A major issue for policy-makers concerns the implications of ageing on health-care expenditure. An important factor determining such expenditures is the extent to which ageing is associated with deteriorating health status and the development of disorders such as dementia. Use of population indicators such as ‘healthy life expectancy’ help to demonstrate the relationship between longer life and changes in health status (Reference Robine and RitchieRobine & Ritchie, 1991). Estimates already exist for several health conditions (Reference Bone, Bebbington and JaggerBone et al, 1995), including dementia (Reference Ritchie, Robine and LetenneurRitchie et al, 1994; Reference Perenboom, Boshuizen and BretelerPerenboom et al, 1996). However, there have been few attempts to link these with age-specific health care utilisation and cost data. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) and the Resource Implications Study (RIS) of the MRC CFAS were designed with these aims partly in mind (MRC CFAS, 1998; Reference McNamee, Gregson and BuckMcNamee et al, 1999). Based on a population subgroup identified as cognitively impaired from these studies, this paper provides estimates of current and future costs of dementia based on calculations of life expectancy with dementia (LEWD) and dementia-free life expectancy (DFLE).
METHOD
Calculation of life expectancy with dementia (LEWD) and dementia-free life expectancy (DFLE)
Both LEWD and DFLE were calculated using Sullivan's method (Reference Mathers and RobineMathers & Robine, 1997): age— and gender-specific prevalence rates for dementia were applied to life tables to divide years of life lived by a period life table cohort at different ages into years with and without dementia. This method produces valid estimates of population health projections, assuming smooth and regular changes in disability prevalence over time (Reference Mathers and RobineMathers & Robine, 1997). The model provides cohort-specific expected dementia years, calculated for people with and without dementia. The underlying assumption of this approach is that individuals move across cohorts over time, with the model reflecting entry to and exit from the population of people with dementia for each cohort.
Population projection, dementia prevalence and case definition
Population estimates were provided by national sources, which assume reductions in future mortality (Office of Population Censuses and Surveys, 1996a ). Life expectancy estimates were produced by extrapolating average age/gender-specific changes during 1961-1991 to 2001-2031 (Office of Population Censuses and Surveys, 1996b ). Age— and gender-specific prevalence rates were estimated using data collected in a multi-centre study of four areas of England (Cambridgeshire, Newcastle, Nottingham and Oxford) and one area in Wales (Gwynedd). Diagnosis of dementia was made using the Geriatric Mental State (GMS version B3; Reference Copeland, Kelleher and KelletCopeland et al, 1976), from which the Automated Geriatric Examination Computer Assisted Taxonomy (AGECAT; Reference Copeland, Dewey and Griffiths-JonesCopeland et al, 1986) could be derived. Prevalence estimates were based on a level of three or more on the organic section of AGECAT, which is approximately equivalent to moderate (or more) clinical dementia. An age-stratified (65-74 years and ≥ 75 years) sample of 2500 individuals was selected randomly from Family Health Services Authority or general practice files in the five centres. Individuals in long-term hospital care were included because they remain registered with their general practitioner (GP) for at least 2 years after admission. A census of long-stay hospitals serving the catchment area of the study populations identified less than 20 long-term residents not already identified from GP lists. Further information on study design is reported elsewhere (MRC CFAS, 1998).
Measurement of service use and costs
Service use and cost data were collected in a 5-year longitudinal Resource Implications Study (RIS), part of the previous multi-centre study. Methods of data collection and unit cost estimation are described in a separate paper (Reference McNamee, Gregson and BuckMcNamee et al, 1999). Briefly, however, data relating to the frequency and/or duration of subjects' use of health care and personal social services over 2 years or until death (whichever was earlier) were collected by fieldworkers at regular intervals using local records between 1991 and 1995. Subjects included people living in private households or resident in long-term care. The services costed were in-patient stays, out-patient visits, residential home care, nursing home care, domiciliary home care, day care, day hospital care, district nursing, sitting services, meals on wheels, respite care, GP care, chiropody, community psychiatric nurse care, physiotherapy, social work care, occupational therapy, health visitor care and laundry and incontinence services.
