Shift-work and night work require individuals to labour outside conventional daytime hours, imposing shifted activity and sleep schedules(Reference Kecklund and Axelsson1). It is estimated that worldwide about 20 % of individuals participate in some form of shift-work. Besides shift- and night workers, another 25 % of young adults worldwide are exposed voluntarily to shifted sleep-activity habits(Reference Roenneberg, Allebrandt and Merrow2), mainly due to the modern life style that promotes social or leisure activities at normal rest times. Individuals engaged in nocturnal leisure activities shift their sleep-activity patterns differentially between weekdays and weekends, leading them to similar conditions as shift-workers. This shifted sleep timing is now referred as social jet-lag(Reference Wittmann, Dinich and Merrow3). Both social jet-lag and shift-work share similar features because in both conditions individuals are awake and active outside conventional daytime hours; they suffer from altered sleep-activity habits, shifted eating patterns and are exposed to light at night(Reference Parsons, Moffitt and Gregory4, Reference Reid and Abbott5). Such conditions cause a conflict with the internal biological clock and promote circadian disruption triggering loss of homeostasis(Reference Escobar, Salgado-Delgado and Gonzalez-Guerra6).
While we know little about the short- or long-term consequences of social jet-lag, a vast number of studies provide information about the short- and long-term effects of night and shift-work. Shift-workers are identified as a population with higher risk to develop adverse health effects including myocardial infarction, ischemic stroke and CVD(Reference Esquirol, Perret and Ruidavets7, Reference Wang, Armstrong and Cairns8). Shift-workers complain of disturbed sleep and excessive fatigue(Reference Kecklund and Axelsson1, Reference Wright, Bogan and Wyatt9), which have adverse consequences on their work performance, leading to high levels of stress(Reference Ma, Andrew and Fekedulegn10, Reference Han, Trinkoff and Storr11) to a reduced reaction time and sleepiness-related accidents(Reference Gumenyuk, Roth and Korzyukov12, Reference Marquie, Tucker and Folkard13). In the long term, shift-work is associated with psychiatric disorders, depression and substance abuse(Reference Meyrer, Demling and Kornhuber14–Reference Trinkoff and Storr16).
Metabolically, shift-work is associated with a higher propensity to develop overweight or obesity(Reference Brum, Filho and Schnorr17, Reference Laermans and Depoortere18) and it is a risk factor for metabolic syndrome(Reference Brum, Filho and Schnorr17, Reference Wang, Zhang and Zhang19), insulin resistance(Reference Lucassen, Rother and Cizza20), dyslipidaemia and type 2 diabetes(Reference Kivimaki, Batty and Hublin21, Reference Zimberg, Fernandes Junior and Crispim22). Due to the worldwide increasing incidence of obesity and metabolic disease, attention has focused on individuals at risk, especially shift-workers and groups exposed to disrupted sleep-activity patterns. In regard to social jet-lag, studies are needed to confirm this association; however, in individuals presenting overweight, a higher BMI was associated with a higher number of shifted hours between weekdays and weekends(Reference Roenneberg, Allebrandt and Merrow2). Clinical and experimental studies have indicated that shift-work and other conditions that cause circadian disruption prime individuals for a higher vulnerability to lose metabolic balance and obesity(Reference Laermans and Depoortere18, Reference Espitia-Bautista, Velasco-Ramos and Osnaya-Ramirez23, Reference McHill and Wright24).
Circadian rhythms are relevant in order to adjust the intensity and efficiency of the organism's response to the daily challenges required by the day–night cycles. Disrupted circadian rhythms will result in a time-deficient response that in the long term will lead to loss of homeostasis and disease. Circadian rhythms are driven by the circadian system, which is a complex internal timing system constituted by a biological clock, the suprachiasmatic nucleus (SCN), and by peripheral oscillators(Reference Buijs, van Eden and Goncharuk25). The circadian system fluctuates, synchronised to the external light–dark cycle, which is the main environmental time reference; however, other inputs, relevant for the group or the individual's survival, can also provide time information, including the internal energetic state and food availability.
At the cellular level, clock mechanisms are driven by transcription–translation feedback loops of several interacting genes better known as clock genes(Reference Takahashi26). Clock genes impose a temporal order to the transcription of other genes necessary for metabolic functions in the cells. The SCN coordinates such rhythms by means of hormonal and autonomic mechanisms, allowing in this way the time signal to reach cells that are not directly exposed to light(Reference Buijs and Kalsbeek27). However, other internal stimuli that provide time information to the cells are elicited by feeding cycles that induce metabolic rhythms. Peripheral organs respond to the changing levels of glucose, insulin, temperature and corticosterone, in different ways, which depend on their function and involvement in metabolic balance(Reference Takahashi26). Therefore, time of food intake has shown to be a powerful signal for the circadian system, driving brain and peripheral oscillators as well as behaviour(Reference Escobar, Cailotto and Angeles-Castellanos28). When time of food does not coincide with the normal sleep–activity cycle, driven by the biological clock, food creates an internal conflict with temporal signals driven by the SCN for the regulation of metabolic efficiency favouring weight gain, obesity and metabolic syndrome(Reference Moran-Ramos, Baez-Ruiz and Buijs29–Reference Johnston31).
Several studies demonstrate that shift-workers develop shifted food intake patterns, with increased consumption towards late at night(Reference Lowden, Moreno and Holmback32, Reference Waterhouse, Buckley and Edwards33), moreover during their shifts they show a preference for high-energetic and high-fat food(Reference Cain, Filtness and Phillips34, Reference Tada, Kawano and Maeda35) creating a shifted time pattern of energy signals to the cells, organs and components of the circadian system. Thus, it is possible that the shifted mealtime may trigger an internal conflict promoting internal desynchrony, leading to a deficient temporal response by organs involved in digestion and metabolic balance, and to a loss of homeostasis. Food intake currently of the normal activity phase may be the solution to counteract the adverse physiological consequences frequently observed in human shift-workers. Founded on this assumption, studies based on chrono-nutrition suggest implementing time-organised eating schedules for shift- and night workers in such a way that food does not represent a conflicting temporal signal with the normal light–dark cycle. More information is necessary in order to dissect the contribution of the time of eating for circadian disruption and to verify if restricted feeding schedules can be a possible intervention for individuals at risk of circadian disruption.
Experimental models in rodents have been used to better understand how shift-work impacts the circadian system, and to uncover factors associated with circadian disruption that exert adverse effects on behaviour and metabolic efficiency. Therefore, experimental protocols have implemented conditions of shifted timing of sleep, shifted timing of activity or shifted timing of food intake, and the exposure to light at night(Reference Opperhuizen, van Kerkhof and Proper36). The advantage of experimental models is that variables can be better controlled and causal relations can be determined. A limitation is that mainly rats or mice, which are nocturnal animals, have been used for these models.
