The number of people with one or more chronic diseases, including type 2 diabetes (T2DM), is rising and lifestyle factors seem to play an important role in this development. Scientific literature suggests that dairy product intake may affect glucose tolerance and hence the development of T2DM.
Mechanistically, beneficial effects of dairy product consumption in the prevention of glucose intolerance and T2DM may be explained by the presence of calcium and protein and their favourable influence on energy balance and body weight maintenance( Reference Astrup, Chaput and Gilbert 1 ). Beneficial links have also been observed between whey protein and the regulation of particular satiety-related hormones, lipid metabolism and insulin secretion( Reference Jakubowicz and Froy 2 , Reference Pal, Ellis and Dhaliwal 3 ). In addition, possible metabolic effects of dairy products have been proposed for Mg (e.g. by promoting insulin sensitivity)( Reference Dong, Xun and He 4 ), conjugated linoleic acid (e.g. body weight regulation)( Reference Salas-Salvado, Marquez-Sandoval and Bullo 5 ) and lactic acid bacteria present in fermented products (e.g. gut microbiota and satiety)( Reference Diaz-Lopez, Bullo and Martinez-Gonzalez 6 – Reference Tremblay, Doyon and Sanchez 8 ). Conversely, unfavourable metabolic effects may occur following the consumption of dairy products with a relatively high energy density, such as full-fat dairy products, for instance via raising blood LDL-cholesterol concentrations( Reference Brassard, Tessier-Grenier and Allaire 9 ). Moreover, given the suggested impact of sugar-sweetened beverages on the development of T2DM( Reference Imamura, O’Connor and Ye 10 ), also adverse effects may result from the consumption of sugar-sweetened dairy products. Given these potential favourable, as well as less favourable, pathways of various dairy product nutrients, it is challenging to value the actual health impact of dairy product consumption as a whole; the different nutrients may strengthen but also weaken each other’s effects.
As a result, several observational studies( Reference Sluijs, Forouhi and Beulens 7 , Reference Chen, Sun and Giovannucci 11 – Reference Brouwer-Brolsma, van Woudenbergh and Oude Elferink 28 ) and meta-analyses( Reference Drouin-Chartier, Brassard and Tessier-Grenier 29 ) investigated associations between dairy product intake and incident T2DM. Chen et al.( Reference Chen, Sun and Giovannucci 11 ) conducted a meta-analysis of prospective cohort studies and concluded that there is no convincing evidence for an association between total dairy product consumption and incidence of T2DM (n 14, relative risk (RR) per one serving of dairy products: 0·98; 95 % CI 0·96, 1·01)( Reference Chen, Sun and Giovannucci 11 ). In contrast, a meta-analysis by Aune et al.( Reference Aune, Norat and Romundstad 30 ) did suggest a link between total dairy product intake and incident T2DM (n 12, RR/400 g 0·93; 95 % CI 0·87, 0·99). Despite the null findings for total dairy product resulting from the meta-analysis by Chen et al., subgroup analyses did show a significant inverse association between yoghurt consumption and T2DM( Reference Chen, Sun and Giovannucci 11 ). This illustrates that analyses of specific dairy product subgroups, rather than total dairy products, may improve the understanding of potential effects of the dairy product matrix. Hence, the research field is now evolving to more detailed analyses including different dairy product subgroups as for instance shown by the recent meta-analysis of Gijsbers et al.( Reference Gijsbers, Ding and Malik 31 ) and systematic review of meta-analyses by Drouin-Chartier et al.( Reference Drouin-Chartier, Brassard and Tessier-Grenier 29 ). This last mentioned research group concluded their work by stating that current epidemiologic evidence largely points towards neutral or beneficial associations between dairy product intake and incident T2DM, but that recommendations to consume low-fat dairy products instead of full-fat products are currently insufficiently supported( Reference Drouin-Chartier, Brassard and Tessier-Grenier 29 ).
As original studies with analyses on the dairy product subgroup level are still scarce( Reference Gijsbers, Ding and Malik 31 ), we explored associations of dairy product intake with pre-diabetes and newly diagnosed T2DM (ND-T2DM) prevalence – defined using the aetiological markers fasting plasma glucose (FPG) and HbA1c% – in a uniquely large population of Dutch adults by subdividing total dairy product intake into a broad variety of dairy product subclasses, including skimmed dairy products, semi-skimmed dairy products, full-fat dairy products, non-fermented dairy products, fermented dairy products, total milk, skimmed milk, semi-skimmed milk, full-fat milk, total yogurt, skimmed yogurt, full-fat yogurt, buttermilk, curd cheese/quark, custard, flavoured yogurt drinks, total cheese, low-fat cheese and regular-fat cheese. We also studied potential effect modification of dairy product intake with age, sex and BMI, and mediation effects by markers of lipid metabolism.
Methods
Participants
This cross-sectional study was performed using data of the Lifelines Cohort Study. Lifelines is a multi-disciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviours of 167 729 persons living in the North of the Netherlands. It uses a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors that contribute to the health and disease of the general population, with a special focus on multi-morbidity and complex genetics( Reference Scholtens, Smidt and Swertz 32 ). Between 2006 and 2013, inhabitants of the three Northern provinces of The Netherlands (Friesland, Groningen and Drenthe) and their families were invited for participation in the study, with the goal to create a three-generation design. The first group of participants, aged 25–50 years old, was recruited through their general practitioner. Exclusion criteria included having a severe psychiatric or physical illness, limited life expectancy (<5 years) and insufficient knowledge of the Dutch language to complete a Dutch questionnaire. When a participant was considered to be eligible to the study, he or she received a baseline questionnaire and was invited to the Lifelines research site for a comprehensive health assessment. During the visit at the research centre, participants were also asked to indicate whether family members would be willing to participate in the study; in case of a positive response, family members were invited as well. In addition to this recruitment strategy, inhabitants of the northern part of The Netherlands could also register themselves via the Lifelines website. A more detailed description of the Lifelines study can be found in the article on the cohort description( Reference Scholtens, Smidt and Swertz 32 ). All participants gave written informed consent. The Lifelines study is conducted according to the principles of the Declaration of Helsinki and in accordance with the research code of the University Medical Centre Groningen. The Lifelines study is approved by the medical ethical committee of the UMCG, the Netherlands.
