Introduction
Intraoperative flash visual evoked potential (FVEP) recording has become possible due to recent refinements in technical and anesthetic methods that have improved FVEP reproducibility. Specifically, the use of restrictive bandpass filtering, optimal reject window settings, mastoid reference site, total intravenous anesthetic (TIVA), and stable retinal stimulation (ensured by concomitant electroretinogram [ERG] recording) have greatly enhanced FVEP reproducibility.Reference Houlden, Turgeon and Polis1–Reference Sasaki, Itakura and Suzuki5 The FVEP was previously considered difficult to obtain during surgery and, when obtained, a poor predictor of postoperative visual function.Reference Cedzich, Schramm and Fahlbusch6–Reference Wiedemayer, Fauser, Sandalcioglu, Armbruster and Stolke10 Now that it is possible to obtain more reproducible intraoperative FVEPs, there is renewed interest in determining its relationship to iatrogenic visual pathway injury.
Neurosurgery performed in areas involving the visual pathway, including orbital, transsphenoidal, and transcranial approaches over the parietal, temporal, and occipital cortices are associated with varying levels of postoperative visual dysfunction.Reference Rajan, Simon and Nair4 For instance, surgical resection of suprasellar meningiomas has been shown to worsen visual function in 14–28% of patients,Reference Andrews and Wilson11–Reference Rosenstein and Symon14 and surgical resection of craniopharyngiomas worsen visual function in 3–11.5% of patients.Reference Caldarelli, Massimi, Tamburrini, Cappa and Di Rocco15, Reference Chakrabarti, Amar, Couldwell and Weiss16 Rare cases of visual loss following surgery for aneurysms (particularly paraophthalmic and superior hypophyseal artery aneurysms) have been reported.Reference Day17–Reference Rizzo19 Manipulation of the optic nerve and interference with the microvasculature during microsurgical dissection is thought to be responsible for the visual loss.
Ultimately, the goal of intraoperative FVEP monitoring is to prevent iatrogenic visual pathway injury through prompt identification of FVEP deterioration at a time when corrective measures may be applied. With the recent improvements in FVEP monitoring, FVEPs could increasingly serve as a reliable tool for reducing the incidence of intraoperative injury to the visual pathway. In this paper we describe the relationship between our intraoperative FVEP results and visual outcome. We also describe surgical maneuvers associated with intraoperative FVEP deterioration and recovery.
Methods
Patient population: FVEPs were attempted in 89 patients (35 males; mean age 52.2 years during 105 surgeries; 12 patients had 2 surgeries and 2 patients had 3 surgeries). FVEPs were recorded in 77 pre-chiasmal (62 via endonasal approach), 25 post-chiasmal (20 by craniotomy and 5 by endovascular approach), and 3 spinal surgeries. Further classification of pathology is shown in Table 1. Patients were selected based on the surgeon’s determination that (a) the visual system was at risk of iatrogenic injury; (b) the intraoperative FVEP monitoring could rapidly detect this injury; and (c) corrective measures could be taken during surgery to reverse it. Three spinal surgeries were monitored because there are reports of blindness after lengthy spinal surgeries in the prone position.Reference Mohammed, Houlden and AlKherayf20
Table 1: Number of cases with FVEP monitoring by pathology

CR = craniotomy; E = endonasal ; SP = spine ; V = endovascular ; PrC = pre-chiasmal ; PoC = post-chiasmal; ACOM = anterior communicating artery; PCOM = posterior communicating artery; PCA = posterior cerebral artery; AVM = arteriovenous malformation.