In this review, we have searched in the experimental protocols modelling shift-work whether the time of food intake could be the cause of circadian disruption and metabolic disease.
Data bases used for this review were Google scholar and PubMed, and keywords used for the bibliographic search were (shift work circadian disruption metabolism rat mice obesity) including ‘sleep deprivation’ and not review = 271 articles; (shift work circadian disruption metabolism rat mice obesity) including ‘forced activity’ and not review = 37 articles; (shift work circadian disruption metabolism rat mice obesity) including ‘light at night’ and not review = 131 articles; (shift work circadian disruption metabolism rat mice obesity) including ‘restricted feeding’ and not review = 262 articles.
Studies published in languages different from English were not considered. Reviews were discarded. Citations referring to human studies were discarded; only studies using repeated manipulations (usually more than 3 d) as a model of shift-work were included, leaving out numerous studies that explore acute effects (manipulations for a single occasion). For models of experimental shift-work, we identified the following protocols: shifted timing of sleep, shifted timing of activity or shifted timing of food intake, as well as the exposure to light at night. From these models, only studies describing circadian disruption and/or overweight and/or metabolic dysfunction were analysed, discarding studies that explored other physiological systems or other mechanisms. Models using shifted light–dark cycles that resemble a jet-lag condition were not included as well as models using knock out or GM mice. For the condition of shifted food intake, a big spectrum of diets and timing schedules were observed ranging from 2 to 16 h food access(Reference Moran-Ramos, Baez-Ruiz and Buijs29), which led to diverse metabolic outcomes. In this analysis, we have included studies using 8–16 h food access with a regular chow or a high-fat, high energetic diet, because shorter access to food promotes a hypoenergetic condition leading animals to lose weight. Considering all criteria, a total of fifty-four studies were included in this review.
Experimental models of shift- and night work: what do they indicate?
Experimental models of chronic sleep disruption
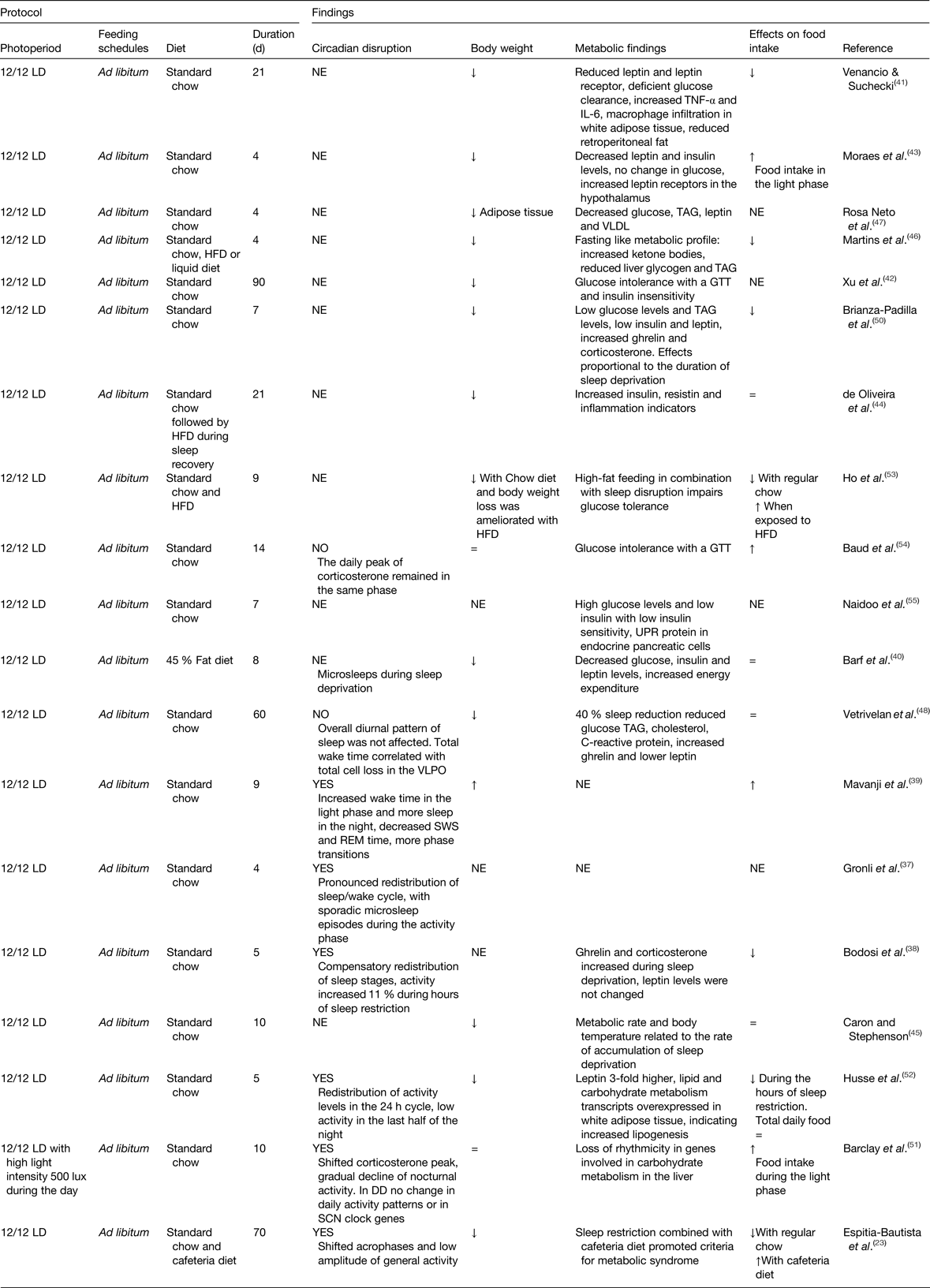
The most well-known feature of shift-work in human subjects is the disruption of the normal sleep-activity patterns; therefore, animal models aimed at mimicking shift-work have used protocols in rodents to chronically reduce or shift the sleep timing. Table 1 summarises studies that explored the consequences of chronic sleep disruption, and the effects on the circadian system, on body weight and/or metabolic function. Studies causing chronic sleep disruption vary in their strategies and in the time employed to produce a chronic sleep deprivation, some (ten studies) reduce total sleep, others inhibit rapid eye movement sleep (seven studies) or induce sleep fragmentation (two studies). In general, all strategies led to a redistribution of sleep–wake phase(Reference Gronli, Meerlo and Pedersen37–Reference Barf, Van Dijk and Scheurink40), suggesting a circadian disturbance. However, the majority of such studies have not assessed circadian rhythms.
