Introduction
Bearded sprangletop is one of the most common and competitive annual semiaquatic grasses, and it is widespread in California rice fields (Brim-DeForest et al. Reference Brim-DeForest, Al-Khatib and Fischer2017; Driver et al. Reference Driver, Al-Khatib and Godar2020a). Bearded sprangletop, an annual grass native to North America (Bryson and DeFelice Reference Bryson and DeFelice2009), relies on seed production to complete its life cycle. Bearded sprangletop produces many seeds, and seedlings generally emerge later than other weedy grasses (Driver et al. Reference Driver, Al-Khatib and Godar2020a; McCarty et al. Reference McCarty, Porter, Colvin, Shilling and Hall1995). Bearded sprangletop can reduce rice grain yield by up to 36% if not controlled (Smith Reference Smith1983). As a result of its prolificity and competitiveness, growers must manage this weed using cultural and chemical tools.
Although flooding rice fields with deep water is a common practice to suppress bearded sprangletop (Driver et al. Reference Driver, Al-Khatib and Godar2020a), herbicides are a major component of California’s weed control strategy to achieve adequate bearded sprangletop control and high rice yields (Yasuor et al. Reference Yasuor, TenBrook, Tjeerdema and Fischer2008). Despite the use of integrated weed management methods, bearded sprangletop biotypes in California have been suspected to be resistant to herbicides such as cyhalofop (Group 1, an ACCase inhibitor), thiobencarb [Group 15, an inhibitor of very-long-chain fatty acids (VLCFA) synthesis], clomazone (Group 13, an inhibitor of 1-deoxy-D-xyulose 5-phosphate (DXP) synthase], benzobicyclon + halosulfuron-methyl [Group 27, 7, 4-hydroxyphenylpyruvate dioxygenase (HPPD) + Group 2, acetolactate synthase (ALS) inhibitor] (Becerra-Alvarez et al. Reference Becerra-Alvarez, Godar, Ceseski and Al-Khatib2023; Brim-DeForest et al. Reference Brim-DeForest, Alarcon-Reverte and Fischer2015; Driver et al., Reference Driver, Al-Khatib and Godar2020b). Only clomazone resistance has been confirmed in bearded sprangletop biotypes (Driver et al. Reference Driver, Al-Khatib and Godar2020b). Preliminary studies by Brim-DeForest et al. (Reference Brim-DeForest, Alarcon-Reverte and Fischer2015) suggested target-site resistance to cyhalofop in bearded sprangletop. Therefore, identification of the resistance mechanisms might be useful in developing quick molecular diagnostic tests that advisors can use to confirm resistance development in bearded sprangletop.
ACCase inhibitors prevent plants from synthesizing fatty acids (Devine Reference Devine1997). Fatty acid synthesis inhibition likely prevents the creation of phospholipids needed to construct new membranes for cell development (Gronwald Reference Gronwald1991). Three catalytic domains make up ACCase: biotin carboxyl transferase (CT), biotin carboxylase (BC), and biotin carboxyl carrier (BCC) (Nikolskaya et al. Reference Nikolskaya, Zagnitko, Tevzadze, Haselkorn and Gornicki1999). These domains are all involved in the two reversible processes of carboxylation of acetyl-CoA. Initially, a biotin group covalently linked to the BCC domain is carboxylated in an ATP-dependent manner by the BC domain. Subsequently, the carboxyl group is transferred from biotin to acetyl-CoA by the CT domain. The biosynthesis of secondary metabolites and fatty acids depends on the produced malonyl-CoA (Harwood Reference Harwood1988). There are two distinct ACCase isoforms found in the plants. The homomeric enzyme cytosolic ACCase, present in all eukaryotes, combines the three domains into a single polypeptide. Most plants contain chloroplastic (plastidic) ACCase, a heterodimeric enzyme with three domains distributed among four subunits. Because their ACCase plastidic isoform is homomeric, plants belonging to the Poaceae family are unique (Konishi et al. Reference Konishi, Shinohara, Yamada and Sasaki1996). The selective binding of the CT domain of grasses’ plastidic isoform by aryloxyphenoxypropionates (FOPs), the cyclohexanediones (DIMs), and the phenylpyrazolin herbicides confers herbicidal effects; other isoforms remain unaffected and insensitive (Kaundun et al. Reference Kaundun, Hutchings, Dale and McIndoe2013; Nikolskaya et al. Reference Nikolskaya, Zagnitko, Tevzadze, Haselkorn and Gornicki1999; Zhang et al. Reference Zhang, Tweel and Tong2004). Long-term usage of FOPs herbicides, particularly in rice production, has exerted selection pressure on weeds and led to resistant bearded sprangletop biotypes (Phongphitak et al. Reference Phongphitak, Maneechote, Rerkasem and Jamjod2014; Rahman et al. Reference Rahman, Ismail and Sofian-Azirun2011). Cyhalofop, quizalofop, and clethodim are typically used to control grass as a FOPs and DIMs herbicides. Cyhalofop is widely used in conventional rice farming systems, and quizalofop is utilized in Provisia® rice farming system to control bearded sprangletop, other weed grasses, and weedy rice (Lancaster et al. Reference Lancaster, Norsworthy and Scott2018).