Population for analyses
In total, 144 095 out of 167 729 participants completed a baseline FFQ. Participants with unreliable dietary data (n 29 413) – that is men with energy intakes <3347 kJ or > 17 573 kJ and women with energy intakes <2092 kJ or > 14 644 kJ – and/or FFQ judged as unreliable by the research dieticians, for example owing to nutrient or food group reports below the possible under or upper limit, or reporting to have diabetes (n 2596) were excluded from the analyses. Finally, 112 086 participants were included in our analyses.
Dietary assessment
Dietary intake was assessed by the ‘flower FFQ’, which has been developed as an alternative for the regular – often time-consuming – FFQ. The name ‘flower FFQ’ has been derived from its design, consisting of one main questionnaire on energy and macronutrient intake (heart), and four complementary food questionnaires (petals) on micronutrients and eating behaviour, with overlapping questions to provide information on covariance. For the current analyses, only data of the flower heart were available, which comprised 110 food items, including all major food groups such as dairy products (further specified in Table 1), bread, pasta, rice, potatoes, fruit, vegetables, legumes, meat, fish, coffee, tea and soda/juice. Portion sizes were estimated using natural portions and commonly used household measures( Reference Donders-Engelen, van der Heijden and Hulshof 33 ). FFQ data were converted into total intakes of energy and nutrients by means of the Dutch Food Composition table 2011 (NEVO)( 34 ). A more detailed description of the Flower FFQ can be found elsewhere( Reference Brouwer-Brolsma, Streppel and van Lee 35 ). Before entering the dietary variables in the statistical models, they were all adjusted for energy intake by means of the residual method( Reference Willett 36 ). The questionnaire also included an item about whether or not participants were on a weight loss diet at the time of the dietary assessment. Currently, researchers are working on the validation of the ‘flower FFQ’.
Table 1 Dairy product group classification

* The first number following the dairy product in the second column indicates the fat quantity (g) per 100 g; the percentage (%) refers to the contribution of that specific dairy product to that category.
Markers of glucose homoeostasis
Fasting blood samples were collected at baseline, processed on the day of collection and either directly analysed or stored at −80°C in a fully automated storage facility. FPG was determined in venous plasma by means of the Roche glucose assay (hexokinase/glucose-6-phosphate dehydrogenase enzymatic reactions) and the Modular P analyser (Roche Diagnostics). HbA1c was determined in whole blood (EDTA-anticoagulated) by means of turbidimetric inhibition immunoassay on a Cobas Integra 800 CTS analyser (Roche Diagnostics Netherland BV), which has been shown to have a coefficient of variation of 2·1 % for a mean HbA1c of 5·5 %, and 1·9 % for a mean HbA1c of 10·6 %( Reference Jansen, Stolk and Nolte 37 ). Subsequently, pre-diabetes was defined as having a FPG between 5·6 and 6·9 mmol/l or an HbA1c of 5·7–6·4 %( 38 ). ND-T2DM was defined as having a FPG ≥7·0 mmol/L or HbA1c ≥6·5 %( 38 ).
Non-dietary covariates
Baseline data on demographic factors, education level (primary, secondary, higher or other education), current and past active smoking behaviour, physical activity (SQUASH)( Reference Wendel-Vos, Schuit and Saris 39 ), ethanol consumption (none, 1–9, 10–19, ≥20 g/d), history and prevalence of diseases (i.e. hypertension and hypercholesterolaemia) and family history of diseases were collected by means of questionnaires. Weight was measured to the nearest 0·1 kg, without shoes and heavy clothing, using a calibrated SECA 761 scale. Height was measured to the nearest 0·1 cm, without shoes, using a calibrated SECA222 stadiometer. BMI was calculated as weight/height squared (kg/m2). Waist circumference was measured twice, to the nearest 0·1 cm, midway between the lowest rib and the top of the iliac crest at the end of gentle expiration, using SECA 200 measuring tape. The mean of the two measurements was used in the analyses( Reference van der Ende, Hartman and Hagemeijer 40 ). Total cholesterol (TC) and HDL-C were assessed in serum using an enzymatic colorimetric method. LDL-C was determined in serum with a colorimetric method. Serum TAG concentrations were measured with a colorimetric UV method. All these cholesterol measurements were done on a Roche Modular P chemistry analyser (Roche)( Reference Slagter, van Vliet-Ostaptchouk and Vonk 41 ).