The epidemiological cost model
Assumptions and methods of calculation are featured in Table 1. Estimation required five stages: first, the total number of years lived for each cohort (Y) was calculated by taking the difference between the product of life expectancy (LE) and the total number of survivors of successive cohorts; second, the number of years lived with dementia (YD) for each cohort was calculated by taking the product of the total number of years lived (Y) and the cohort-specific dementia prevalence rate; third, DFLE was calculated by subtracting the number of years lived with dementia (YD) from the total remaining number of years lived (Y), followed by division of the total number of dementia-free years (Y—YD) by the total population alive at the beginning of each cohort; fourth, cohort-specific LEWD per person was estimated by subtracting the DFLE from the remaining life expectancy (LE); and finally, cohort-specific care costs per year were applied to each cohort's LEWD, which was then multiplied by the total number of people with dementia for that cohort, to produce aggregate estimates of cohort-specific care costs.
Table 1 Parameters used to calculate baseline 1994 estimates

| Age (years) | Population (× 103) | Dementia prevalence (%) | Mean costs per year (£) | Life expectancy (years) | Dementia-free life expectancy (years) | Expectation of life with dementia (years) | Total costs per year (£ million) |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 65-69 | 1110 | 0.014 | 7097 | 14.8 | 14.1 | 0.7 | 76 |
| 70-74 | 999 | 0.031 | 8554 | 11.4 | 10.7 | 0.7 | 183 |
| 75-79 | 581 | 0.056 | 3201 | 9.0 | 8.1 | 0.9 | 89 |
| 80-84 | 404 | 0.102 | 6333 | 6.6 | 5.7 | 0.9 | 230 |
| 85+ | 228 | 0.196 | 10921 | 3.9 | 3.1 | 0.8 | 373 |
| Women | |||||||
| 65-69 | 1253 | 0.015 | 2670 | 18.5 | 17.0 | 1.5 | 75 |
| 70-74 | 1282 | 0.022 | 3248 | 14.6 | 13.2 | 1.4 | 129 |
| 75-79 | 891 | 0.071 | 6646 | 11.5 | 9.7 | 1.8 | 767 |
| 80-84 | 765 | 0.141 | 10105 | 8.5 | 6.7 | 1.8 | 1940 |
| 85+ | 689 | 0.275 | 9762 | 4.8 | 3.5 | 1.3 | 2440 |
Cost projections to 2031
To extrapolate costs to four different periods between 2001 and 2031, different population and life expectancy values were used, which were provided by national sources (Office of Population Censuses and Surveys, 1996a , b ) holding age/gender-specific prevalence rates, service utilisation and costs constant. The effects of these assumptions on the results were tested by specifying different prevalence rates and levels of service utilisation. In the absence of clear evidence on age— and gender-specific time trends, analysis was conducted to determine the required change in prevalence rates in order to produce constant costs over time.
To determine factors associated with service use and costs, two separate multivariate analyses were undertaken. For individuals in their own homes, the log of the total cost was used as the dependent variable in a multiple regression analysis. For the total study sample, logistic regression was undertaken to explore the relationship between residence in long-term care and a range of covariates (see Table 2). Parameter estimates from these models were used to determine the effects of changes in service utilisation on cost projections.
