Introduction
The Red-legged Cormorant Phalacrocorax gaimardi is a species endemic to South America. This species is found along the Pacific coast from Northern Peru (Isla Foca) to Southern Chile (Punta Elefante, Peninsula de Taitao), and along the Atlantic coast, where its range is restricted to Santa Cruz Province, Argentina (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002, Frere et al. Reference Frere, Quintana and Gandini2005). The world’s breeding population was estimated at 15,000 individuals (Frere et al. Reference Frere, Gandini, Ruiz and Vilina2004), while BirdLife International (2013) estimates a total population of 30,000 individuals. This small population has shown rapid declines owing to interactions with fishers and fisheries (entanglement in gear, competition with fishers and also when people take adults, chicks and eggs at a subsistence level), and for these reasons this species is classified as ‘Near Threatened’ (BirdLife International 2013). In the north of its range, the species has been detrimentally affected by adverse oceanographic conditions caused by El Niño Southern Oscillation (ENSO) events (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002). Particularly in northern Peru, dramatic declines of Red-legged Cormorant populations have been recorded owing to kelp die-off caused by rises in sea temperature (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002, BirdLife International 2013). While in 2000 the Peruvian population was estimated at 1,500-2,100 birds, between 1968 and 2000, the numbers at 10 localities in northern and central Peru declined from 3,229 to 69 birds. This sharp decline showed that the number of Red-legged Cormorants in Peru is seriously threatened (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002). For the year 2000, the total population estimated for Chile ranged between 5,018 and 5,218 breeding pairs (Frere et al. Reference Frere, Gandini, Ruiz and Vilina2004), but unsurveyed sites have been recently described in South Chile. Therefore, the total population of Chile might be a few thousand individuals (R. Barros and F. Diaz, pers. comm.). In Argentina, colonies of Red-legged Cormorants are found at 13 localities, from Monte Loayza (47°04’S, 66°17’W) to Monte León (50°23’S, 68°55’W). Colony size is variable, ranging between three and 600 breeding pairs (Millones et al. Reference Millones, Frere and Gandini2008). In Argentina, this species is considered as ‘Endangered’ (López-Lanús et al. Reference López-Lanús, Grilli, Coconier, Di Giacomo and Banchs2008). Although there are no estimates of mortality associated with human activities, about 93% of the Argentine population breeds near coastal cities, where the increase of urban and fish waste is believed to have favoured its main predator, the Kelp Gull Larus dominicanus (Frere et al. Reference Frere, Gandini, Ruiz and Vilina2004), which is known to heavily impact on breeding success (Frere and Gandini Reference Frere and Gandini2001).
Breeding seabirds can be affected by several oceanographic characteristics such as sea surface temperature and marine primary productivity (Brooke Reference Brooke, Schreiber and Burger2002, Croxall et al. Reference Croxall, Trathan and Murphy2002, Schreiber and Burger Reference Schreiber, Burger, Schreiber and Burger2002). Short- and long-term changes in sea surface temperatures influence seabird species abundances, breeding success and assemblages (Hunt et al. Reference Hunt, Priddle, Whitehouse, Veit and Heywood1992, Schreiber Reference Schreiber, Schreiber and Burger2002, Inchausti et al. Reference Inchausti, Guinet, Koudil, Durbec, Barbraud, Weimerskirch, Cherel and Jouventin2003). Changes in reproductive success and chick growth rates of seabirds have been related to changes in sea surface temperatures worldwide, probably due to changes in the distribution of fish (the most frequent prey for seabirds) (Schreiber Reference Schreiber, Schreiber and Burger2002). Long-term studies of seabirds indicate that inter-annual decreases in fledging success are correlated with sea surface temperatures above long-term averages in El Niño years (Schreiber and Schreiber Reference Schreiber and Schreiber1984, Schreiber Reference Schreiber1994, Smithers et al. Reference Smithers, Peck, Krockenberger and Congdon2003). In the Pacific Ocean, warming of sea water has been linked with a reduction in marine productivity (Peck et al. Reference Peck, Smithers, Krockenberger and Congdon2004, Quillfeldt et al. Reference Quillfeldt, Strange and Masello2007), which has in turn led to a high mortality of seabirds, lack of reproduction and dispersion (Apaza and Figari Reference Apaza, Figari, Tarazona and Castillo1999, Zavalaga et al. Reference Zavalaga, Frere and Gandini2002). On the Argentine coast, sea productivity has been identified as a factor that appears to influence the pattern of use of the breeding habitat by Red-legged Cormorants (Millones and Frere Reference Millones and Frere2012).