The fundamental source of the weeds’ resistance is attributed to the development of target- and/or non–target-site resistance mechanisms to herbicides (Délye et al. Reference Délye, Jasieniuk and LeCorre2013). Target-site resistance and non–target-site resistance mechanisms can contribute to weed survival, depending on the selections made to its genetic changes (Délye et al. Reference Délye, Jasieniuk and LeCorre2013). Target-site and non–target-site resistance mechanisms can also coexist in a single individual or population, raising their resistance to one herbicide or giving them multiple resistance to various herbicides (Garcia et al. Reference Garcia, Palma-Bautista, Rojano-Delgado, Bracamonte, Portugal, Alcantara-de la Cruz and De Prado2019). Target-site resistance constitutes the most common resistance mechanism to ACCase (Powles and Yu Reference Powles and Yu2010). Herbicide target-site amino acid substitution in the CT domain of ACCase has caused herbicide resistance in various weed species (Laforest et al. Reference Laforest, Soufiane, Simard, Obeid, Page and Nurse2017). Several amino acid substitutions have been reported in the ACCase gene region in resistant Leptochloa spp. such as Ile-1781-Leu, Ile-1781-Trp, Trp-1999-Cys, Trp-2027-Ser, Trp-2027-Leu, Trp-2027-Cys, Ile-2041-Asn, Asp-2078-Gly, Cys-2088-Arg, Gly-2096-Ala (Deng et al. Reference Deng, Cai, Zhang, Chen, Chen, Di and Yuan2019; Peng et al. Reference Peng, Pan, Liu, Cheng, Ma, Li, Liu, Wang and Bai2020; Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007, Reference Yu, Gao, Pan, Yao and Dong2017; Yuan et al. Reference Yuan, Di, Chen, Chen, Cai and Deng2019; Zhang et al. Reference Zhang, Chen, Xu, Song, Yao, Gao and Wu2020; Zhao et al. Reference Zhao, Jiang, Li, Gao, Zhang, Liao and Cao2022).
Recently, suspected herbicide-resistant bearded sprangletop biotypes have become a common problem in California rice fields. In annual survey studies conducted in California, Becerra-Alvarez et al. (Reference Becerra-Alvarez, Godar, Ceseski and Al-Khatib2023) observed an increase in suspected cyhalofop-resistant bearded sprangletop biotypes. This research examines the resistance of three bearded sprangletop biotypes to ACCase-inhibiting herbicides. Specifically, this study aimed to confirm resistance and determine the resistance level of the three suspected bearded sprangletop biotypes through the development of dose–response curves to cyhalofop, quizalofop, and clethodim. The second objective was to establish whether a mutation in the target-site gene was responsible for resistance.