Table 1. Experimental models of chronic sleep disruption
DD, constant dark; GTT, glucose tolerance test; HFD, high-fat diet; LD, light–dark; NE, not explored; REM, rapid eye movement; SCN, suprachiasmatic nucleus; UPR, unfolded protein response; VLPO, ventrolateral preoptic nucleus; =, similar to controls; ↑ increased; ↓decreased.
Due to sleep deprivation, food ingestion was decreased (seven out of fifteen studies), or not changed (four out of fifteen), while in some studies (four studies) this behaviour was not monitored (Table 1). In the majority of the studies, a decrease in body weight gain was observed, which is suggested to be the result of increased energy expenditure(Reference Barf, Van Dijk and Scheurink40) due to the exhausting conditions and physiological stress imposed by the extended protocols (from 18 to 20 h) of sleep deprivation(Reference Barf, Van Dijk and Scheurink40–Reference Rosa Neto, Lira and Venancio47). Studies observing a reduced body weight reported metabolic changes indicating a catabolic state or fasting like state, with reduced levels of glucose, low TAG, low cholesterol, low leptin levels, and in some studies accompanied by high levels of ghrelin and corticosterone(Reference Bodosi, Gardi and Hajdu38, Reference Barf, Van Dijk and Scheurink40–Reference Brianza-Padilla, Bonilla-Jaime and Almanza-Perez50). Thus, an anabolic state was associated with sleep deprivation.
Importantly, studies that have implemented a milder strategy of sleep restriction by using randomised loud noise or reducing the period and hours of sleep restriction observed increased body weight using a regular diet(Reference Mavanji, Teske and Billington39, Reference Caron and Stephenson45). Using gentle handling for 6 h at the start of the rest phase resulted in disrupted circadian rhythmicity of clock and metabolic genes in the liver(Reference Barclay, Husse and Bode51), altered glucose and TAG blood levels, as well as modifications in adipocytes transcription profile(Reference Husse, Hintze and Eichele52). While some studies did not find a significant effect on body weight, they observed glucose intolerance, insulin insensitivity(Reference Venancio and Suchecki41, Reference Xu, Wang and Zhang42, Reference Ho, Barf and Opp53, Reference Baud, Magistretti and Petit54) and increased circulating insulin(Reference de Oliveira, Visniauskas and Sandri44). An important feature of protocols using milder strategies for sleep deprivation is that animals were able to maintain a normal feeding rate. An example is the study by Caron and Stephenson(Reference Caron and Stephenson45) in which, by using milder sleep deprivation and giving rats the opportunity to have short recovery sleep bouts, this reduced the sleep debt and improved temperature regulation, food intake and metabolic efficiency.
The relevance of the time of food intake on body weight and the metabolic outcome was assessed in five studies(Reference Espitia-Bautista, Velasco-Ramos and Osnaya-Ramirez23, Reference Bodosi, Gardi and Hajdu38, Reference Moraes, Venancio and Suchecki43, Reference Barclay, Husse and Bode51, Reference Husse, Hintze and Eichele52) combining sleep deprivation with daytime feeding, where food consumption was shifted to the hours that animals were kept awake. The effects of shifted food intake are not consistent; it did not increase body weight using a regular diet(Reference Bodosi, Gardi and Hajdu38, Reference Barclay, Husse and Bode51); in two studies it resulted in decreased bodyweight(Reference Moraes, Venancio and Suchecki43, Reference Husse, Hintze and Eichele52), and in one study it promoted overweight when combined with a highly palatable energy-dense diet, better known as cafeteria diet(Reference Espitia-Bautista, Velasco-Ramos and Osnaya-Ramirez23). Overweight remained during the recovery period after chronic rapid eye movement sleep deprivation combined with high-fat diet(Reference de Oliveira, Visniauskas and Sandri44). Metabolic effects were also worsened when combining cafeteria diet with sleep deprivation leading to a metabolic syndrome(Reference Espitia-Bautista, Velasco-Ramos and Osnaya-Ramirez23, Reference Ho, Barf and Opp53), and in aged mice, the combination of a high-fat diet with sleep deprivation led to damage to the pancreas(Reference Naidoo, Davis and Zhu55).
All together, chronic disrupted sleep causes metabolic alterations favouring in some cases a metabolic syndrome and in others reflecting a fasted state, even when body weight is reduced or not affected. Only a few studies using milder strategies for sleep disruption report increased and shifted food intake towards the forced hours of wake time and this was associated with indicators of metabolic syndrome.