Statistical analyses
Participant characteristics are reported as mean values and standard deviations, numbers and percentages. Medians and interquartile ranges (IQR) were used to report skewed variables. Differences over tertiles of total dairy product intake were tested by means of χ 2 tests in case of categorical variables, one-way ANOVA in case of normally distributed continuous variables and Kruskal–Wallis test in case of skewed continuous variables. Logistic regression analysis was conducted to calculate OR for pre-diabetes and ND-T2DM per dairy product intake tertile, using the lowest tertile as the reference group. OR per 100 g/d or serving increase in dairy product intake were calculated as well. Models were adjusted for age (years), sex (model 1), model 1+alcohol (0, 1–9, 10–19, ≥20 g/d), smoking (never, former, current), education (primary, secondary, higher, other), physical activity (number of days/week of at least moderate intensity physical activity) (model 2), model 2+total energy intake (kJ/d), intake of energy adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/juice, other dairy product groups (g/d) (model 3), model 3+BMI (kg/m2) and waist circumference (cm) (model 4). Potential mediation by markers of lipid metabolism was examined by adding TC, HDL-cC, LDL-C and TAG to model 4 (model 5). The P for trend across medians of dairy product intake tertiles was calculated to study potential dose–response associations of dairy product intake with prevalent pre-diabetes and ND-T2DM. Possible interactions between dairy product intake and age, sex and BMI in association with FPG and HbA1c were tested through the inclusion of a cross-product term in linear models and visualised through stratified analyses. A two-sided P value≤0·05 was considered to be statistically significant for all analyses. Analyses were performed using the statistical package SPSS, version 22 (IBM SPSS Inc.).
Results
The characteristics of the population are described in Table 2. Comparison of the top and bottom tertile of total dairy product intake shows that participants in the top tertile were more likely to be older, women, former smokers, overweight, to be diagnosed with hypertension and hypercholesterolaemia and to have a higher intake of fruits. Analyses on the key variables in this study showed that 25 549 (23 %) participants had pre-diabetes and 1305 (1 %) had ND-T2DM. Median dairy product intake of the total population was 324 (IQR 227) g/d. Participants consumed more semi-skimmed dairy products than skimmed or full-fat products, and higher quantities of non-fermented dairy products than fermented dairy products. On the product level, milk was the largest contributor to the total sum of dairy products – that is 98 (IQR 170) g/d.
Table 2 Baseline characteristics according to tertiles (T) of total dairy product intake of 112 086 participants without self-reported diabetes (Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)
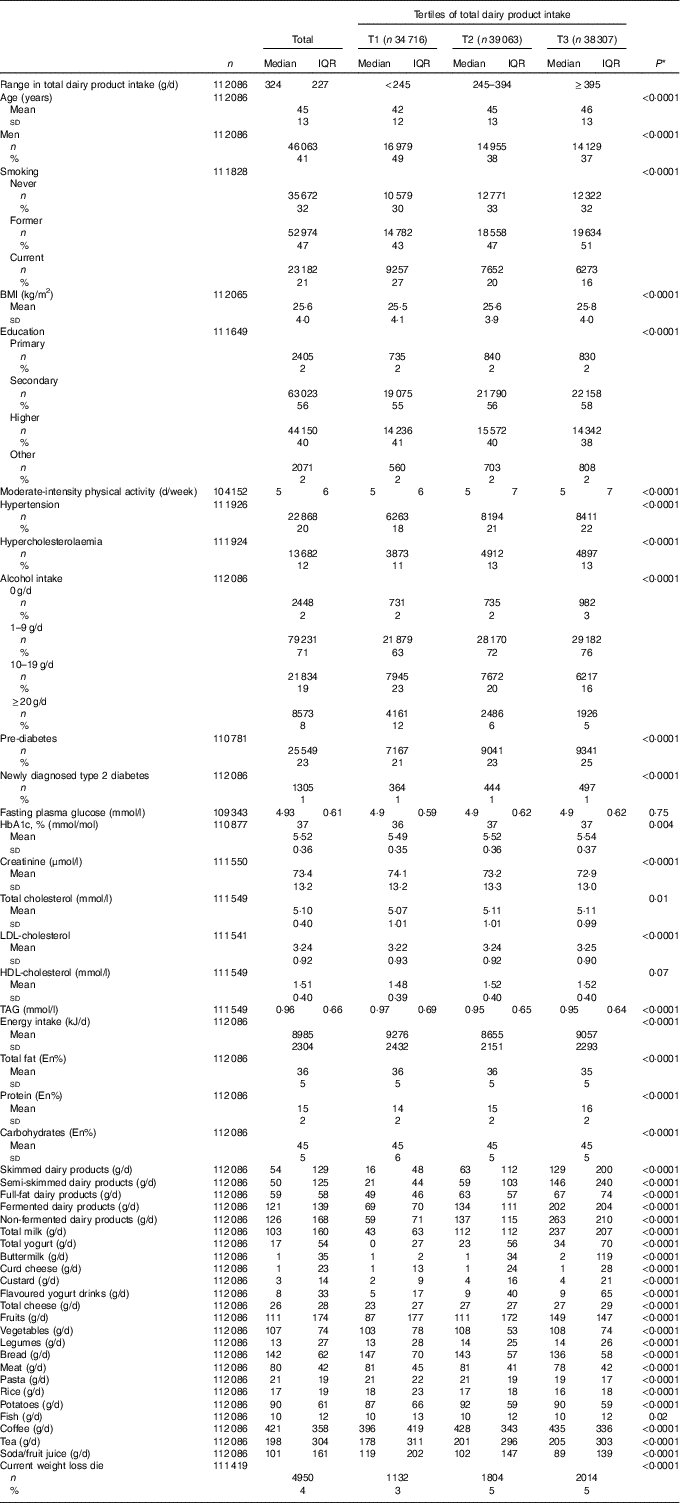
* Differences across quintiles are investigated using ANOVA in case of normally distributed continuous variables, Kruskal–Wallis test in case of skewed continuous variables and χ 2 tests in case of categorical variables.