Materials and Methods
Plant Material
A total of four bearded sprangletop biotypes, one known susceptible (S1) and three suspected resistant (R1, R2, and R3) to cyhalofop, were studied. Bearded sprangletop biotype S1 (ST-HR-2015) was collected from California Rice Experiment Station in Biggs, CA (39.451999°N; 121.72417°W) in 2015, and its new generation was produced in the greenhouse in 2019. Suspected resistant bearded sprangletop biotypes were collected from rice fields with a history of cyhalofop use and where bearded sprangletop survived the herbicide treatment. R1 (ST-19-10) was collected from Butte County, CA (39.379639°N; 121.744028°W) in 2019; R2 (ST-20-02) was collected from Glenn County, CA (39.626306°N; 122.03722°W) in 2020; and R3 (ST-21.07) was collected from Colusa County, CA (39.318667°N; 122.121722°W) in 2021. The seeds were stored at 4 C until utilized in the experiments. To break bearded sprangletop seed dormancy, seeds were placed in a freezer at –20 C for 3 mo before being placed in a refrigerated test tube at 4 C and soaked in deionized water. The water in the tubes was changed daily for 2 wk (Driver et al. Reference Driver, Al-Khatib and Godar2020b). The seeds were then placed on wet filter paper and incubated for 16 h at 40 C. Germinated seeds were transplanted in 8-cm by 8-cm by 6-cm pots in the greenhouse on Orchard Park Drive at UC Davis. Three seedlings were placed in each pot. Greenhouse temperature was 23 to 34 C, relative humidity was 65% to 70%, and 14-h/10-h day/night photoperiod. The supplemental light was 400 µmol m–2 s–1. Sterilized media soil was used, composed of one part compost (redwood shavings and turkey manure), one part coarse sand, one part peat moss, and 1.23 kg m–3 dolomite. The soil pH was 6.6, and the soil nutrient was 125 mg kg–1 available N, P2O5 46 mg kg–1 Olsen P, 759 mg kg–1 extractable K, 1,041 mg kg–1 calcium, 578 mg kg–1 magnesium, 7.6 mg kg–1 copper, 13 mg kg–1 zinc, 58 mg kg–1 manganese, 57 mg kg–1 iron, and 0.51 mg kg–1 boron. Plants were irrigated as needed.
Dose-Response Experiment
Herbicides were applied at three- to four-leaf stage. Cyhalofop, quizalofop, and clethodim doses were selected 16-fold below and above the label dose of herbicide with the control application, thus, 0×, 1/16×, 1/8×, 1/4×, 1/2×, 1×, 2×, 4×, 8×, and 16× (Table 1). Cyhalofop-p-butyl ((2R)-2-[4-(4-cyano-2-fluorophenoxy) phenoxy] propanoate) formulation was Clincher CA (Corteva Agriscience, Indianapolis, IN), which contained 29.6% of active ingredient. Cyhalofop was applied with 2.5% crop oil concentrate (COC). Quizalofop-p-ethyl (2-[4-(6-chloroquinoxalin-2-yl) oxyphenoxy] propanoate) formulation was Targa (Nissan Chemical Corporation, Japan), which contained 10.3% active ingredient. Quizalofop was applied with 1% COC. Clethodim (2-[(E)-N-[(E)-3-chloroprop-2-enoxy]-C-ethylcarbonimidoyl]-5-(2-ethylsulfanylpropyl)-3-hydroxycyclohex-2-en-1-one) herbicide formulation was Select Max (Valent U.S.A. LLC, San Ramon, CA), which contained 12.6% of the active ingredient. Clethodim was applied with 0.25% nonionic surfactant. Adjuvants were selected according to product labels, and their application was based on percent volume per volume (%v/v) concentration. Herbicides were applied in a spray chamber (Technical Machinery Inc., Berkeley, CA) with one Teejet XR8002VS flat-fan nozzle (TeeJet Tech., Springfield, IL) calibrated to deliver 187 L ha–1 at 275 kPa pressure; application height of the nozzle was 72 cm, and speed was 1.34 m s–1. After herbicide application, pots were placed back inside the greenhouse and irrigated 48 h later. Pots were maintained until harvest, and the aboveground plants were harvested 28 d after treatment. Plants were dried at 70 C for 3 d. The herbicide dose required to control 50% of the test biotypes (ED50) was calculated from dry-plant weight and converted to dry-biomass percentage compared to the nontreated control for presentation (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995). The experiment was conducted twice as a randomized block design with three replications.