Table 2 Variables used in multivariate analyses

| Label | Description |
|---|---|
| Subjects in own home | |
| Dependent | |
| Log cost | Log cost per week |
| All subjects | |
| Dependent | |
| Long-term care | =1 if in own home throughout study period, =0 otherwise |
| Independent | |
| Structure1 | =1 if lives alone, =0 otherwise |
| Carer input1 | =1 if daily, =2 if two or three times per week, =3 if at least weekly, =4 if at least monthly, =5 if less often |
| Network1 | =1 if locally self-contained or private restricted, =0 otherwise |
| Frailty type | =1 if physically and mentally frail, =0 otherwise |
| Housing tenure | =1 if owned, =0 otherwise |
| Died | =1 if died during study period, =0 otherwise |
| Education | Years in full-time education |
| ADL | Activities of Daily Living Scale |
| MMSE | Mini-Mental State Examination Scale |
RESULTS
Current and projected costs, assuming no changes in prevalence and health status
Total direct health care and personal social service costs amounted to £ 0.95 billion for men and £5.35 billion for women in 1994. Assuming that the national population projections for 2031 are reasonable estimates and that there are no changes in dementia prevalence, levels of mental and physical functioning, service utilisation and the unit costs of resources, costs in 2031 are forecast to be £2.34 billion (men) and £ 11.20 billion (women) at 1994/1995 prices. This represents a greater than twofold increase, compared with a rise in population aged 65 years or over of 53%. Figure 1 shows that steady rises of £1-1.4 billion occur every decade up to 2021, after which more dramatic increases occur, mostly due to greater numbers of women aged 80 years or over.
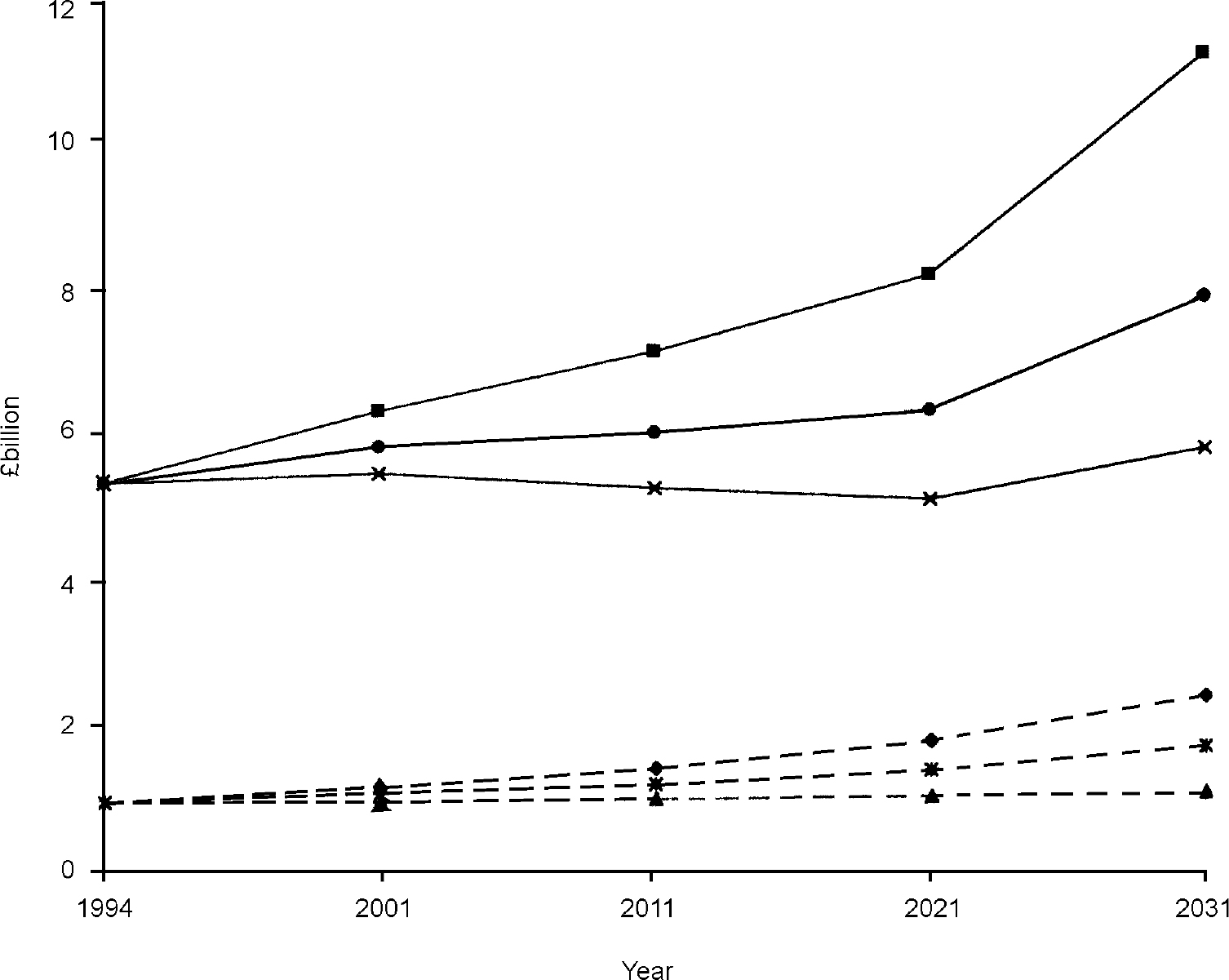
Fig. 1 Projected change in costs of dementia: (♦) men, base case; (▪) women, base case; (▴) men, reduced prevalence; ([UNK]) women, reduced prevalence; ([UNK]) men, improved health; (•) women, improved health.
Effects of changes in dementia prevalence rates on costs