Pre-diabetes
After full adjustment (model 4), significant inverse associations were observed of skimmed (OR per 100 g (OR100 g) 0·98; 95 % CI 0·97, 1·00; P=0·02 and OR of the third tertile (ORT3) 0·95; 95 % CI 0·92, 0·99; P=0·02) and fermented dairy product intake (OR100 g 0·98; 95 % CI 0·97, 0·99; P=0·004 and ORT3 0·94; 95 % CI 0·90, 0·98; P=0·004) with pre-diabetes, showing a 2 % lower odds of pre-diabetes with each 100-g increase in dairy product intake for both dairy product subclasses. Positive associations were observed for full-fat (OR100 g 1·03; 95 % CI 1·01, 1·06; P=0·004 and ORT3 1·10; 95 % CI 1·06, 1·15; P<0·0001) and non-fermented dairy products (OR100 g 1·01; 95 % CI 1·00, 1·02; P=0·30 and ORT3 1·05; 95 % CI 1·00, 1·09 P=0·03) with pre-diabetes. On the product level, a significant inverse association was observed between buttermilk (ORserving/150 g 0·97; 95 % CI 0·94, 1·00; P=0·04 and ORT3 0·99; 95 % CI 0·95, 1·04; P=0·68) and pre-diabetes, whereas a positive association was observed for custard with pre-diabetes (ORserving/150 g 1·13; 95 % CI 1·03, 1·24; P=0·01 and ORT3 1·05; 95 % CI 1·01, 1·10; P=0·02) (Table 3). No associations were observed for the intake of total dairy products, semi-skimmed dairy products, milk, yogurt, curd cheese, yogurt drinks or cheese with pre-diabetes. However, more specific analyses for milk, yogurt and cheese that were further subdivided based on fat content did show positive associations for full-fat milk (ORserving/150 g 1·03; 95 % CI 0·99, 1·08; P=0·19 and ORT3 1·07; 95 % CI 1·02, 1·11; P=0·002) and full-fat yogurt (ORserving/150 g 1·09; 95 % CI 0·99, 1·19; P=0·08 and ORT3 1·07; 95 % CI 1·02, 1·12; P=0·007), whereas an inverse association was observed for low-fat cheese (ORserving/20 g 0·97; 95 % CI 0·95, 0·99; P=0·004 and ORT3 0·96; 95 % CI 0·92, 1·00; P=0·08) (Table 4). Including markers of lipid metabolism (model 5) – that is potential intermediates – did not affect the associations between dairy product intake and pre-diabetes (data not shown).
Table 3 Associations between dairy product consumption and pre-diabetes (fasting plasma glucose (FPG) 5·6–6·9 mmol/l or HbA1c 5·7–6·4 %) in Lifelines (n 110781) (Odds ratios and 95 % confidence intervals)
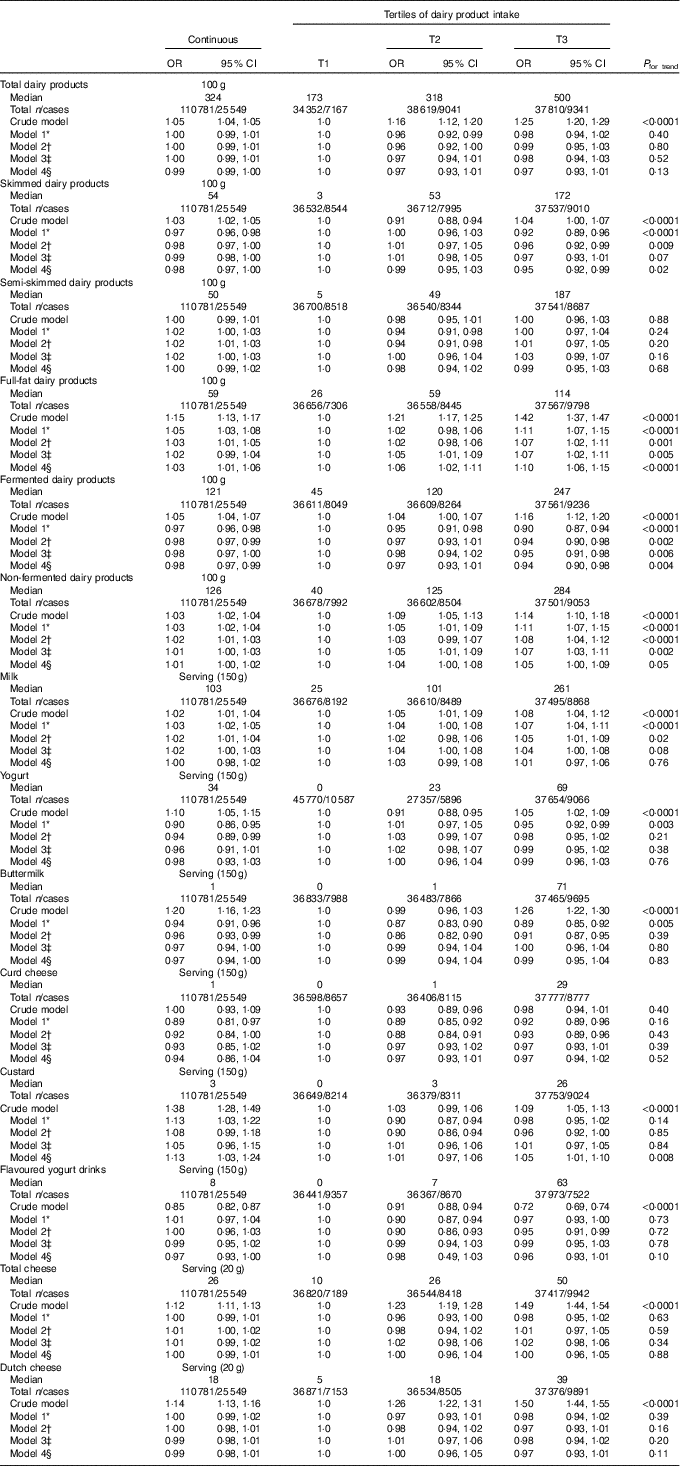
T, tertile.