Experimental models of forced activity
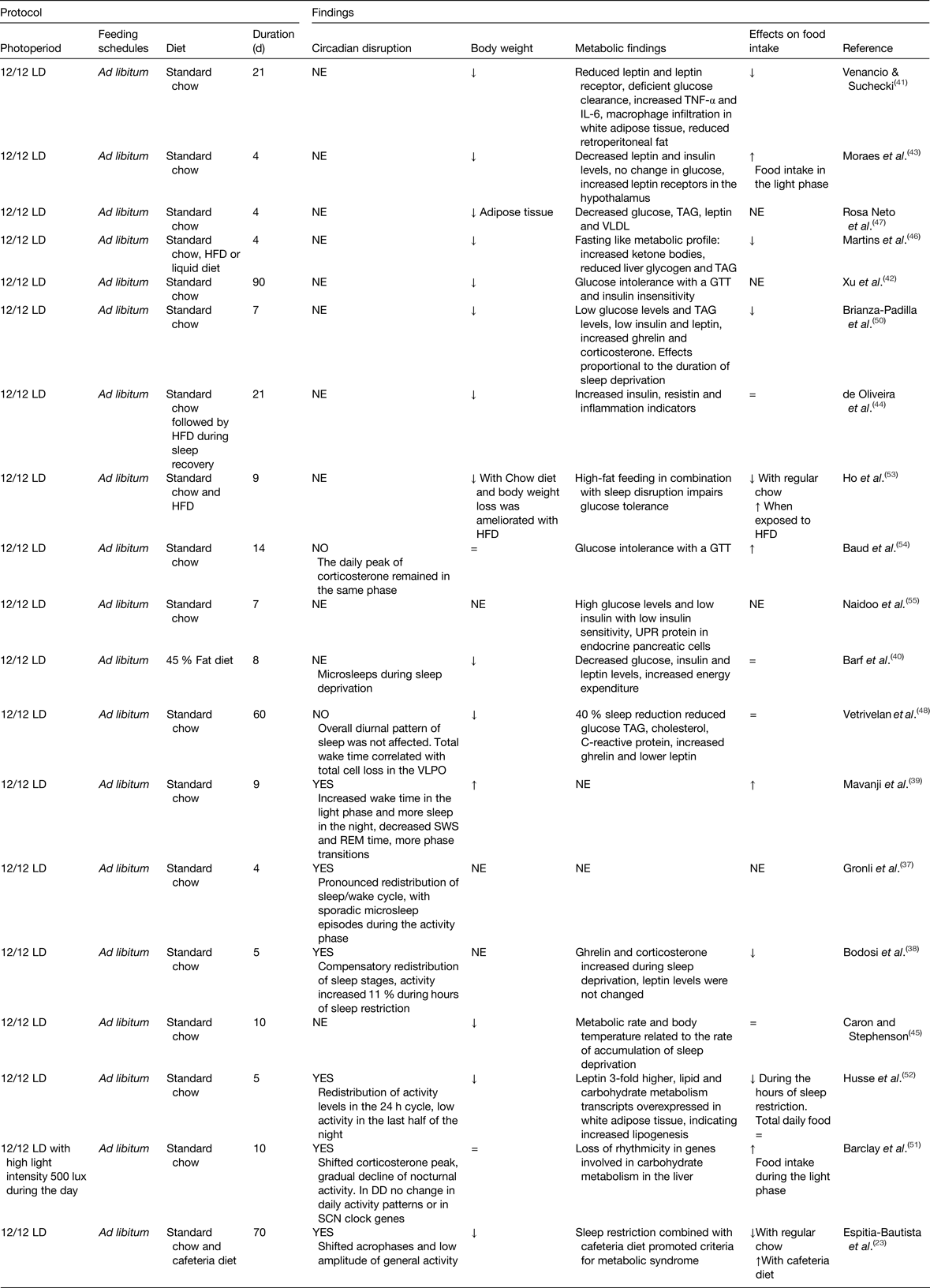
Animal models that shift activity to the resting phase are scarce (Table 2). A main difficulty in defining such models is that some protocols that shift activity coincide with manipulations used for sleep deprivation.
Table 2. Experimental models of forced activity
LD, light–dark; NE, not explored; rpm, revolutions per minute; =, similar to controls; ↑ increased; ↓decreased.
The strategies used to keep animals awake and active mainly consist of motorised wheels that vary in their construction. Similar to the studies for sleep deprivation, we found a variety of studies requiring from the animals different intensities of activity and effort (Table 2), which is reflected by the number of revolutions per minute, by the frequency of locomotor adjustments or the number of responses of the animal. The majority of studies required from the animals a strong effort during their forced activity schedules, keeping continuous alertness and emitting effortful movements that mimic more of an exercise routine(Reference Tsai and Tsai56). Some schedules represented a stressful condition(Reference Leenaars, Kalsbeek and Hanegraaf57) or even drove animals to exhaustion and to a torpor state due to the negative metabolic state driven by the exhausting protocol(Reference Hut, Pilorz and Boerema58). Models of forced activity induced disrupted circadian rhythms mainly by shifting activity towards the rest phase.
Body weight was decreased (five out of eight studies)(Reference Tsai and Tsai56–Reference Murphy, Wideman and Nadzam60), in two studies body weight was mildly (7 %) increased(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Buijs62) and one study did not assess it(Reference Dallmann and Mrosovsky63). Interestingly, only two groups have explored the metabolic outcome of shifted forced activity using a mild strategy to enforce activity. Both groups report shifted feeding patterns towards the rest phase and a resulting disrupted metabolism(Reference Marti, Meerlo and Gronli59, Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Buijs62). The study by Marti et al.(Reference Marti, Meerlo and Gronli59) imposed this protocol for only 4 d and could already observe in the liver a disturbed pattern of genes encoding insulin sensitivity and lipid metabolism. A series of studies by our group, using slow rotating wheels, reported that activity during the resting phase for 4 weeks induced rats to gain more bodyweight and to develop abdominal obesity accompanied by liver steatosis and glucose intolerance(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Buijs62).
With this protocol, rats developed disrupted circadian rhythms characterised by a shift in core body temperature, in general activity and serum TAG, a loss of the rhythm in glucose, in the rhythm in clock genes in the liver and no change in corticosterone rhythm.
Importantly, forced activity in the slow rotating wheel induced a shift in the timing of food consumption to the light phase, suggesting this as a possible factor inducing circadian disruption and loss of metabolic balance(Reference Salgado-Delgado, Angeles-Castellanos and Saderi64). To confirm this association, rats were prevented from ingesting food during the forced activity hours and only had access to food during the night (which is the normal activity phase for rats). This procedure prevented circadian disruption and the adverse metabolic effects observed in rats exposed to the working schedule. Moreover, daytime food access alone recapitulated the effects of this working schedule on metabolism(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64). A possible effect of food intake in other protocols of forced activity in the rest phase was not assessed.
All together, most of the experimental models using forced activity in the rest phase have not provided evidence that this factor may cause circadian disruption and metabolic dysfunction. This is probably due to the exhausting protocols used to induce activity. Similar as observed with the sleep deprivation studies, milder protocols favouring rats to eat during the rest phase induced increased body weight and adverse metabolic changes in the direction of metabolic syndrome, pointing out shifted food intake as an important risk factor for circadian and metabolic disruption.
Experimental models for light at night
In rodents, light exposure at night has been used as a strategy to mimic one of the most disrupting and common conditions experienced by human shift-workers. Light is a signal that immediately activates the SCN; however, for nocturnal rodents, light is a rest signal, and for human subjects, light is associated with activity. Table 3 summarises studies that explored the metabolic consequences of continuous light exposure in mice and rats either implementing constant light intensity throughout 24 h (LL) or alternating bright light during the day with dim light exposure at night (L/DL).
Table 3. Experimental models for light at night

BAT, brown adipose tissue; HIP, human islet amyloid polypeptide; L/DL, light/dim light; L/L, light/light; lx, lux; NE, not explored; SCN, suprachiasmatic nucleus; =, similar to controls; ↑ increased; ↓ decreased.