In order to keep costs approximately constant, future dementia prevalence rates would need to decline smoothly over each decade by 0.5%, 1% and 2% for people aged 75-79 years, 80-84 years and 85 years or over, respectively. The effects on costs resulting from this level of changing prevalence for those aged 75 years or over are shown in Table 3, along with the effects of a similar increase in prevalence. An increase was shown to lead to greater divergence from base case estimates over time. Decreases in prevalence led to relatively stable costs of £91-77 million (£773-760 million), £211-197 million (£1840-1950 million) and £391-381 million (£2630-2780 million) for men (women) over the period 2001-2031, respectively. Increases in prevalence produced steadily increasing costs of £132-372 million (£1030-2450 million), £315-1060 million (£2450-6340 million) and £ 589-2160 million (£3520-9210 million) for men (women) over the period 2001-2031, respectively.
Table 3 Effect of changes in prevalence on costs

| Age (years) | Year | Men | Women | ||
|---|---|---|---|---|---|
| % Dementia prevalence | Total costs (£ million) (base case) | % Dementia prevalence | Total costs (£ million) (base case) | ||
| 75-79 | 2001 | 0.051-0.061 | 91-132 (111) | 0.066-0.076 | 773-1030 (896) |
| 2011 | 0.046-0.066 | 82-175 (124) | 0.061-0.081 | 697-1240 (947) | |
| 2021 | 0.041-0.071 | 81-255 (156) | 0.056-0.086 | 708-1680 (1140) | |
| 2031 | 0.036-0.076 | 77-372 (197) | 0.051-0.091 | 760-2450 (1490) | |
| 80-84 | 2001 | 0.092-0.112 | 211-315 (261) | 0.131-0.151 | 1840-2450 (2140) |
| 2011 | 0.082-0.122 | 202-451 (314) | 0.121-0.161 | 1730-3080 (2360) | |
| 2021 | 0.072-0.132 | 184-630 (374) | 0.111-0.171 | 1640-3930 (2660) | |
| 2031 | 0.062-0.142 | 197-1060 (541) | 0.101-0.181 | 1950-6340 (3830) | |
| 85+ | 2001 | 0.255-0.295 | 391-589 (485) | 0.255-0.295 | 2630-3520 (3060) |
| 2011 | 0.235-0.315 | 402-921 (635) | 0.235-0.315 | 2610-4680 (3570) | |
| 2021 | 0.215-0.335 | 381-1350 (791) | 0.215-0.335 | 2490-6040 (4070) | |
| 2031 | 0.195-0.355 | 381-2160 (1090) | 0.195-0.355 | 2780-9210 (5530) | |
The implications of reductions in prevalence are also highlighted in Fig. 1, showing that sustained reduction in prevalence among the group aged 75 years or over are required to ensure that costs remain similar to 1994 levels. Similar increases in prevalence increase the total costs year-on-year, rising to £4.26 billion and £18.5 billion by 2031 for men and women aged 65 years or over, respectively (not shown in Fig. 1).