* Model 1 was adjusted for age (years, continuous) and sex (men/women).
† Model 2 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical) and physical activity (moderate intensity exercise, d/week).
‡ Model 3 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical), physical activity (moderate intensity exercise, d/week), total energy intake (kJ/d, continuous) and the intake of energy-adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/fruit juice and other dairy product groups (g/d, continuous).
§ Model 4 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical), physical activity (moderate intensity exercise, d/week), total energy intake (kJ/d, continuous), the intake of energy-adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/fruit juice, other dairy product groups (g/d, continuous), BMI (kg/m2, continuous) and waist circumference (cm, continuous).
Table 4 Associations of milk, yogurt and cheese classified on the basis of fat content with pre-diabetes (PD) (fasting plasma glucose (FPG) 5·6–6·9 mmol/l or HbA1c 5·7–6·4 %) (n 110 781) and newly diagnosed type 2 diabetes (ND-T2DM) (FPG≥7·0 mmol/l) (n 112 086) in Lifelines*(Odds ratios and 95 % confidence intervals)
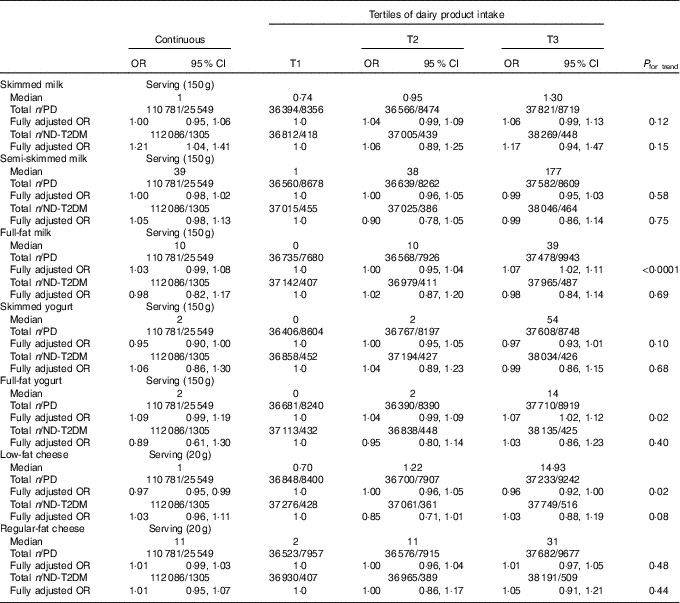
T, tertile.
* The fully adjusted OR was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical), physical activity (moderate intensity exercise, d/week), total energy intake (kJ/d, continuous), the intake of energy-adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/fruit juice, other dairy product groups (g/d, continuous), BMI (kg/m2, continuous) and waist circumference (cm, continuous).
Newly diagnosed type 2 diabetes
Exploration of the associations between dairy product intake and ND-T2DM showed significant positive associations between full-fat (OR100 g 1·04; 95 % CI 0·96, 1·13; P=0·29; ORT2 1·18; 95 % CI 1·01, 1·37; P=0·03 and ORT3 1·16; 95 % CI 0·99, 1·35; P=0·07) and non-fermented dairy product (OR100 g 1·05; 95 % CI 1·01, 1·09; P=0·01 and ORT3 1·10; 95 % CI 0·95, 1·27; P=0·21) with ND-T2DM (Table 5). On the product level, a significant positive association was observed between milk and ND-T2DM (ORserving/150 g 1·08; 95 % CI 1·02, 1·15; P=0·006 and ORT3 1·10; 95 % CI 0·95, 1·27; P=0·19), which was predominantly driven by skimmed milk consumption (ORserving/150 g 1·21; 95 % CI 1·04, 1·41; P=0·01 and ORT3 1·17; 95 % CI 0·94, 1·47; P=0·16). No associations were observed for the consumption of total, skimmed, semi-skimmed and fermented dairy product, yogurt, buttermilk, curd cheese, custard, flavoured yogurt drinks and cheese with ND-T2DM. Including markers of lipid metabolism (model 5) did not influence the associations between dairy product intake and – ND-T2DM (data not shown).