In this study, we provide information about recent population trends of the Red-legged Cormorant on the Argentine coast. We also discuss whether population trends could be related to sea temperature and productivity.
Methods
In Argentina, colonies of Red-legged Cormorants start to be occupied in late August and September. Egg-laying occurs from mid-October to mid-November and hatching occurs from mid-November to early December (Frere and Gandini Reference Frere and Gandini2001, Frere et al. Reference Frere, Quintana and Gandini2005). We analysed information available for the 13 colonies of the Red-legged Cormorant in Argentina. Data on the number of active nests of this species, obtained during the laying period, were available from 1990 to 2009. Data from 1990 were obtained from Yorio and Harris (Reference Yorio and Harris1997).
To detect the overall population trend (for the 13 colonies combined), available time series combined with missing observations were modelled using the program TRIM (Trends and Indices for Monitoring Data; Pannekoek and van Strien Reference Pannekoek and van Strien2005). This software is specifically developed to analyse monitoring data from incomplete counts, which is commonplace in ecological surveys. For our study, we used a model with a site-effect and a linear (on the log-scale) effect of time. To identify the changes in population trends across years, we started the analysis with a model with change points at each time-point, and used the stepwise selection procedure to identify change points with significant changes in slope based on Wald tests with a significance-level threshold value of 0.05 (Pannekoek and van Strien Reference Pannekoek and van Strien2005). We took into account over-dispersion and serial correlation, since they can have important effects on standard errors, although they usually have only a small effect on the estimates of parameters (Pannekoek and van Strien Reference Pannekoek and van Strien2005).
Annual population changes (λ) of the entire population and each individual colony were calculated as:
where Nt is the number of pairs breeding at time t, Nt+1 is the number of pairs breeding at time t+1 (Sibly and Home Reference Sibly and Home2002). Nt, and Nt+1 were given by TRIM as imputed counts (observation plus estimated values for missing counts).
We considered sea surface temperature and chlorophyll a concentration as suitable covariates to test for environmental effects on the variation of breeding population sizes. For the entire Red-legged Cormorant breeding range, we obtained monthly average data on sea surface temperatures. We used values from September and October of each breeding season and up to about 60 km from the coast. Data were obtained from the Physical Oceanography Distributed Active Archive Center Web (PO. DAAC), with a resolution of 18 km. We used chlorophyll a concentration (mg/m3) data in the sea as an index of ocean primary production along the breeding range of the Red-legged Cormorant (see Boersma et al. Reference Boersma, Rebstock, Frere and Moore2009). Values (with 0.6-degree spatial resolution), only available from 1997 to 2009, were obtained from the Ocean Productivity Website (http://www.science.oregonstate.edu/ocean.productivity). From September and October of each year, we obtained monthly average chlorophyll a concentration data close to the coast (up to about 60 km). The resolution of the values of sea surface temperature and chlorophyll a concentration we obtained was not high enough to show variations between neighbouring colonies. We decided to arrange all colonies into three coastal sectors: north, central and south. For each coastal sector and breeding period, we obtained mean surface temperature and mean chlorophyll a concentration close to the coast. The effects of sea surface temperature and chlorophyll a concentration on the breeding population size were examined by fitting generalised linear mixed models with Poisson error structure and log-link function. The analysis corresponded to data from 1997 to 2009. We found no significant correlation between these two environmental variables (P Pearson > 0.05). To avoid pseudoreplication, we introduced coastal sector and colony identity (nested in coastal sector) as random factors in this analysis. As expected, population time series were significantly positively autocorrelated with one year lag (autocorrelation function: P < 0.05). To account for autocorrelation, we included an autocorrelation structure of order 1 into each model. The models that best fit the data were selected using the Akaike’s Information Criterion with the small-sample bias adjustment (AICc) (Burnham and Anderson Reference Burnham and Anderson2002). For the particular analyses of La Mina colony, where data showed normality, we performed linear models with normal error structure and an autocorrelation structure of order 1. For model selection we used Akaike’s Information Criterion with the small-sample bias adjustment (AICc).
