al, 1997) has also reported an association between atrial fibrillation and cognitive impairment after adjusting for age, gender, education and vascular risk factors, but it was only apparent in women, and weakly so, when stroke cases were excluded. We found a univariate association between ECG-measured arrhythmia and cognitive decline in this longitudinal study. However, no independent association was found when arrhythmia was added to the multivariate model adjusting for alcohol consumption and systolic blood pressure decline, suggesting possible confounding in previous reports.
We found no association between baseline systolic or diastolic blood pressure and cognitive outcome, in accordance with previous findings on this cohort (Reference Bird, Blizard and MannBird et al, 1990; Reference Prince, Lewis and BirdPrince et al, 1996a ). Interestingly, we did find an association between less decline in systolic blood pressure and poorer cognitive outcome. However, this should be interpreted cautiously. A survivor effect cannot be ruled out, as we had previously reported no effect of anti-hypertensive treatment on cognition, compared to placebo, despite large randomisation group effects on blood pressure levels (Reference Prince, Bird and BlizardPrince et al, 1996b ), after 5-year follow-up of the entire cognitive sub-study cohort. Further-more, blood pressure did not predict cognitive decline over the 54 initial months of this study (Reference Prince, Lewis and BirdPrince et al, 1996a ). Nevertheless, both the Framingham (Reference Elias, Wolf and D'AgostinoElias et al, 1993) and the Honolulu (Reference Launer, Masaki and PetrovitchLauner et al, 1995) studies have provided evidence of an association between higher mid-life systolic blood pressure levels and cognitive decline in late life. In the Framingham study this association was most apparent in those left untreated (Reference Farmer, Kittner and AbbottFarmer et al, 1990). Our finding is also consistent with the notion that treating hypertension may provide a means for a modest preventive intervention (Reference StewartStewart, 1999).
Non-steroidal anti-inflammatory drugs (NSAIDs)
We could not replicate our earlier finding of a protective effect for NSAIDs against cognitive decline on the PALT over the first 5 years of the MRC trial (Reference Prince, Rabe-Hesketh and BrennanPrince et al, 1998). Any beneficial effect of NSAIDs may not be sustained. The effect of NSAIDs in cognitive function is unresolved, as other studies have found NSAIDs not to be associated with cognitive function, or even to be a risk factor for cognitive decline (Reference Saag, Rubenstein and ChrischillesSaag et al, 1995).
Genetic/familial factors
Although APOE ϵ4 is associated with dementia in this sample (Reference Prince, Lovestone and CervillaPrince et al, 2000), in univariate analysis it was only weakly associated with cognitive outcome. Neither did APOE modify the effect of any other variable in the final model. This finding is comparable with those of most previous studies (Reference Feskens, Havekes and KalmijnFeskens et al, 1994; Reference SmallSmall, 1998) as the association between APOE and cognitive impairment or decline is certainly less clear than is the case when studying Alzheimer's disease or dementia cases, especially among older old populations (Reference SmallSmall, 1998). A loading for family history of dementia did predict poorer cognitive outcome, even among those free of dementia and after adjusting for the effect of APOE. Our results support the ‘continuum hypothesis’ (Reference Brayne and CallowayBrayne & Calloway, 1988) and suggest a multi-factorial polygenic model, with the summation of the small effects of multiple genes influencing propensity for cognitive deterioration across a braod continuum of ‘normal’ and ‘pathological’ age-related change.
Smoking and alcohol
We found a trend for smoking to protect against poorer cognitive outcome, which was not borne out in multivariate analysis. The association between smoking and lower cognitive outcome was found to be confounded by the Time 1 cognitive factor, alcohol use and, to a lesser extent, by premorbid IQ. Low to moderate alcohol consumption may have a protective role in cognitive impairment (Reference Launer, Feskens and KalmijnLauner et al, 1996; Reference Dufouil, Ducimetière and AlperovitchDufouil et al, 1997). We found that those who were abstinent before the age of 60 had poorer cognitive outcomes than did those who drank mildly or moderately. However, it could also be argued that those subjects who drank more and survived till this phase of the study could be a healthier group.
CLINICAL IMPLICATIONS AND LIMITATIONS
CLINICAL IMPLICATIONS
-
▪ This study identifies factors that predict failing cognition long before the onset of clinically significant deterioration. This may assist in the early recognition of cognitive disorder.
-
▪ Both moderate alcohol consumption and a reduction in raised systolic blood pressure could be potential targets for preventive interventions aimed at reducing cognitive dysfunction.
-
▪ We replicate longitudinally both the ‘continuum’ and the ‘brain reserve’ hypotheses, which may also have implications for diagnosis and prevention of dementia.
LIMITATIONS
-
▪ Although the cohort is based on the primary care network, it consists of mildly hypertensive volunteers, which limits the generalisability of our findings.
-
▪ Only 58.6% of survivors responded, reflecting the old age and frailty of the cohort and the logistic difficulties of the long-term follow-up period.
-
▪ The instruments used to measure cognitive function at baseline differed from the MMSE, which was used at follow-up.
ACKNOWLEDGEMENTS
The Medical Research Council supported this study. We thank the MRC Working Party and the MRC Epidemiology and Medical Care Unit for allowing us to continue to investigate trial subjects, and the MRC Framework general practitioners and research nurses for collecting much of the data. We would like to thank Carsten Russ and John Powell of the Department of Neuroscience at the Institute of Psychiatry for genotyping the sample; and Angela Thomas and Jody Raab from the Royal Free Hospital for their technical support.






eLetters
No eLetters have been published for this article.