From studies involving LL, seven out of eight reported clear circadian disruption based on arrhythmic locomotor activity patterns, on low SCN neuronal activation or disturbed corticosterone and melatonin rhythms(Reference Fonken, Workman and Walton65–Reference Dauchy, Dauchy and Tirrell71). From the other two studies, we may assume that the circadian system was also affected because they report low melatonin levels, which is also an indicator of disruption at the level of the biological clock(Reference Dauchy, Dauchy and Tirrell71, Reference Wideman and Murphy72). To note is that two studies using alternating bright light during the day with dim light at night did not induce circadian arrythmicity at least in general activity and corticosterone levels(Reference Fonken, Workman and Walton65, Reference Borniger, Maurya and Periasamy73), and a third study did not explore it(Reference Aubrecht, Jenkins and Nelson74).
The effects of LL on body weight are inconsistent: in two studies body weight was increased(Reference Fonken, Workman and Walton65, Reference Coomans, van den Berg and Houben66), in four studies animals remained similar to controls(Reference Kooijman, van den Berg and Ramkisoensing67, Reference Qian, Yeh and Rakshit68, Reference Gale, Cox and Qian70, Reference Dauchy, Dauchy and Tirrell71) and in two studies body weight gain is not reported(Reference Polidarova, Sladek and Sotak69, Reference Wideman and Murphy72). Interestingly, metabolic dysfunction was consistently reported in all studies independently of the body weight outcome(Reference Fonken, Workman and Walton65–Reference Wideman and Murphy72). Rodents in LL developed increased glucose levels, glucose intolerance, decreased insulin sensitivity, increased fat mass deposition, elevated plasma fatty acids, decreased activity of brown adipocytes, higher RER during the subjective day and decreased energy expenditure(Reference Fonken, Workman and Walton65–Reference Dauchy, Dauchy and Tirrell71). Adverse effects of LL are also described in organs involved in energy balance. Qian et al.(Reference Qian, Yeh and Rakshit68) reported disrupted pancreatic islet architecture as well as increased apoptosis due to LL. Loss of circadian rhythmicity in clock genes in the liver and the colon were also reported while rhythmicity in the duodenum was preserved(Reference Polidarova, Sladek and Sotak69, Reference Wideman and Murphy72).
In the three studies alternating bright light with dim light at night, consistent increased body weight gain was observed, together with higher RER, glucose intolerance, increased insulin levels during the light phase and decreased energy expenditure(Reference Fonken, Workman and Walton65, Reference Aubrecht, Jenkins and Nelson74, Reference Borniger, Weil and Zhang75), similar as observed in LL.
Interestingly only two of the cited studies using LL (Table 3) assessed the 24 h pattern of food consumption. Polidarova et al.(Reference Polidarova, Sladek and Sotak69) reported loss of circadian rhythms in feeding behaviour, Wideman and Murphy(Reference Wideman and Murphy72) indicated that rats in LL consumed less food throughout the 24 h cycle, however attained a positive feed efficiency value (g body weight change/g food intake), which suggests that this condition may favour body weight gain. Contrasting, only the study by Fonken et al.(Reference Fonken, Workman and Walton65) reported that mice exposed to alternating bright light during the day with dim light at night shifted their feeding patterns and consumed a higher amount of food in the day.
Altogether, studies using constant light report a consistent disruptive effect of light on metabolism leading to increased adiposity and disruptive glucose balance. Because few studies assessed patterns of food ingestion, the contribution of food timing to metabolic dysfunction is not clear. Nevertheless, eating while the nocturnal animal is exposed to light suggests a circadian conflict, which requires further studies.
Experimental models of shifted food timing
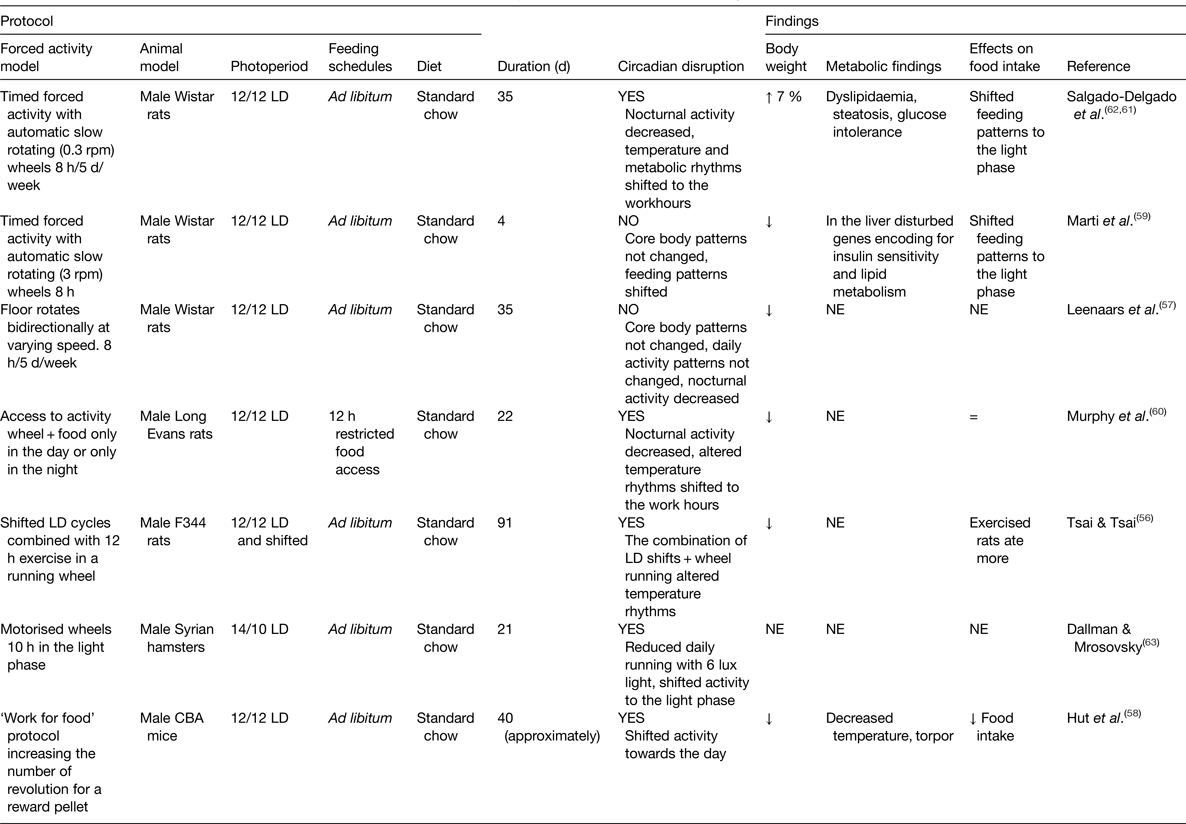
Restricting food access to the rest phase has been used in rodents as a strategy to reproduce the shifted feeding schedule of human shift-workers. Studies that explored the metabolic consequences of shifted feeding schedules are summarised in Table 4. The majority of the studies used a protocol of restricting food access to 12 h during the day, which is the rest phase, and compared this with food access for 12 h during the night. Diets mainly consist of standard chow; however, some studies also have used high-fat diet or high-fat and high-sucrose diets. There are also some studies using shortened food access for 4 or 5 h during the day; however, we have not included them in this review because this daily brief access to food can lead to food entrainment, resets circadian metabolism and induce energy restriction resembling a fasted day(Reference Moran-Ramos, Baez-Ruiz and Buijs29).