Table 1. Clethodim, cyhalofop-p-butyl and quizalofop-p-ethyl application doses used in this study on the suspected herbicide-resistant bearded sprangletop biotypes.

Nucleotide Substitution Experiment
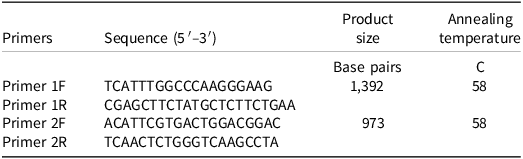
Fresh leaf tissue from five plants of each bearded sprangletop biotype were collected at 28 d after treatment. Cetyltrimethylammonium bromide DNA extraction method was used (Doyle and Doyle Reference Doyle and Doyle1987). Two primer pairs were designed based on sequences of Leptochloa chinensis (L.) Nees (GenBank: QWJ75145.1) from the National Center for Biotechnology Information (Table 2). The expected coverage of the two pairs was 98.9% and the 643 number of nucleotides they overlap. Polymerase chain reaction (PCR) amplification was performed using the Qiagen Taq PCR master mix (Qiagen, N.V., Netherlands), which contained 25 μL TAq Master mix, 1 μL of each primer (10 μM), 1 μL genomic DNA mixed in ddH2O in 50 μL. Thermal was included initially as denaturing step at 95 C for 5 min, followed by 35 cycles of 45 s denaturation at 95 C, 45 s annealing at 60 C, 60 s elongation at 72 C, and a final extension of 5 min at 72 C. Electrophoresis was performed at 120 V for 1 h. Plant DNA was purified by QIAquick PCR Purification Kit (Qiagen, N.V., Nederlands). Sequencing was performed at UC Davis Genomic Center. The sequencing data were analyzed by using MEGA 11: Molecular Evolutionary Genetics Analysis version 11 (Tamura et al. Reference Tamura, Stecher and Kumar2021).
Table 2. Primers of the ACCase gene fragment of bearded sprangletop.
Statistical Analyses
A four-parameter log-logistic model (Eq. 1) was used to establish the dose of each herbicide that result in 50% dry-weight reduction (ED50) (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995). The ED50 estimations were computed using the R DRC package (v4.3-1; Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) for statistical analysis.
where Y is biomass, b is the slope at the inflexion point (ED50), C and D are the lower and higher boundaries of the asymptote, respectively, and x is the herbicide dose. ANOVA was used to examine the P value that indicated a significant difference between the S and R biotypes. The resistance index (RI) was computed by dividing the ED50 of the resistant biotype by that of the susceptible biotype (Guo et al. Reference Guo, Lv, Zhang, Li, Wu, Lu, Liu and Wang2016).
Results and Discussion
Dose–Response Study
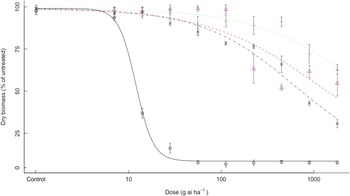
All three bearded sprangletop biotypes were found to exhibit high-level resistance to cyhalofop. The ED50 value for susceptible bearded sprangletop (S1) treated with cyhalofop was 27.4 g ai ha–1; however, the R1, R2, and R3 resistant biotypes had ED50 values higher than 4,480 g ai ha–1 cyhalofop (16×), resulting in RI values more than 164-fold (Table 3). The exact ED50 values could not be calculated for R1, R2, and R3, resistant to cyhalofop (Table 3, Figure 1), as bearded sprangletop survived at all cyhalofop doses applied with no 50% reduction in dry weight.
Table 3. Average cyhalofop, quizalofop, and clethodim dose that cause 50% dry-weight reduction (ED50) and resistance index (RI) of bearded sprangletop biotypes.

a SE, standard error, which is an average of two runs. >4,480 and >1,792, the data did not allow for the estimation of ED50 values, as all doses of cyhalofop and quizalofop were not sufficient to cause a 50% reduction in dry weight.
b RI, resistance index ED50 value of resistant bearded sprangletop biotype divided by that of susceptible biotype.