FVEP methods: Methods for reproducible intraoperative FVEP recording have been previously described and the technical and physiologic factors outlined.Reference Houlden, Turgeon and Polis1 In brief, left then right eyes were stimulated with flashing red light stimulation at a rate of 1.41 Hz using a Cadwell goggle stimulator (Cadwell Laboratories, Kennewick, WA, USA). Stimulation was delivered via three LEDs on each side, 640 nm peak wavelength, 10 ms pulse width, 3000 mCd of luminous intensity reflected back on an angle to give uniform illumination across the whole red plastic lens. FVEP recordings were obtained from subdermal corkscrew electrodes placed at Oz-linked mastoid and Oz–Fz (international 10–20 system) using a 32-channel Cadwell Elite machine (Cadwell instruments, Kennewick WA). The ground electrode was placed on the upper leg. Electroretinogram (ERG) was concomitantly recorded from a subdermal needle electrode placed just above the bridge of the nose and referenced to Fz. The electrode positioned above the bridge of the nose (between the eyes) allowed us to record ERG from each eye separately after left then right eye stimulation. Simultaneous monitoring of ERG and FVEP allowed for detection of post-retinal visual pathway compromise (FVEP change without ERG change) that was easily distinguished from retinal compromise or inadequate retinal stimulation (simultaneous FVEP and ERG change). When simultaneous loss of FVEP and ERG occurred during reflection of the frontal skin flap over the stimulating goggles, a downwardly displaced goggle from skin flap pressure was usually the culprit (as determined by visual inspection of the goggle position under the drape). The sweep duration was 300 ms and 50–150 repetitions were included in each average. The amplifier gain was 50,000 and the recording bandpass was 10–100 Hz for all channels. The reject window setting was 20 µV (peak-to-peak) for Oz-linked mastoid montage and 30 µV for Oz–Fz montage. If the FVEP was not reproducible, then the low cut filter was increased to 30 Hz. Applying tight reject window settings during signal acquisition blocked transient high-amplitude artifacts. This led to improvement in signal quality and decreased the number of stimulus repetitions necessary for a reproducible FVEP. The FVEP amplitude was measured from the first negative peak after 60 ms (N1) to the following positive peak (P1). A >50% decrease in the intraoperative FVEP N1–P1 amplitude recorded from Oz- linked mastoid (without a change in ERG amplitude) was considered a significant FVEP change and reported to the surgeon. We did not consider transient FVEP N1–P1 amplitude change without new postoperative visual deficit to be a false-positive result, rather an indication that there was reversible electrophysiological dysfunction within visual pathways contributing to the FVEP.
Anesthetic methods: Total intravenous anesthesia (TIVA) was used through the whole period of monitoring in all patients except two. Stable level of anesthesia was maintained by the continuous infusion of propofol (100–150 µg/kg/min), remifentanil (0.2–0.5 µg/kg/min), and ultra-low dose of ketamine (0.1–0.2 mg/kg/h).Reference Choi, Lee, Park, Lee, Kim and Baik21, Reference Weinbroum22 Low-dose vasopressor infusion (phenylephrine or norepinephrine) was added if required to maintain mean arterial pressure in the range of 70–100 mm Hg. In two patients, inhalation agents (desflurane or sevoflurane), remifentanil, and occasional boluses of propofol were used. All changes in anesthetic medications were recorded in the neuromonitoring log.
Visual testing: Pre- and postoperative visual testing results were obtained retrospectively. To include as many FVEP-monitored patients as possible, we analyzed only the most commonly performed tests of visual function (administered by the neurosurgeon and/or ophthalmologist blinded to the intraoperative FVEP findings). These included pre- and postoperative color vision, visual acuity, and confrontational visual field testing. The confrontational visual field test, also referred to as Donder’s test, was conducted by having the patient place a hand over one eye and maintaining a fixed gaze while simultaneously reporting awareness of the examiner’s hand moving to various positions in the patient’s visual field. In addition, the patient reported on the number of fingers presented in quick succession, in all four visual quadrants, to assess finger counting ability in each visual field quadrant. Postoperative visual testing was typically performed 1 month after surgery.
Research adhered to the tenets of the Declaration of Helsinki (Code of Ethics of the World Medical Association).