Table 4. Experimental models of shifted food timing
HFD, high-fat diet; LH-PeF, lateral hypothalamus-perifornical area; LD, light–dark; MCH, melanin-concentrating hormone; α-MSH, α-melanocyte-stimulating hormone; NE, not explored; ORX, orexin; OVLT, organum vasculosum lamina terminalis; ↑ increased; ↓ decreased.
From the studies examined here, twelve out of fourteen reported circadian disturbances, while the other two studies did not explore this feature(Reference Arble, Bass and Laposky76, Reference Oosterman, Foppen and van der Spek77). Disrupted circadian rhythms were observed in the fluctuations of clock genes in the liver, muscle and heart(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Bray, Ratcliffe and Grenett78–Reference Yasumoto, Hashimoto and Nakao83). Studies also report loss of temperature rhythms, shifted locomotor activity towards the rest phase, shifted glucose, TAG, leptin and ghrelin rhythms and decreased leptin immunoreactivity rhythms in the organum vasculosum of the lamina terminals(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64, Reference Bray, Ratcliffe and Grenett78–Reference Rocha, de Matos and de Souza86). In the study of Ramirez-Plascencia et al.(Reference Ramirez-Plascencia, Saderi and Escobar87), authors described shifted or blunted activity rhythms of orexin, melanin-concentrating hormone and α-melanocortin-stimulating hormone neurons in the hypothalamus. Therefore, it is a consistent finding that shifted food access to the rest phase affects brain and peripheral clocks involved in metabolic regulation. Such findings emphasise the importance of food as a powerful circadian synchroniser.
The effect of shifting food to the light period on body weight gain is quite clear. In ten studies using 12 h day feeding, animals gained significantly more body weight(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64, Reference Arble, Bass and Laposky76–Reference Bray, Ratcliffe and Grenett78, Reference Mukherji, Kobiita and Chambon81–Reference Reddy and Jagota84, Reference Ramirez-Plascencia, Saderi and Escobar87); in four studies, animals remained similar to controls(Reference Opperhuizen, Wang and Foppen79, Reference Reznick, Preston and Wilks80, Reference Shamsi, Salkeld and Rattanatray85, Reference Rocha, de Matos and de Souza86). In two studies, where authors did not find differences in body weight gain(Reference Opperhuizen, Wang and Foppen79, Reference Reznick, Preston and Wilks80), this was associated with decreased food consumption. In the case of the studies by Shamsi et al.(Reference Shamsi, Salkeld and Rattanatray85) (8 or 16 h day feeding) and Rocha et al.(Reference Rocha, de Matos and de Souza86), animals’ body weight remained similar to controls; however, animals developed metabolic alterations (Table 4).
Among rodents that gained weight, metabolic dysfunction was reported, mainly fat mass accumulation, dyslipidaemia, high glucose levels and glucose intolerance, decreased insulin sensitivity, lower RER, decreased energy expenditure and disrupted circadian rhythms of metabolic genes in the liver and muscle(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Arble, Bass and Laposky76–Reference Bray, Ratcliffe and Grenett78, Reference Mukherji, Kobiita and Damara82–Reference Reddy and Jagota84, Reference Ramirez-Plascencia, Saderi and Escobar87).
All together, studies using restricted food access to the rest phase reported circadian disruption in organs related with metabolic function. This loss of temporal order among organs regulating metabolism may be the cause of a disturbed metabolism that can have potential health consequences, such as metabolic syndrome, obesity and diabetes(Reference Escobar, Salgado-Delgado and Gonzalez-Guerra6, Reference Bass and Takahashi88).
What do experimental models indicate about the association between circadian disruption, overweight and metabolic disturbance?
All models described here induce circadian disruption and affect the metabolic state in one or another direction (Table 5), indicating a clear association between conditions that affect daily cycles and the development of an adverse metabolic condition. In some studies due to the lack of circadian assessment or metabolic follow-up, this association is not always evident. However, protocols using light exposure at night or shifted food to the rest phase highlight the adverse effects of food intake at the wrong time or light at night on metabolic health. Both factors are present in the modern life style and affect the shift-worker.
Table 5. Proportion of studies providing evidence for the relationship between circadian disruptions and adverse metabolic function
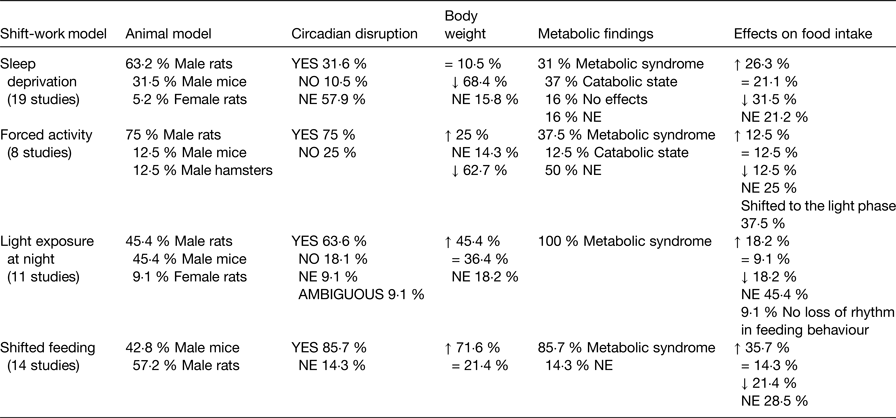
Metabolic syndrome = overweight or obesity, increased adipose tissue, glucose intolerance, increased TAG, increased cholesterol; NE, not explored; =, similar to controls; ↑ increased; ↓ decreased.