Figure 1. Effect of cyhalofop on the growth biomass of R1 (Δ), R2 (+), R3 (×), and S (o) biotypes of bearded sprangletop. S was the susceptible biotype; R1, R2, and R3 were resistant biotypes. Each point represents the average of six measurements (two runs and three replications) with standard error of the mean. Dose–response curves were generated by nonlinear regression using a log-logistic model. Vertical error bars represent the 95% confidence intervals at ED50.
It was determined that all three bearded sprangletop biotypes showed high levels of resistance to quizalofop. Whereas all resistant biotypes showed dry-weight reduction between 30% and 70% even at the highest dose of 1,792 g ai ha–1 (16×) quizalofop, no S1 plants could not maintain their dry weight at 112 g ai ha–1 (1×) quizalofop (Table 3, Figure 2). The S1 biotypes had ED50 of 12.0 g ai ha–1 quizalofop, but the R2 resistant biotype had ED50 values higher than 1,792 g ai ha–1 (16×), resulting in RI values more than 150-fold (Table 3). The exact ED50 values could not calculated for resistant R2 biotype as a result of high resistance to quizalofop and no 50% reduction in dry weight (Figure 2). Bearded sprangletop biotypes R1 and R3 exhibited ED50 values of 1,107.8 and 602.1 g ai ha–1 to quizalofop, respectively.
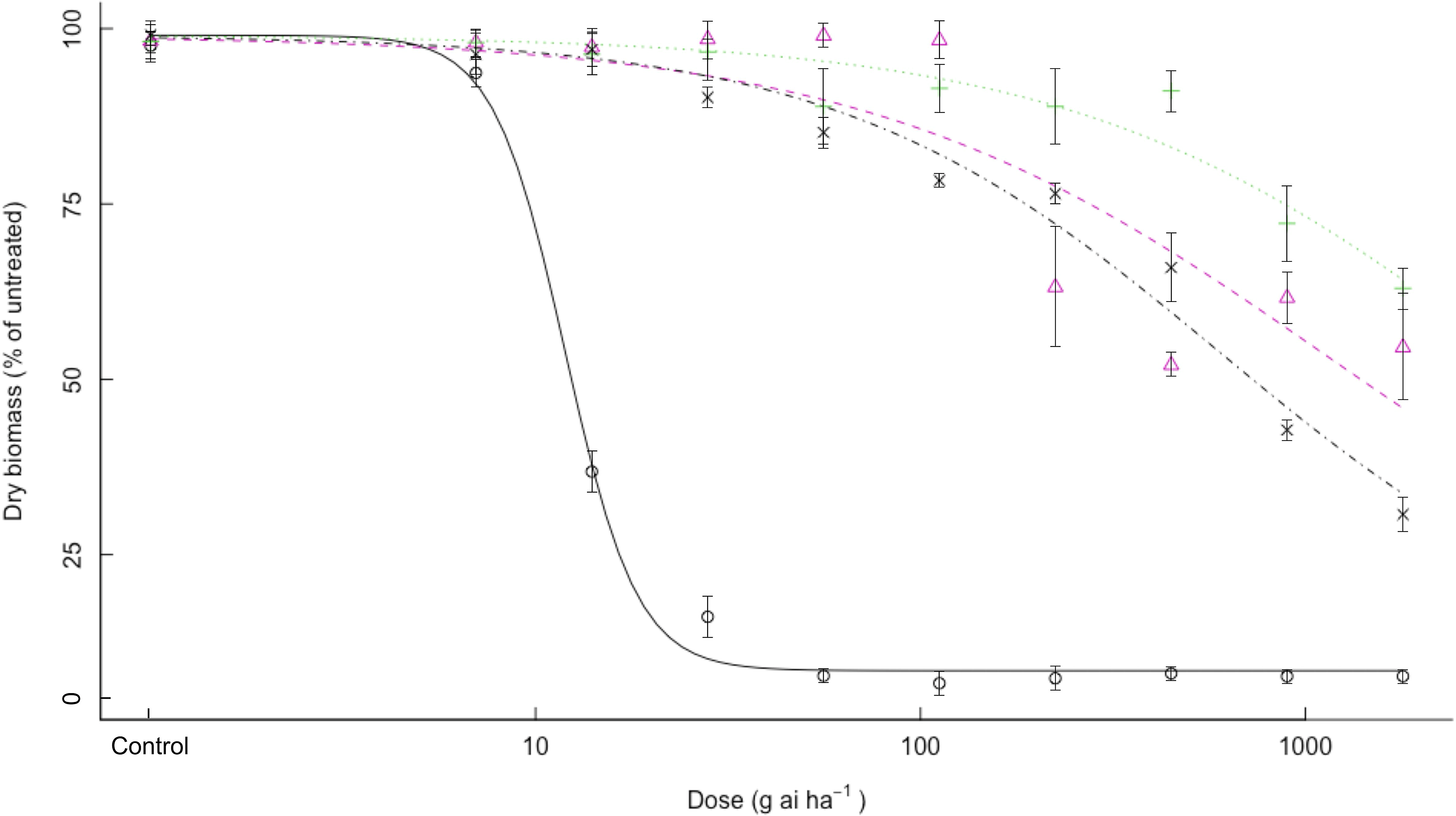
Figure 2. Effect of quizalofop on the growth biomass of R1 (Δ), R2 (+), R3 (×), and S (o) biotypes of bearded sprangletop. S was the susceptible biotype; R1, R2, and R3 were resistant biotypes. Each point represents the average of six measurements (two runs and three replications) with standard error of the mean. Dose–response curves were generated by nonlinear regression using a log-logistic model. Vertical error bars represent the 95% confidence intervals at ED50.