Results
Out of a possible 210 eyes, 31 (14%) were not monitored and considered non-contributive cases. The reasons were as follows: 16 eyes had absent or irreproducible baseline FVEPs (all had preoperative visual deficits); 7 eyes lost their FVEP (and ERG) due to intraoperative goggle movement; 1 patient (2 eyes) had FVEP amplitude decrease related to unanticipated anesthetic changes; FVEPs were not attempted in 4 eyes due to anticipated goggle movement related to reflection of the frontal skin flap; 1 patient (2 eyes) had intraoperative removal of goggles by neuromonitoring staff after the advent of an oculocardiac reflex. Every eye with an absent or irreproducible baseline FVEP had some form of preoperative visual deficit (typically profound deficits in visual acuity or dense visual field loss), but no degree of visual loss guaranteed an absent baseline FVEP. The relationship between FVEP N1–P1 amplitude change and visual outcome was determined from the remaining 179 eyes. Our success rate for obtaining reproducible intraoperative FVEPs was 86%. This would have been slightly higher if inadvertent intraoperative goggle movement did not occur (confirmed by loss of ERG and FVEP and by visual inspection, described in Methods section). Reproducible FVEPs were typically obtained in <1.5 minutes from each eye.
One eye (0.5%) with normal preoperative vision had a permanent FVEP loss (absent N1–P1 and subsequent FVEP waveforms, without change in ERG) during resection of a suprasellar tumor (Figure 1a and b). That eye had only light perception after surgery (a true positive). Seven eyes (4%) had transient FVEP N1–P1 amplitude decrease (without change in ERG) that was related to either manipulation of tumor, aneurysm, or nerve (see Table 2). All of those eyes did not have new postoperative visual deficits except one that had mild changes in visual acuity (and optic neuropathy) after clipping of a paraophthalmic aneurysm. In that case, the FVEP amplitude transiently decreased by 65% (to 35% of baseline) for a period of just under 10 minutes (while dissecting around the aneurysm) and returned fully to baseline amplitude following the event. There was no change in the ERG, indicating stable retinal stimulation (Figure 2). All of the other eyes had no significant change in FVEP N1–P1 amplitude and no new postoperative visual deficits (no false-negatives; Table 3). There were no false-positives. If we exclude the transient changers from our analysis, then the sensitivity is 1.0 (95% CI = 2.5, 100) and specificity is 1.0 (95% CI = 97.9, 100). If we include them, then the sensitivity is 0.5 (95% CI = 1.3, 98.8) and specificity is 1.0 (95% CI = 97.9, 100), respectively.
Table 2: Data from five patients with transient intraoperative FVEP amplitude decrease (>50% decrease in N1–P1 amplitude compared with baseline)
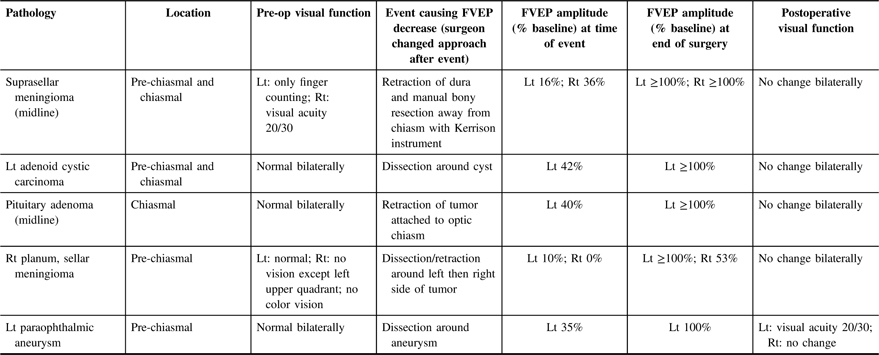
Lt = left; Rt = right.