Results
The long-term trend of the overall population (the 13 colonies combined) showed a slight decline. Between 1990 and 2009, the breeding population of the Red-legged Cormorant showed a decrease of 1.2% per year (overall multiplicative slope = 0.9880 ± 0.005; CI 95% 0.9782-0.9978). Between these years, the overall estimated number of total breeding pairs declined from 1067 ± 66 to 945 ± 75 (Figure 1). Two significant change points were detected: 1997 and 2002 (Wald tests P = 0.0001 and 0.0007 respectively; Figure 1). Therefore the long-term trend can be separated into three major periods: i) between 1990 and 1997, during which the population was stable (overall multiplicative slope = 1 ± 0.0001); ii) between 1997 and 2002, during which the number of total breeding pairs declined from 1,106 ± 55 to 723 ± 61, at an average rate of -6.07 %; and iii) between 2002 and 2009, during which the number of breeding pairs increased from 723 ± 61 to 945 ± 75, with an annual average change of 3.3%.

Figure 1. Estimates of annual breeding population of Red-legged Cormorants from 1990 to 2009. Black dots indicate the number of annual breeding pairs for the entire population in Argentina. White dots indicate the number of annual breeding pairs for La Mina colony. Estimates were given by TRIM as imputed counts. Error bars are ± SE.
Median size and average population change were different between colonies (Table 1). Two colonies showed an average population change > 1 (∼ 2% of the entire population), three colonies showed an average population change < 1 and > 0 (∼ 62% of the entire population), and seven colonies showed an average population change < 0 (∼36% of the entire population). For the particular case of the colony of Cañadón Torcido, it was not possible to calculate the average population change. The colony of La Mina represented more than 55% of the entire breeding population of Red-legged Cormorants in Argentina. We observed that the entire population and the population of La Mina showed similar interannual variations (Figure 1).
Table 1. Location of Red-legged Cormorant colonies, their median size (number of breeding pairs) and average population change (%) between 1990 and 2009.

Neither of the environmental factors analysed (sea surface temperature and chlorophyll a concentration) affected the variation in the total estimated annual breeding pairs of Red-legged Cormorants. The global model was the best model describing the variation of breeding population sizes (Table 2), but neither environmental variable was an important predictor (all confidence intervals encompassed zero) (Table 3). Since La Mina is the largest colony and its trend was very similar to that of the entire population, we decided to analyse the effect of sea surface temperature and chlorophyll a concentration on its breeding population size (in a radius of approximately 30 km) for each month (September and October). When we considered sea surface temperature and chlorophyll a concentration for September, when individuals begin to settle, we observed that the lowest AICc model suggested a positive effect of chlorophyll a concentration on La Mina colony. Although the global model was the second-ranked (with ΔAICc = 4.4), only chlorophyll a concentration was an important predictor of the population sizes in La Mina colony (parameter estimates = 189.83 ± 75.31; CI 95% 39.22–340.45, excluding zero) (Tables 4a and 5a). When we considered sea surface temperature and chlorophyll a concentration for October, when egg laying occurs, although the best models included chlorophyll a concentration, this variable was not an important predictor of the population sizes in La Mina colony (parameter estimates = -25.38 ± 37.46; CI 95% -100.30–49.53, encompassed zero) (Tables 4b and 5b).
Table 2. Generalised linear mixed models explaining variation in the breeding population size of the Red-legged Cormorant from 1997 to 2009.
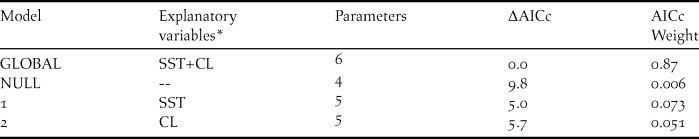
* SST: mean sea surface temperature; CL: mean chlorophyll a concentration.