Metabolic effects largely depended on the specific manipulations by each model, i.e. duration, intensity of activity, feeding schedule and type of diet. Regarding sleep deprivation and forced activity models, the majority of the studies report decreased body weight gain associated with a catabolic metabolism which, as previously discussed, may be related to the physical requirements and stressful conditions of the protocols. Importantly, studies using milder protocols for sleep disruption or for enforced activity are scarce, however report metabolic changes that suggest the development of metabolic syndrome(Reference Barclay, Husse and Bode51, Reference Husse, Hintze and Eichele52, Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64). Contrasting, light exposure at night has proved to be an effective and consistent model to mimic the metabolic alterations observed in human shift-workers. Most of the studies using light at night reported increased body weight gain and all of them described alterations reflecting a dyslipidaemia and criteria for a metabolic syndrome. Likewise models using shifted food to the rest phase found consistent metabolic dysfunction and showed to be effective to produce body weight gain, in spite of a few reports that observed no difference from the control.
The relevance of shifted food intake as a cause of circadian and metabolic disorder in shift-work model
When looking at all models of shift-work, it is clear that many studies did not explore the possible role of the time of food intake as a risk factor for circadian disruption and metabolic alterations (Table 5). Evidently, the models implementing shifted food access to the rest phase have paid attention to this factor and reported consistently changes in the metabolic state towards dyslipidaemia and glucose intolerance. Other models that observed shifted food consumption to the normal rest hours have provided strong evidence about this association. In the studies by Salgado-Delgado et al.(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64), animals shifted their food intake to the rest phase and this had an adverse metabolic outcome. When animals were not allowed to eat at the wrong phase, the adverse metabolic effects were prevented, pointing out the relevance of food timing as a main risk factor for metabolic problems as observed in human shift-workers. One of the studies using dim light at night also observed a shift in meal patterns, and this shift was associated with overweight and metabolic changes(Reference Fonken, Workman and Walton65).
Food has proved to be a powerful entraining signal for the circadian system. Metabolic signals elicited by food intake impact organs at the cellular level and provide timing to cellular processes and genes involved in glucose and lipid metabolism(Reference Escobar, Cailotto and Angeles-Castellanos28). Under shift-work conditions several external and internal timing signals are shifted due to abnormal exposure to activity, to light and to food, resulting in an internal conflict with time signals from the SCN transmitted to organs and cells via the autonomic nervous system. This affects differentially organs and regulatory genes depending on their dependence on metabolism, endocrine or autonomic signals(Reference Escobar, Cailotto and Angeles-Castellanos28). The loss of an internal temporal order among different regulatory systems leads to incorrect or deficient adaptive responses to external demands, which can be the cause of a loss of homeostasis and a higher propensity to disease(Reference Buijs, van Eden and Goncharuk25).
Main internal signals that influence metabolic function
Food intake as a time signal for the circadian system
Studies implementing shifted feeding schedules towards the rest phase described shifted rhythms of clock and metabolic genes in organs involved in metabolic balance, especially in the liver(Reference Salgado-Delgado, Saderi and Basualdo Mdel61, Reference Opperhuizen, Wang and Foppen79, Reference Mukherji, Kobiita and Damara82, Reference Yasumoto, Hashimoto and Nakao83, Reference Shamsi, Salkeld and Rattanatray85), in the muscle and adipose tissue(Reference Bray, Ratcliffe and Grenett78, Reference Reznick, Preston and Wilks80). As we have mentioned earlier, clock genes impose a temporal order to the transcription of other genes necessary for metabolic functions in the cells.
In the study by Salgado-Delgado et al.(Reference Salgado-Delgado, Angeles-Castellanos and Saderi64), where forced activity in slow rotating wheels induced a shifted food intake towards the day, also shifted and blunted clock genes in the liver were reported, suggesting a circadian disruption at the cellular level. Such studies indicate the relevant effect of the time of food intake as a potent disrupting factor, when time of food does not coincide with the light–dark cycle. The disruption of clock gene expression in the liver is associated with disturbed liver metabolism and development of liver steatosis (see for review(Reference Sabath, Baez-Ruiz and Buijs89)).
The circadian conflict at the cellular level is suggested to occur between the shifted food-related signals (glucose, insulin) and the biological clock transmitting light–dark information to peripheral organs and cells(Reference Buijs and Kalsbeek27). Melatonin and corticosterone are the main hormonal pathways used by the biological clock to transmit time information of the light–dark cycle to peripheral organs(Reference Menaker, Murphy and Sellix90, Reference Pevet and Challet91) and may be the source of conflict with the food-entrained rhythms.
Corticosterone
Corticosterone is proposed as an internal timing signal for a variety of organs(Reference Leliavski, Dumbell and Ott92) and reaches peak levels at the beginning of the active phase, which in rodents corresponds to the beginning of the night. In the liver(Reference Torra, Tsibulsky and Delaunay93, Reference Pezuk, Mohawk and Wang94), corticosterone influences gluconeogenesis as demonstrated in vivo while in vitro exerts synchronizing effects on fibroblasts(Reference Balsalobre, Brown and Marcacci95) and adipose tissue(Reference Gomez-Abellan, Diez-Noguera and Madrid96).
In rats, sleep deprivation induced increased levels of corticosterone during the protocol(Reference Bodosi, Gardi and Hajdu38), mild forced activity, as well as exposure to food restriction to the resting phase, induced a peak of corticosterone at the beginning of the schedule in addition to the normal peak at the beginning of the night(Reference Bodosi, Gardi and Hajdu38, Reference Salgado-Delgado, Angeles-Castellanos and Saderi64, Reference Reznick, Preston and Wilks80). Similarly, animals exposed to LL exhibit increased levels of corticosterone along the 24 h period with a loss of the circadian rhythmicity(Reference Fonken, Workman and Walton65, Reference Coomans, van den Berg and Houben66). Such high levels of glucocorticoids affect glucose homeostasis and promote gluconeogenesis in the liver(Reference Kuo, McQueen and Chen97). It is also known that high doses of glucocorticoids result in increased weight gain, glucose intolerance and high insulin and TAG levels(Reference Karatsoreos, Bhagat and Bowles98). Elevated corticosteroid levels and dampened or disrupted glucocorticoid rhythmicity have been reported in obese adults and in genetically obese Zucker rats and db/db mice(Reference Leliavski, Dumbell and Ott92). Moreover, the shifted timing of corticosterone release may function as an altered time signal and exert a disruptive effect on the circadian system.
Melatonin
It is suggested that the SCN uses the nocturnal melatonin secretion to distribute circadian signals within the brain or the periphery, to organs and cells possessing melatonin receptors(Reference Pevet and Challet91). In rodents, daily administration of melatonin entrains activity rhythms in free-running rats(Reference Cassone and Natesan99–Reference Slotten, Pitrosky and Pevet101), and some studies suggest that melatonin can entrain adipocytes(Reference Alonso-Vale, Andreotti and Mukai102) and protein synthesis in hepatocytes(Reference Brodsky and Zvezdina103). In three of the experimental models of light at night, altered timing and/or levels of melatonin were observed(Reference Gale, Cox and Qian70–Reference Wideman and Murphy72).