Table 3: Intraoperative flash visual evoked potential changes and relationship to patient outcome

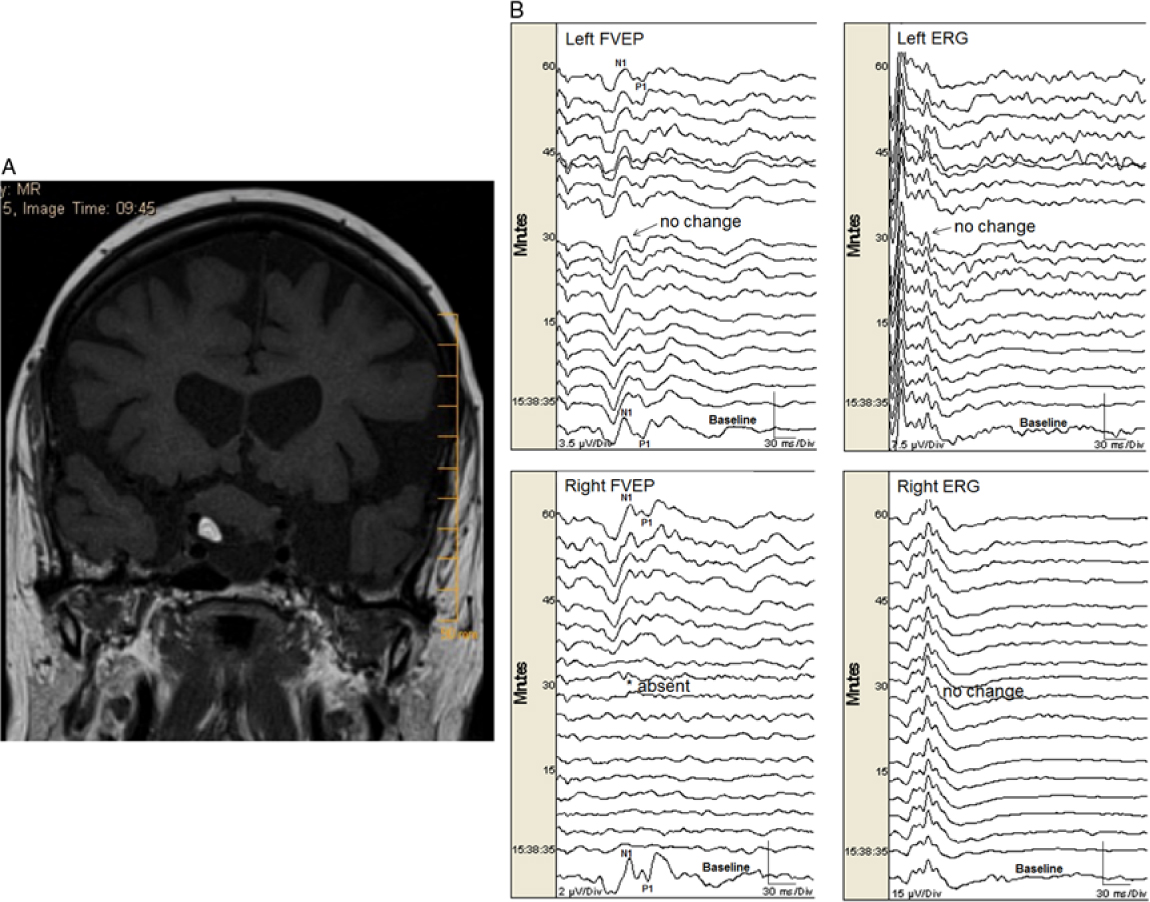
Figure 1: (A). A heterogeneous mass lesion in the suprasellar region (slightly more right of midline) measuring approximately 20 × 25 × 19 mm (AP × transverse × craniocaudal) likely splaying the optic nerves and chiasm. There was a focus of fat noted within the lesion. There was also an inferior cystic component. No significant enhancement noted in the post gadolinium images. (B). Permanent FVEP loss in this patient undergoing endonasal resection of a midline suprasellar teratoma. Preoperatively the patient had decreased visual acuity bilaterally with left worse than right eye. When working near the right optic nerve, the right FVEP was suddenly lost (bottom left panel). There was no change in the left FVEP (top left panel) and ERG bilaterally (top right and bottom right panels, respectively). At that time, the surgeon could not delineate a margin between the tumor and the nerve. Surgery ceased but the right FVEP remained absent. Postoperatively, the patient had worsened visual function in the right eye (only light perception) and improved visual function in her left eye.