Table 3. Estimated parameters (± SE) and 95% confidence interval limits (CIL) for explanatory variables describing the variation in the breeding population size of the Red-legged Cormorant from 1997 to 2009.

* SST: mean sea surface temperature; CL: mean chlorophyll a concentration.
Table 4. Linear models explaining variation in La Mina breeding population size during (A) September and (B) October, from 1997 to 2009.

* SST: mean sea surface temperature; CL: mean chlorophyll a concentration.
Table 5. Estimated parameters (± SE) and 95% confidence interval limits (CIL) for explanatory variables describing the variation in La Mina breeding population size during (A) September and (B) October, from 1997 to 2009.

* SST: mean sea surface temperature; CL: mean chlorophyll a concentration.
Discussion
We could recognise some periods of stability and others of increase, however our results suggest that the long-term trend (two decades) showed that the population of the Red-legged Cormorant decreased. A strong decline was observed between 1997 and 2002. In 2002, the breeding population was 32% lower than at the beginning of the study period. After that, the entire population showed a moderate increase, but insufficient to compensate for the decreases. In 2009, the breeding population of this species remained 11% lower than in 1990. Between 1990 and 2009, populations in seven of the colonies of Red-legged Cormorants on the Argentine coast showed a moderate decrease whereas populations in the other six colonies showed a stable state or moderate increase. The largest colony, located in La Mina, showed temporal fluctuations similar to those of the entire breeding population. The population in La Mina decreased between 1998 and 2002 (almost 50%), and then increased until 2009. The temporal fluctuations of this colony seem to determine the entire population trend on the Argentine coast. La Mina represents a key locality for the conservation of Red-legged Cormorants in this country. In 2012, the Marine Park Makenke, which includes this colony, was created and thus may contribute to the long-term conservation of the species.
In Peru, Red-legged Cormorants underwent a marked decline between 1968 and 2000. In several sites the numbers of individuals of this species declined by more than 95% (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002). These drastic declines occurred after the El Niño Southern Oscillation events (Zavalaga et al. Reference Zavalaga, Frere and Gandini2002). A characteristic of these events is the occurrence of above average sea surface temperatures that have been linked with a reduction in marine productivity (Peck et al. Reference Peck, Smithers, Krockenberger and Congdon2004, Quillfeldt et al. Reference Quillfeldt, Strange and Masello2007). Although we could not detect a direct relationship between the two oceanographic factors analysed (sea surface and ocean primary production close to the coast) and the overall number of breeding pairs of Red-legged Cormorants in Argentina, we detected a positive effect of ocean productivity close to the coast at a local spatial scale, and at the beginning of the breeding seasons. We observed a positive effect of ocean productivity close to the coast on the breeding population size of La Mina colony. The effect observed was detected only for September. During this month, colonies of Red-legged Cormorants start to be occupied. Variations in sea productivity should affect Red-legged Cormorant’s prey at the beginning of its breeding seasons, deterring several pairs to breed at their colonies. In a previous study, we observed that high productivity close to the coast should be beneficial for this species (Millones and Frere Reference Millones and Frere2012), which feeds in inshore waters, closer to their breeding colonies than other shags and cormorants (Frere et al. Reference Frere, Quintana, Gandini and Wilson2008,).
Further studies are needed to fully understand determinant factors affecting temporal variations in the Red-legged Cormorant populations. Probably, a complex combination of factors (human interactions, predation and environmental factors) is responsible for the Red-legged Cormorant population trend observed on the Argentine coast. In this study, we linked population variations with the ocean productivity at a local spatial scale and for a particular moment of the breeding season, suggesting that the coastal ocean productivity could be one of the determinant factors affecting temporal variations in the Argentinian population.
Acknowledgements
The Universidad Nacional de la Patagonia Austral, Wildlife Conservation Society and Fundación Antorchas funded this work. We thank Julio Lancelotti, Javier Ciancio, Adrián Pérez, Natalia Collm, Juan Pablo Martin, Néstor Juanola and Annick Morgenthaler for field assistance. We also thank V. Eusevi for English language assistance.