Table 5 Associations between dairy product consumption and newly diagnosed type 2 diabetes (fasting plasma glucose (FPG)≥7·0 mmol/l) in Lifelines (n 112086) (Odds ratios and 95 % confidence intervals)
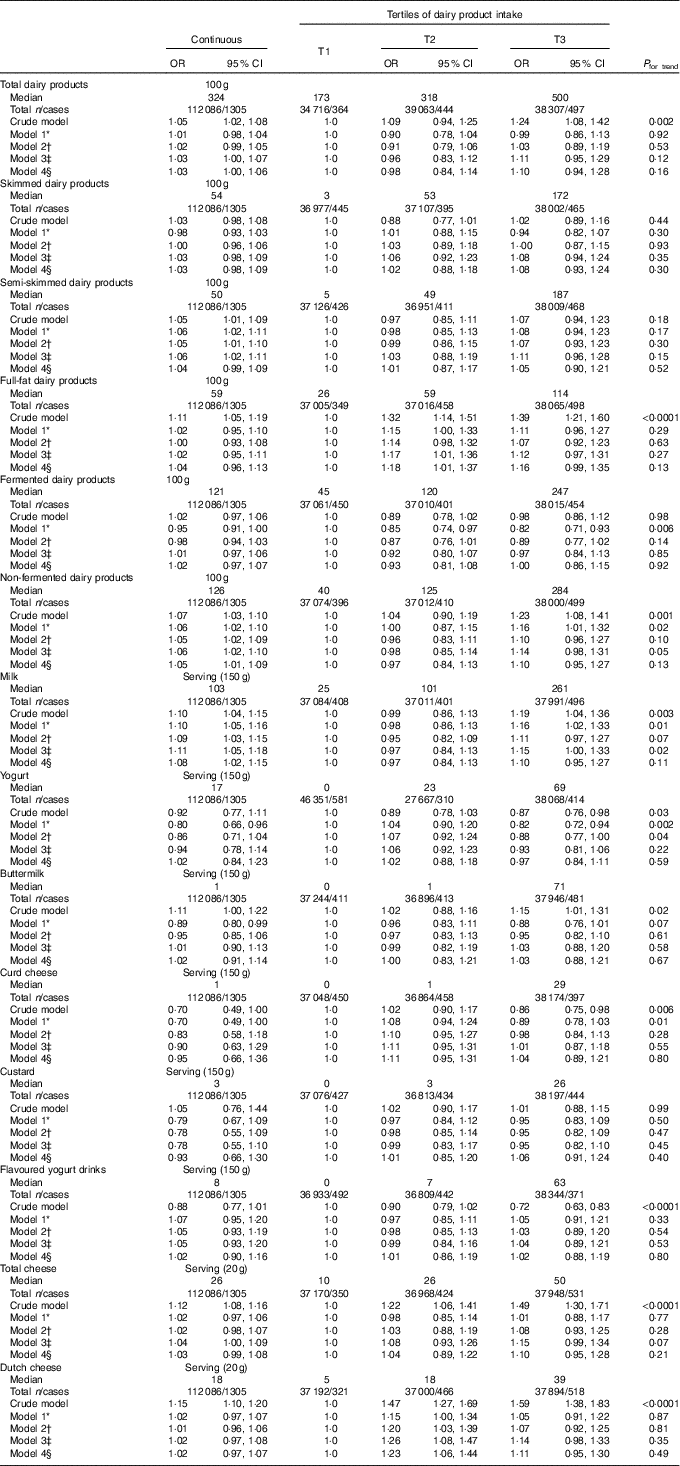
T, tertile.
* Model 1 was adjusted for age (years, continuous) and sex (men/women).
† Model 2 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical) and physical activity (moderate intensity exercise, d/week).
‡ Model 3 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical), physical activity (moderate intensity exercise, d/week), total energy intake (kJ/d, continuous) and the intake of energy-adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/fruit juice and other dairy product groups (g/d, continuous).
§ Model 4 was adjusted for age (years, continuous), sex (men/women), alcohol (categorical), smoking (categorical), education (categorical), physical activity (moderate intensity exercise, d/week), total energy intake (kJ/d, continuous), the intake of energy-adjusted bread, pasta, rice, potato, fruit, vegetables, legumes, meat, fish, coffee, tea, soda/fruit juice, other dairy product groups (g/d, continuous), BMI (kg/m2, continuous) and waist circumference (cm, continuous).
Moreover, although our analyses showed several significant interactions between dairy product intake and age, sex and/or BMI in relation to FPG and HbA1c, no consistent patterns could be identified for these three elements (online Supplementary Table S1).
Discussion
Our analyses of dairy product intake with pre-diabetes and ND-T2DM among Dutch adults in the Lifelines Cohort Study showed inverse associations of skimmed dairy products, fermented dairy products, buttermilk and low-fat cheese with pre-diabetes. Positive associations were observed for full-fat dairy products, non-fermented dairy products, custard, full-fat milk and full-fat yogurt with pre-diabetes. The observed associations for dairy product intake and ND-T2DM were less convincing, but did show positive associations for full-fat dairy products, non-fermented dairy products, total milk and skimmed milk. Our analyses did not point towards effect modification by age, sex and BMI, or mediation through markers of lipid metabolism.
When comparing our data on skimmed, semi-skimmed and full-fat dairy products with other prospective studies and meta-analyses, our findings on pre-diabetes are partly in line with data of the Black Women’s Health Study and the Women’s Health Study( Reference Liu, Choi and Ford 17 , Reference van Dam, Hu and Rosenberg 23 ). These two studies also showed an inverse association between low-fat dairy products and T2DM incidence( Reference Liu, Choi and Ford 17 , Reference van Dam, Hu and Rosenberg 23 ). However, no such association was observed for high-fat dairy products( Reference Liu, Choi and Ford 17 , Reference van Dam, Hu and Rosenberg 23 ). Moreover, no difference between low-fat and high-fat products in association with incident T2DM was observed in several other prospective studies( Reference Chen, Sun and Giovannucci 11 , Reference Louie, Flood and Rangan 18 , Reference Soedamah-Muthu, Masset and Verberne 21 , Reference Struijk, Heraclides and Witte 22 ). Yet, a meta-analysis of thirteen studies showed a 4 % lower risk of incident T2DM per 200 g/d low-fat dairy product intake (RR 0·96; 95 % CI 0·92, 1·00), whereas no significant association was observed for high-fat dairy product intake (RR 0·98; 95 % CI 0·93, 1·04)( Reference Gijsbers, Ding and Malik 31 ). This meta-analysis also showed a 12 % lower risk of incident T2DM for an intake of 40 g of fermented dairy products per day (n 5)( Reference Gijsbers, Ding and Malik 31 ). Although we did not observe an association between fermented dairy product intake and ND-T2DM, we did observe a 6 % lower odds of having pre-diabetes for participants in the highest fermented dairy products intake tertile. To note, as there was quite some overlap between the consumed products in the fermented dairy products and skimmed dairy product groups in our study, the inverse associations of skimmed and fermented dairy products with pre-diabetes may partly be explained by the consumption of the same products.