Figure 2: Transient significant FVEP N1–P1 amplitude change in a patient undergoing surgery for clipping of a left paraophthalmic aneurysm. Preoperatively the patient had normal vision. When dissecting around the aneurysm, the left FVEP N1–P1 amplitude decreased by 65% (to 35% of baseline; left panel). The surgeon stopped resecting and the amplitude recovered 10 minutes later. There was no concomitant change in left ERG (right panel), indicating adequate retinal stimulation. The right FVEP and ERG did not change (not shown). Postoperatively, the patient experienced mild optic neuropathy in the form of decreased left-sided visual acuity (20/30). This was thought to be the result of segmental interruption of the blood supply to the posterior optic nerve.
Illustrative study showing transient FVEP deterioration (Table 2, case 4): A 39-year-old female, 38 weeks gestation, presented with progressive right visual field loss leading to a car accident. There were no symptoms of pituitary hormone deficiencies or excess, and she had no headaches, nausea, or vomiting. There were no other cranial nerve palsies on examination. On imaging she was found to have a suprasellar mass (Figure 3a). Due to loss of vision in the right eye, an urgent cesarean section was performed and 5 days later she had an expanded endoscopic endonasal approach for tumor resection (pathology revealed a transitional type meningioma; WHO grade 1). Despite right visual field loss preoperatively (no vision except left upper quadrant; no color vision), we were able to obtain reproducible FVEP bilaterally. There were significant transient changes in FVEP bilaterally associated with retraction near left, then right optic nerves (Figure 3b). Postoperatively, she developed pan-hypopituitarism, but her right eye vision improved. Three months later, all visual fields were normal to confrontation testing and she was deemed safe to drive.

Figure 3: (A). A sellar/suprasellar mass, separate from the pituitary gland, measuring approximately 17 × 16 × 21 mm (AP × transverse × craniocaudal) abutting the optic chiasm (not separate from it) displacing the optic chiasm postero-superiorly. The cisternal component of the optic nerves was stretched. The infundibulum was not identified. The A1 segments of the anterior cerebral artery were displaced superiorly. The lesion had homogenous enhancement. (B). Transient significant FVEP N1–P1 amplitude change in this patient undergoing endonasal resection of a right planum, sellar meningioma. Preoperatively the patient had normal vision in the left eye but no vision in the right eye except from the left upper quadrant. When working near the left optic nerve, the left FVEP N1–P1 amplitude decreased to 10% of baseline (top left panel; time 15:03). When the surgeon decreased retraction and slowed resection, the amplitude recovered over the next 10–15 minutes. Later, there was a loss of the right FVEP while working near the right optic nerve (bottom left panel; time 16:06). The right FVEP N1–P1 amplitude recovered to 53% of baseline after decreased retraction and slowed resection. There was no significant change in ERG bilaterally (top right and bottom right panels, respectively). Postoperatively, there were no new visual deficits bilaterally.