Effects of changes in health status on service use and costs
Multiple regression analysis demonstrated that levels of mental and physical functioning, measured by the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) and the Activities of Daily Living Score (ADL; Reference Bond and CarstairsBond & Carstairs, 1982), were the factors most significantly associated (adjusted R 2=25%, F=31.3, d.f.=181; P<0.0001) with variation in expected log costs per week. The regression estimate was:
In other words, for each one-point increase in ADL and MMSE score, the data suggest that predicted log average weekly costs decrease by 15% and 9%, respectively.
The probability of living in long-term care (LTC) was related significantly to mental and physical health, and was calculated as:
This equation suggests that for MMSE 5 (10) and ADL 5 (10), the probability that a respondent lives in long-term care is 0.64 (0.42).
These equations were used with age/gender-specific sample means for ADL, MMSE, community and long-term care costs to estimate the effect on costs of changes in ADL and MMSE. Projecting these changes through 2001-2031, using an assumption that ADL and MMSE both improve smoothly by 0.5 per decade, Fig. 1 depicts the effect on costs. Compared with the baseline, costs are shown to rise but at a much slower rate. In particular, costs for women remain reasonably stable through 2001-2021 but rise steeply thereafter to £7.87 billion in 2031.
DISCUSSION
Comparison of the results with previous studies
Using Sullivan's method to calculate DFLE, costs of formal care for older people with dementia total £6.3 billion per year. Owing to variations in methodology, it is difficult to compare our estimates with previous studies (Reference Wimo, Ljunggren and WinbladWimo et al, 1997). However, when allowance is made for type of population and service coverage, they appear larger than previous estimates produced for England and Wales that report formal care costs of £1 billion for Alzheimer's disease (Reference Gray and FennGray & Fenn, 1993) and £ 4.4 billion for people with advanced cognitive impairment (Reference Kavanagh, Schneider and KnappKavanagh et al, 1993). Examination of variation in survey design suggests that recall bias and the time period in which data were collected could explain such differences.
Three previous estimates of DFLE for the UK have been reported. Compared with estimates produced in this paper, LEWD was lower in Liverpool and Cambridge (Reference Jagger, Ritchie and Bronnum-HansenJagger et al, 1998), whereas in Melton Mowbray it was higher (Reference Bone, Bebbington and JaggerBone et al, 1995). One explanation for these differences relates to study design in the diagnosis of dementia. In the case of Liverpool, case definition was made at the screening phase, which led to different assumptions with respect to missing data. This resulted in lower prevalence estimates across all age groups for both genders. For Melton Mowbray, estimates were made inclusive of mild dementia, which led to higher prevalence and dementia life-year estimates. The lower Cambridge estimate is more difficult to explain, because this relates to earlier work conducted in one of the centres using the same study design. The most likely cause relates to low prevalence for a number of vascular problems (MRC CFAS, 1998).
Assumptions of the epidemiological cost model
Our baseline projections of formal care costs reveal that, considering only changes in the number of older people, the percentage increase in total costs can be expected to outstrip the percentage increase in the total number of older people. However, this finding is dependent on assumptions made about prevalence rates and levels of mental and physical health. The precision of the five centre prevalence rates used in the baseline estimates is unknown because no published confidence intervals are available. However, prevalence estimates for this sample show the same relationship with age and are within the range of estimates produced by other comparable studies (Reference KayKay, 1995).