It is evident that the cyhalofop and quizalofop doses used in this study were not high enough to significantly reduce the dry weight of the resistant biotypes. However, the doses used in this study were similar to those used in previous research that examined herbicide resistance in Leptochloa spp. (Brim-Deforest et al. Reference Brim-DeForest, Alarcon-Reverte and Fischer2015; Deng et al. Reference Deng, Cai, Zhang, Chen, Chen, Di and Yuan2019; Peng et al. Reference Peng, Pan, Liu, Cheng, Ma, Li, Liu, Wang and Bai2020; Tehranchian et al. Reference Tehranchian, Norsworthy, Korres, McElroy, Chen and Scott2016; Yuan et al. Reference Yuan, Tian, Li, Qian, Guo and Shen2021; Zhang et al. Reference Zhang, Chen, Song, Zhang, Xu and Wu2022).
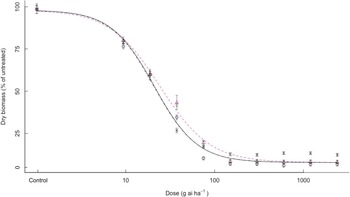
For clethodim, all bearded sprangletop biotypes were killed at 1× dose. S1, R1, R2, and R3 biotypes had ED50 values of 20.9, 24.3, 23.6, and 20.7 g ai ha–1 clethodim, respectively (Figure 3). Therefore, the R biotypes were susceptible to clethodim despite being resistant to the two APP (FOP) herbicides.

Figure 3. Effect of clethodim on the growth biomass of R1 (Δ), R2 (+), R3 (×), and S (o) biotypes of bearded sprangletop. S1, R1, R2, and R3 were all susceptible to clethodim. Each point represents the average of six measurements (two runs and three replications) with standard error of the mean. Dose–response curves were generated by nonlinear regression using a log-logistic model. Vertical error bars represent the 95% confidence intervals at ED50.