Illustrative study showing permanent FVEP loss: A 77-year-old female with a history of multiple myeloma presented with bilateral visual loss over a period of 5 years but more recent progressive worsening of visual acuity in her left eye. Imaging revealed a suprasellar mass with heterogeneous content and significant chiasmal and optic nerve compression (more on the right side) (Figure 1a). She had an expanded endoscopic endonasal approach for tumor resection of the lesion, with dissection of the lesion from cranial nerves II and III as well as from the frontal lobes (pathology revealed a mature teratoma). Intraoperatively, there was a plane between the optic tract and the lesion bilaterally. Nevertheless, the most anterior part of the lesion was adherent to the right optic nerve, and traction on the lesion was associated with sudden FVEP loss (Figure 1b), so the procedure was aborted. The FVEP did not recover by the end of surgery. Postoperatively, she had only light perception in the right eye, while visual acuity improved in her left eye.
Discussion
Our FVEP findings relate strongly to visual outcome. We had one eye with permanent FVEP loss associated with severe deterioration of visual function (0.5% of all eyes monitored) and one eye with transient FVEP change associated with mild postoperative deterioration of visual acuity (0.5% of all eyes monitored). In the former case, the right FVEP N1–P1 (and all subsequent FVEP waveforms) permanently disappeared (Figure 1b). At that time, the margin between optic nerve and tumor was ill-defined. The surgeon was promptly informed of the FVEP loss and he stopped resecting and retracting, but the FVEP did not return. In contrast, we had seven eyes (4%) with significant transient FVEP amplitude deterioration related to surgical manipulation. In all of those cases the surgeon was promptly informed of the FVEP deterioration and stopped resecting and/or retracting near the offended optic nerve or chiasm. The FVEP amplitude recovered within 10–15 minutes in all cases (Table 2). All but one of those eyes had unchanged postoperative visual function (one had a mild deterioration in visual acuity postoperatively despite full recovery of the FVEP). Accordingly, we believe prompt reporting of FVEP deterioration resulted in prompt surgical action that was important for FVEP amplitude recovery, which, in turn, may have accounted for the low postoperative visual deficit rate (1%) in this series. We believe that transient change should be excluded from the analysis of sensitivity and specificity because it reflects functional compromise of the visual pathway that is undergoing some degree of recovery and, as such, is not categorically a positive or negative finding. Consequently, our sensitivity and specificity were 1.0 (excluding the transient changers).
Careful exclusion of eyes that produced low amplitude, irreproducible baseline FVEP (despite good ERG) from our analysis (non-contributive cases; 14% of all eyes tested) likely helped to eliminate false-positive and false-negative results. Lack of false-negatives in our study (excluding transient changers from false-negative calculations for the same reasons described above) may also be explained by our relatively few post-chiasmal operations (24% of surgeries). Other investigators have associated false-negative FVEPs to new partial field defects after post-chiasmal surgery.Reference Sasaki, Itakura and Suzuki5, Reference Luo, Regli, Bozinov and Sarnthein23 Intraoperative deterioration of visual function in a partial field may be difficult to detect with FVEP monitoring because the FVEP is generated from all quadrants of the retina. As such, the FVEP likely relates more to a measure of gross visual function. Some investigators have described no relationship between FVEP and visual acuity.Reference Cedzich, Schramm and Fahlbusch6, Reference Mauguiere and Fischer24 Others have shown that intraoperative FVEP changes are associated with changes in visual acuity more than changes in visual field testing,Reference Kodama, Goto, Sato, Sakai, Tanaka and Hongo3 and our results support this (both patients with FVEP change and new visual deficits had changes in visual acuity). A recent study showed that if the criterion for significant FVEP change is decreased to ≥20% amplitude reduction (instead of ≥50%) while using multiple recording sites (O1, O2, and Oz) and white light stimulation, then there may be improved detection of iatrogenic injury resulting in quadrantanopia.Reference Gutzwiller, Cabrilo, Radovanovic, Schaller and Boëx25