It is well described that low light intensities at night are sufficient to inhibit melatonin(Reference Dauchy, Dauchy and Tirrell71); however, this hormone under the dim light at night schedule has not been evaluated. Likewise in animal protocols that restrict food access to the day, melatonin has not been evaluated. Importantly, the three studies involving LL that reported low melatonin levels associated the hormone levels with metabolic alterations(Reference Gale, Cox and Qian70–Reference Wideman and Murphy72).
Melatonin in addition to being an important regulator of circadian rhythms is also involved in the regulation of glucose metabolism(Reference Navarro-Alarcon, Ruiz-Ojeda and Blanca-Herrera104). The relevance of melatonin for metabolic balance is further demonstrated under conditions of low hormone concentrations such as ageing, in which, melatonin supplementation effectively decreased fat mass accumulation, body weight gain and restored insulin and leptin levels(Reference Wolden-Hanson, Mitton and McCants105, Reference Rasmussen, Boldt and Wilkinson106). In diabetic rats and mice, melatonin treatment also improved glucose and TAG levels, diminished body weight and insulin levels(Reference Amin, El-Missiry and Othman107, Reference Favero, Stacchiotti and Castrezzati108). Based on this evidence, we suggest that low melatonin levels resulting from circadian disruption may promote the metabolic dysfunction; however, more data from the experimental models will be necessary to support this association.
Main contributions of experimental models, limitations and perspectives
The analysis of the four experimental models for shift-work indicates that the strategies and variables assessed by different groups are diverse with respect to intensity, time of exposure and variables used to assess circadian and/or metabolic state, leading in some studies to contrasting or inconclusive findings. It is important to indicate that studies here reported have included a significant number of subjects in their experimental designs, which permits to draw conclusions; however, the variability in the use of factors has led to different outcomes. Moreover, not all studies performed a circadian screening, not all determined the temporal order of food intake and only a few studies have explored the association between the time of food intake as the cause of circadian and metabolic disruption. Importantly, studies that explored this relationship report that the time of food intake is indeed essential for the development of metabolic disturbances.
A growing body of epidemiological evidence in human populations indicates that short sleep is a risk factor for the development of obesity and metabolic disturbance. Animal studies exploring the effects of restricted sleep indeed observed a reduction in insulin sensitivity and changed levels of hormones involved in appetite and neuroendocrine regulation, such as ghrelin, leptin and insulin(Reference Colles, Dixon and O'Brien109, Reference Goel, Stunkard and Rogers110). However, experimental models using sleep deprivation have not provided conclusive effects of the contribution of shifted feeding schedules because very few have explored the possibility of a shifted food intake. Moreover, models for sleep deprivation or forced activity require more uniformity, using mild protocols in order to provide conclusive results about metabolic mechanisms.
As a model of shift-work, light at night exposure has proved to be an effective and a consistent model to mimic the metabolic alterations observed in human shift-workers, highlighting the importance of being exposed to dark nights in order to promote metabolic health. The fact that light at night will activate neurons in the SCN that are normally inactive and inhibit melatonin secretion when melatonin is normally high indicates that light at night is a strong circadian disruptive signal. In such conditions, scheduled food has shown to ameliorate metabolic conditions and to exert strong entraining signal for metabolic genes in the liver(Reference Sabath, Salgado-Delgado and Guerrero-Vargas111). More evidence is needed to determine the role of food timing under constant light. The majority of studies exploring experimental shift-work have been performed in nocturnal animals, which is a limitation when translating their findings to the diurnal human species. As indicated earlier, melatonin is involved in the regulation of glucose metabolism, and in nocturnal rodents, melatonin treatment improved the metabolic state in obese mice(Reference Favero, Stacchiotti and Castrezzati108) suggesting that low melatonin levels resulting from circadian disruption may be partly the cause of metabolic dysfunction. In nocturnal rodents, melatonin release coincides with their active phase and with the time of food intake, while in human subjects, melatonin release coincides with the rest and sleep phase and not with the moments of maximal digestion and food absorption. This important difference requires a better understanding for the role that melatonin could play linking circadian disruption and metabolism. In a similar way, light at night has shown to be a disruptive signal for the circadian system, and while nocturnal rodents are normally active at night, human subjects sleep, thus the disrupting effect on sleep-activity patterns is inverted. Therefore, a next necessary step for the experimental models of shift-work is to use diurnal species in order to confirm such adverse effects and to better translate experimental results to the problem of the human shift-worker.
Interestingly, the majority of studies here reported have used male animals and only two studies were found that explored the response in females(Reference Xu, Wang and Zhang42, Reference Aubrecht, Jenkins and Nelson74). In the study by Xu et al.(Reference Xu, Wang and Zhang42), authors do not discuss a possible difference in their outcomes associated with the sex of the animals. In this regard, Aubrecht et al.(Reference Aubrecht, Jenkins and Nelson74) discuss their previous work using male animals and stated that dim light affects in a similar way body mass and likely metabolic function in both males and females. Other studies testing a high-fat diet in male and female rodents indicate a differential influence associated with sex in rats(Reference Dearden and Balthasar112, Reference Vital, Larrieta and Hiriart113), mice and hamsters(Reference Trefna, Goris and Thissen114, Reference Ingvorsen, Karp and Lelliott115). This is partly explained by a differential response to metabolic signals in brain areas involved with metabolic regulation(Reference Clegg, Riedy and Smith116). Since women are also exposed to shift-work, further studies exploring the effects of circadian disruption and shifted food timing in female rodents are necessary.
We conclude that so far experimental evidence confirms the association of circadian disruption with metabolic alterations, hereby some models indicate that the shifted time of food intake may be a determining factor for the loss of internal synchrony because of the differential response of individual organs to internal entraining signals. Thus, the adverse consequences of shift-work on metabolism may be explained by a loss of coordinated rhythmicity among different organs due to shifted food elicited signals, low melatonin and shifted or increased corticosterone levels(Reference Mirick, Bhatti and Chen117).
Acknowledgements
For the development of experimental models of shift-work and the role of meal time, the group receives support from DGAPA-PAPIIT UNAM IG200417 and CONACyT 239403.
Financial Support
Support was received from DGAPA-PAPIIT UNAM IG200417 (to R. M. B. and C. Ee) and CONACyT 239403 (to C. E.).
Conflicts of Interest
None.
Authorship
All authors contributed to the search of bibliography, the writing and review of the manuscript.