One of the main herbicides used since 2003 in California rice fields to control bearded sprangletop has been cyhalofop. ACCase inhibitor–resistant biotypes may emerge after 6 to 10 yr of selection pressure, especially in cropping systems where the ongoing use of these herbicides is the sole method of controlling grass weeds (Devine Reference Devine1997). With the frequent and intense use of cyhalofop in California rice fields, the continuous rice cultivation year after year, and a limited number of available herbicides, it is not surprising that bearded sprangletop has developed resistance to cyhalofop (Becerra-Alvarez et al. Reference Becerra-Alvarez, Godar, Ceseski and Al-Khatib2023; Brim-DeForest et al. Reference Brim-DeForest, Alarcon-Reverte and Fischer2015). This study revealed that suspected resistant bearded sprangletop biotypes, R1, R2, and R3, had a high level of resistance to cyhalofop and quizalofop but not to clethodim in California rice fields. This study also suggested that quizalofop, used in the newly developed Provisia rice technology, will have problems combating resistant bearded sprangletop in California. The Provisia system features a non-GMO herbicide-tolerant rice, allowing growers to safely apply quizalofop (Mankin et al. Reference Mankin, Neuteboom, Whitt, Schoefl, Hong, Wenck, Carlson, McElver and Stevenson–Paulik2021). Although clethodim is not registered in rice, it is understood to be used successfully to control bearded sprangletop and other economically important weeds with spot spray applications (Unan et al. Reference Unan, Galvin, Becerra-Alvarez and Al-Khatib2024). In addition, this study may indicate that clethodim could be a useful tool to control bearded sprangletop if clethodim-resistant rice is developed. However, one must consider the possibility that other biotypes might have resistance to clethodim after selection with cyhalofop and that clethodim, if used the same way, could exert a strong selection pressure.
Target-Site Resistance: ACCase Mutation Detection
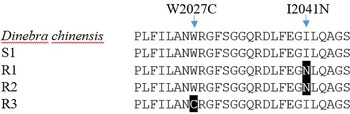
Each biotype of bearded sprangletop provided partial ACCase gene sequences. The results of a sequence alignment revealed a 98.8% similarity between the ACCase gene sequences from bearded sprangletop and Leptochloa chinensis (L.) Nees (GenBank:QWJ75145.1). All tested plants of the R1 and R2 biotypes displayed a substitution of Ile (ATT) to Asn (AAT) at position Ile-2041-Asn, whereas R3 biotype displayed a substitution of Trp (TGG) to Cys (TGC) at position Trp-2027-Cys compared with the S1 biotype and Leptochloa chinensis (L.) Nees. The plants from the S1 biotype, meanwhile, showed no signs of any known mutation.
Target-site–based resistance mechanisms frequently make for cross-resistance to herbicides that have the same mode of action (Beckie and Tardif Reference Beckie and Tardif2012). Several ACCase mutation points in bearded sprangletop have been reported so far. The present study identified two distinct ACCase mutations, Trp-2027-Cys and Ile-2041-Asn, for bearded sprangletop (Figure 4). The Ile-2041-Asn substitution was found in R1 and R2, whereas the Trp-2027-Cys substitution was found in R3, but these substitutions have been reported before as conferring plants with resistance to FOPs and susceptibility to clethodim (Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007). Though the Trp-2027-Cys substitution has been documented before in bearded sprangletop from California (Brim-DeForest et al. Reference Brim-DeForest, Alarcon-Reverte and Fischer2015), this is the first occurrence of the Ile-2041-Asn substitution. Tehranchian et al. (Reference Tehranchian, Norsworthy, Korres, McElroy, Chen and Scott2016) previously identified mutations in the Amazon sprangletop in Trp-2027-Cys in Arkansas. Zhao et al. (Reference Zhao, Jiang, Li, Gao, Zhang, Liao and Cao2022) identified Trp-2027-Cys substitution in Leptochloa chinensis sprangletop and noted that it was resistant to cyhalofop. In addition, Peng et al. (Reference Peng, Pan, Liu, Cheng, Ma, Li, Liu, Wang and Bai2020) reported Trp-2027-Ser and Ile-2041-Asn mutations in which it was resistant to cyhalofop in Leptochloa chinensis. Moreover, Yuan et al. (Reference Yuan, Di, Chen, Chen, Cai and Deng2019) detected Gly-2096-Ala substitution in Diplachne fusca, but this substitution was not detected in our study.