The high success rate in this study was mostly related to the use of restrictive filtering, optimal reject window settings, mastoid reference, concomitant ERG recordings, and TIVA anesthesia.Reference Rajan, Simon and Nair4 In a previous paper, we showed that FVEPs are more reproducible in the presence of low-amplitude EEG than high-amplitude EEG.Reference Houlden, Turgeon and Polis1 EEG amplitude can be strategically decreased using a mastoid reference (instead of Fz) and setting the low-cut filter to a minimum of 10 or 15 Hz.Reference Luo, Regli, Bozinov and Sarnthein23 We acknowledge that the use of 10 Hz low-cut filter will reduce low-frequency contributions to the FVEP, but this compromise is necessary for recording reproducible intraoperative FVEPs.Reference Houlden, Turgeon and Polis1 Accordingly, in the current study we mainly used a 10 Hz low-cut filter and a 20 µV (peak-to-peak) reject window for the Oz-linked mastoid recording montage. The reject window was optimal for excluding artifacts, so they could not contaminate the FVEP response. Previous investigators have used a wide variety of techniques to varying degrees of success (see Table 1 in Rajan et al.).Reference Rajan, Simon and Nair4 Our recording method (described above) incorporates the parameters used by those who had most success in obtaining reproducible intraoperative FVEP monitoring and combines them with the use of a reject window to further improve FVEP reproducibility and detection of FVEP change. Although our FVEPs were not obtained in real time (due to imperative signal averaging), in some cases we were able to generate a reproducible FVEP after averaging 10 stimuli (in less than 7 seconds), while the longest acquisition time was 71 seconds. In the majority of cases the acquisition time was <35 seconds. This facilitated prompt communication of FVEP deterioration to the surgeon so timely corrective measures could be initiated.
Surgical resection of lesions adjacent to or adherent to the optic apparatus has always created much trepidation for neurosurgeons due to the inability to obtain immediate physiological feedback during removal. Surgery involving the sensorimotor pathways can be monitored with somatosensory and motor evoked potentials, and surgery involving speech pathways may be mapped during awake surgery. Unfortunately, the definitive assessment regarding the morbidity of visual pathway surgery has relied on postoperative assessment, so surgery is often more conservative in nature for fear of yielding a permanent visual deficit. In this study, we have shown that reproducible intraoperative FVEP recording is possible and, when deteriorations occur, corrective surgical measures can be undertaken to improve FVEPs resulting in improved or stable visual function postoperatively. Accordingly, reproducible intraoperative FVEP monitoring may keenly affect surgical decision-making and contribute to the prevention of postoperative visual dysfunction while allowing more total resection of tumors when monitoring is stable.
Due to the retrospective nature of the present study, our analysis was limited to the tests of visual function that were performed during the pre- and postoperative period in all patients (color vision, visual acuity, confrontational field testing). Unfortunately, most patients did not have Goldman perimetry testing; due to the retrospective nature of the study, the opportunity for obtaining it was lost. This is a limitation of our study because Goldman perimetry testing is better than confrontational testing in detecting visual field defects. Better detection of field defects may have decreased the sensitivity of our study because intraoperative FVEP monitoring does not always detect intraoperative visual deterioration in a single quadrant.Reference Kodama, Goto, Sato, Sakai, Tanaka and Hongo3, Reference Sasaki, Itakura and Suzuki5, Reference Luo, Regli, Bozinov and Sarnthein23 This may be related to the fact that the goggles themselves are unable to stimulate each quadrant of the visual field separately. In addition, the bulky goggle stimulators used in our study sometimes became displaced during craniotomy due to pressure of the frontal skin flap over the eyes. Lower profile eye patch stimulators (not yet approved for use in North America) are less prone to movement during skin flap retraction than goggle stimulators and should improve the success rate of FVEP monitoring in these cases.
Future studies should be designed to compare FVEP amplitude, latency, morphology, and reproducibility between eye patch stimulation and goggle stimulation, so intraoperative neurophysiology practitioners know what to expect from each stimulation technique. In addition, the effect of different flash intensity and color on intraoperative FVEP reproducibility and visual outcome should be studied.
Disclosures
The authors have no conflicts of interest to declare.