As potential dairy product effects may be related to particular product-specific nutrients, we hypothesised that more detailed analyses on the product level could provide more insight in the potential link between dairy product intake and T2DM. For instance, milk and yogurt are important sources of whey protein, which have been associated with lower postprandial glucose concentrations in patients with T2DM risk( Reference Frid, Nilsson and Holst 42 ). Moreover, both whey and casein have been shown to decrease food intake, body weight and body fat, and beneficially affect glucose tolerance and gut hormones in diet-induced obese rats( Reference Pezeshki, Fahim and Chelikani 43 ). Beneficial associations as previously observed for fermented products and T2DM risk( Reference Diaz-Lopez, Bullo and Martinez-Gonzalez 6 , Reference Sluijs, Forouhi and Beulens 7 ) may be related to potential effects on gut microbiota and satiety( Reference Tremblay, Doyon and Sanchez 8 ). In addition, ruminant trans-fatty acids have been associated with beneficial effects on glucose homoeostasis as well, where the suggested pathways include modulation of the hepatic fat content, expression of PPAR-γ and PPAR-α, and inflammatory state( Reference Tremblay and Rudkowska 44 ).
Our analyses on the product level showed an inverse association for buttermilk with pre-diabetes and a positive association for custard intake and pre-diabetes; no associations were observed for milk, yogurt, curd cheese, flavoured yogurt drinks or cheese intake. Milk consumption, particularly skimmed milk, was positively associated with ND-T2DM, whereas none of the other dairy products were associated with ND-T2DM. Evaluation of the literature with respect to the different dairy product groups shows that our null findings for milk in relation to pre-diabetes are in line with several other observational studies( Reference Sluijs, Forouhi and Beulens 7 , Reference Elwood, Pickering and Fehily 12 , Reference Kirii, Mizoue and Iso 15 , Reference Soedamah-Muthu, Masset and Verberne 21 , Reference Struijk, Heraclides and Witte 22 , Reference Vang, Singh and Lee 24 ), but in contrast to two observational studies in Asian populations, with relatively low milk intakes, showing inverse associations( Reference Villegas, Gao and Dai 25 , Reference Zong, Sun and Yu 27 ). None of the other studies observed a positive association between milk consumption and T2DM. Moreover, a recent meta-analysis including 11 studies did not show a significant link between milk intake and T2DM risk either (RR 0·97 per 200 g/d; 95 % CI 0·93, 1·02; P=0·25)( Reference Gijsbers, Ding and Malik 31 ). Although we observed significant associations of higher fermented dairy product and buttermilk intakes and a lower odds of pre-diabetes, we did not observe associations between yogurt, curd cheese or flavoured yogurt drinks and T2DM or pre-diabetes. However, full-fat yogurt was positively associated with pre-diabetes. Other cohort studies that investigated associations between the intake of yogurt and T2DM showed varying results, ranging from no association( Reference Chen, Sun and Giovannucci 11 , Reference Grantham, Magliano and Hodge 14 , Reference Soedamah-Muthu, Masset and Verberne 21 , Reference Struijk, Heraclides and Witte 22 ), borderline non-significant inverse associations( Reference Sluijs, Forouhi and Beulens 7 , Reference Chen, Sun and Giovannucci 11 , Reference Kirii, Mizoue and Iso 15 ) to significant inverse associations( Reference Chen, Sun and Giovannucci 11 , Reference Liu, Choi and Ford 17 , Reference Margolis, Wei and de Boer 19 ). In contrast to our findings, meta-analysis of eleven studies does suggest a significant inverse association between yogurt intake and risk of T2DM (RR for 80 g/d: 0·86 compared with 0 g/d; 95 % CI 0·83, 0·90; P<0·0001)( Reference Gijsbers, Ding and Malik 31 ). Finally, in line with our findings on total cheese intake, most other studies exploring the association between cheese intake and the development of T2DM, although not all( Reference Sluijs, Forouhi and Beulens 7 , Reference Chen, Sun and Giovannucci 11 ), do not point towards an association( Reference Chen, Sun and Giovannucci 11 , Reference Grantham, Magliano and Hodge 14 , Reference Kirii, Mizoue and Iso 15 , Reference Soedamah-Muthu, Masset and Verberne 21 , Reference Struijk, Heraclides and Witte 22 , Reference Vang, Singh and Lee 24 ). In line, a recent meta-analyses by Gijsbers and colleagues (2016) did not detect a significant relationship for this dairy product and incident T2DM (n 12, RR 1·00 per 10 g/d)( Reference Gijsbers, Ding and Malik 31 ). However, our analyses did show a significant inverse association for low-fat cheese and pre-diabetes. Conversely, our data did not indicate that the association between Dutch cheese and glucose homoeostasis is any different from the impact of total cheese.
It may be noted that, in contrast to the suggested favourable effect of trans-ruminant fatty acids on glucose homoeostasis( Reference Tremblay and Rudkowska 44 ), our data showed positive associations for full-fat dairy products as a whole, as well as various full-fat dairy products. We do not have a clear-cut explanation for the positive associations as observed in our study other than that full-fat dairy products have a higher energy content and hence may contribute to weight gain and as such glucose intolerance. On the contrary, adding BMI did not change the associations, which does not support this speculation on energy content. In addition, the positive association for full-fat dairy products with pre-diabetes in this population was predominantly driven by the subgroups with the lowest fat content within this full-fat dairy product subclass – that is full-fat milk (3·5 g fat) (fully adjusted OR per serving (150 g): 1·03, 95 % CI 0·99, 1·08), full-fat yogurt (2·9 g fat) (fully adjusted OR per serving (150 g) 1·09; 95 % CI 0·99, 1·19) and milk-based ice cream (12 g fat) (fully adjusted OR per serving (75 g) 1·31; 95 % CI 1·16, 1·48), whereas associations for the three food groups with the highest fat content within this full-fat dairy product subclass – that is cream (35 g fat) (fully adjusted OR per serving (30 g) 1·17; 95 % CI 0·94, 1·44), regular-fat cheese (≥24 g fat) (fully adjusted OR per serving (20 g) 1·01; 95 % CI 0·99, 1·03) and chocolate milk (1·9 g fat) (fully adjusted OR per serving (150 g) 0·98; 95 % CI 0·91, 1·06) – with pre-diabetes were less pronounced or even absent. These findings stress the confusing aspect of dairy food categorisation based on ‘fat content’ in association with diabetes-related outcomes and call for future studies investigating the impact of dairy products in even more detail (i.e. individual dairy products).