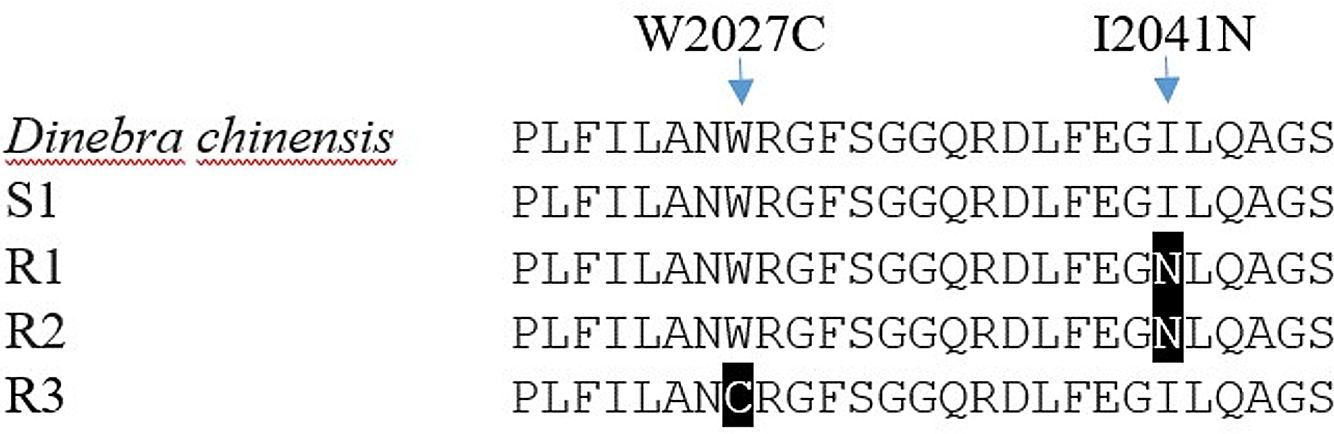
Figure 4. ACCase amino acid sequences of the amplified fragment of Leptochloa chinensis (L.) Nees, the susceptible (S1) and resistant (R1, R2, and R3) biotypes of bearded sprangletop. The black boxes illustrate the amino acid substitution from tryptophan (W) 2027 to cytosine (C) in R1 and R2, and from isoleucine (I) 2041 to leucine (N) in R3. The Leptochloa chinensis (L.) Nees (GenBank: QWJ75145.1) and susceptible bearded sprangletop (S1) ACCase sequence were used as references.
Cyhalofop is the only postemergence herbicide available in California to control bearded sprangletop; however, there are preemergence herbicides to control this weed, including clomazone, thiobencarb, and benzobicyclon (Becerra-Alvarez et al. Reference Becerra-Alvarez, Godar, Ceseski and Al-Khatib2023). If cyhalofop resistance is known in the field, then using preemergence herbicides becomes essential.
Practical Implications
In conclusion, bearded sprangletop resistance to selected ACCase inhibitors is present in California rice fields. The bearded sprangletop biotypes under study were resistant to cyhalofop and quizalofop, but not to clethodim. Target-site resistance was identified as the primary factor contributing to the resistance to cyhalofop and quizalofop for bearded sprangletop. The Trp-2027-Cys and Ile-2041-Asn target-site substitutions play a crucial role in the resistance to cyhalofop and quizalofop for bearded sprangletop in California. The results can aid in creating scientific approaches for the integrated management of resistant biotypes to ACCase inhibitors in bearded sprangletop. This study also revealed that all tested biotypes were susceptible to clethodim. The possibility of resistant bearded sprangletop genotypes becoming widespread in the coming years may cause greater problems. It might be suggested to rice farmers that integrated weed management such as crop rotation, certified clean seeds, deep flooding (Driver et al. Reference Driver, Al-Khatib and Godar2020a), tilling the soil no more than 20 cm deep, and spot spray application (Unan et al. Reference Unan, Galvin, Becerra-Alvarez and Al-Khatib2024) to control of resistant bearded sprangletop.
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. [897192], (project HerbaRice). This paper was given in part during the “100th Anniversary Turkish Herbology Congress” in 2023.
The authors declare no conflicts of interest.