In addition to above summarised studies, our findings display important resemblances with the recently published cross-sectional (Dutch) Maastricht Study with data of 2391 participants( Reference Eussen, van Dongen and Wijckmans 45 ), which also showed significant inverse associations of skimmed dairy products (ORT3 0·73; 95 % CI 0·55, 0·96) and fermented dairy products (ORT3 0·74; 95 % CI 0·54, 0·99) with impaired glucose metabolism, whereas no associations for skimmed dairy products and fermented dairy products were observed for ND-T2DM. Moreover, in line with our findings, the Maastricht study also showed a positive association between full-fat dairy product (ORT3 2·01; 95 % CI 1·16, 3·47) consumption and ND-T2DM. In contrast to the Maastricht Study, we did not observe a significant inverse association between total dairy product consumption and ND-T2DM. Even with the important resemblances, it needs to be noted that the associations observed in the Maastricht Study are substantially stronger than the associations observed in the Lifelines population. Although we do not have a straightforward explanation for this difference, the cut-offs for the lowest tertiles in the Maastricht Study are markedly lower than the cut-offs in our study, which may partly explain the difference in strength of the associations. Another explanation may be that the Maastricht Study was conducted among adults between 40 and 75 years of age, while we included men and women aged 18 years and over. As suggested by the meta-analysis of Gijsbers et al.( Reference Gijsbers, Ding and Malik 31 ), associations between dairy product intake and glucose homoeostasis tend to be stronger in older populations. Then again, we did not observe consistent interactions between markers of glucose homoeostasis and age. Moreover, dairy product intake was not associated with any dairy product subclass in older Dutch adults aged ≥55 years participating in the Rotterdam study( Reference Brouwer-Brolsma, van Woudenbergh and Oude Elferink 28 ). Finally, we do not have a direct explanation for the different findings for pre-diabetes and ND-T2DM as shown in these two studies. It may be postulated that the null associations for ND-T2DM are related to the low number of ND-T2DM cases and hence reflect a power issue. This idea is strengthened by the fact that Lifelines data do show significant associations for non-fermented dairy products (5 % higher odds of ND-T2DM per 100 g) and milk (8 % higher odds of ND-T2DM per serving/150 g) with ND-T2DM when analysed continuously.
A limitation of this study is that we only had cross-sectional data. Therefore, it may be that it was not dairy product consumption that affected glucose homoeostasis, but that people with impaired glucose homoeostasis made other decisions regarding their dietary behaviours and hence their dairy product intake. However, as we had the possibility to study pre-diabetes and ND-T2DM defined based on aetiologic markers rather than self-report, where all self-reported diabetics were excluded to prevent the introduction of reverse causation, we feel that we successfully prevented the introduction of reverse causation. Specifically, analyses on dairy product intake and self-report T2DM within this study showed clear patterns of reverse causation, including a positive association between semi-skimmed dairy products and self-reported T2DM and inverse associations of full-fat dairy products and custard with self-reported T2DM (data not shown), whereas our analyses using the aetiologic markers to define pre-diabetes/T2DM did not. Important advantages of the current analyses include the detailed inquiry of dairy product intake (i.e. ranging from the intake of skimmed dairy products to full-fat dairy products, non-fermented to fermented dairy products and milk to flavoured yogurt drinks), the relatively large range in dairy product intake, its huge sample size (n≥100 000) and the possibility to conduct well-powered stratified analyses for age (<50, 50–65 and ≥65 years), sex and BMI (<25, 25–30, ≥30 kg/m2). Moreover, the dairy product intake in this population was very comparable to the dairy product intake as estimated in the most recent Dutch Food Consumption Survey (i.e. 355 g/d)( Reference van Rossum 46 ), suggesting that the Lifelines population is a representative sample with respect to Dutch dairy product intakes. Finally, we had the possibility to include many potential covariates, including all other major food groups, while retaining sufficient power.
In conclusion, these detailed cross-sectional data on dairy products intake within the Lifelines Cohort Study showed inverse associations of skimmed dairy products, fermented dairy products and buttermilk with pre-diabetes. Moreover, positive associations were observed for full-fat dairy products, non-fermented dairy products and custard, and pre-diabetes. Finally, full-fat dairy products, non-fermented dairy products and milk were positively associated with ND-T2DM. On the basis of our results, it may be speculated that the aspect of fermentation is important to determine whether dairy products is beneficial for diabetes prevention or increases the risk. Future prospective analyses, focusing on a wide range of dairy products, within Lifelines, as well as other mega-cohorts, are wanted to verify the findings of the current study.
Acknowledgements
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines and all the study participants.
The epidemiological analyses were supported by a grant from FrieslandCampina. FrieslandCampina had no role in the design, analysis or writing of this article.
E. J. M. F. designed the research and had primary responsibility for final content. E. M. B.-B. analysed data and wrote the paper. D. S., C. M. S.-P. and E. J. M. F. reviewed and contributed to the manuscript. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003762