The relative impact of different assumptions on the results was explored, although the plausibility of the selected ranges requires comment. With respect to prevalence rates, no apparent upward or downward trend over time is noted in the literature. We therefore determined what rates were required to produce approximately constant costs given a projected increase in population size. As such, their plausibility can be tested only empirically. As a result of reported trends (Reference Manton, Corder and StallardManton et al, 1997), improvement in physical health over time is perhaps more likely than sustained changes in prevalence. Such changes also could lead to better cognitive functioning, which could be facilitated by development of new drug therapies. Ultimately, however, exercises of this type are speculative and are conducted in order to test the extent to which baseline estimates change in relation to the magnitude of changes in assumptions.
Related to the above point, it is important to note that projections are based on national life tables adjusted for future decreases in mortality. It has been shown elsewhere that population growth usually is underestimated, especially at higher ages (Reference Ritchie, Robine and LetenneurRitchie et al, 1994). If all else remains equal, this is likely to mean that our estimates are conservative.
Future changes in dementia prevalence, health status, service use and costs
The baseline estimate projects that costs will amount to £13.5 billion in 2031 at 1994/1995 prices. Because evidence of changes in prevalence and incidence over time is contradictory (Reference Kokmen, Beard and O'BrienKokmen et al, 1993), the effects of both upward and downward trends were modelled. It is apparent, however, that evidence of the beneficial effect of time spent in formal education and the development of dementia (Reference KatzmanKatzman, 1993), together with reductions in vascular and cerebrovascular risk factors (Reference Skoog, Lenfelt and LandahlSkoog et al, 1996), suggests that the underlying trend could be downward. However, prediction of whether changes in risk factors and incidence will produce changes in prevalence depends on trends in survival times.
An equally important source of change is likely to relate to changes in age-specific utilisation rates of formal care, particularly care in residential and nursing homes. Logistic regression demonstrated that subjects in long-term care were more likely to have poorer mental and physical health. Improvements in physical health among older people over time have been demonstrated elsewhere (Reference Bone, Bebbington and JaggerBone et al, 1995; Reference Manton, Corder and StallardManton et al, 1997) and could be expected to reduce the probability of admission to long-term care. Equally, however, other changes in the structure of the population might be expected to reduce the supply of non-spouse carers (Reference Allen and PerkinsAllen & Perkins, 1995). In addition, there is some evidence that admission to long-term care has shown an increasing trend over recent years (Reference Grundy and GlaserGrundy & Glaser, 1997).
Future sustainability of public funding for the care of frail older people
As highlighted by the Royal Commission on Long Term Care (1999), a key policy question is whether growing numbers of frail older people relative to the size of the labour force might require changes in the proportion of public v. private financing of health and social care. To assess this issue, a number of factors likely to exhibit change over time need to be considered.
First, it is apparent that reasonably small reductions in prevalence rates year-on-year would be required to produce an equivalent ratio between the population of working age and the number of people with dementia aged 65 years or over in 2031 compared with 1994. Based on a working age population of 30.3 million and 12.6 million people aged 65 years or over in 2031 (Office of Population Censuses and Surveys, 1996a ), the overall dementia prevalence rate would have to reduce by 2.5% (equivalent to 0.06-0.07% per annum) to 4.1% in the population aged 65 years or over.
A further factor to consider is that age at death may be associated negatively with expenditure (Reference Lubitz, Beebe and BakerLubitz et al, 1995). Age at death has been increasing in developed countries (Reference Wilmoth, Wachter and FinchWilmoth, 1997). It will be important to monitor these changes to assess the implications for projections in healthy life expectancies and health-care expenditure.
Finally, the above projections are necessarily partial in nature. The health and health-care implications of population ageing can be expected to have wider effects on the economy, such as levels of saving, which can be expected to influence rates of economic growth (Reference DisneyDisney, 1996). Studies considering such relationships therefore are required to assess the capacity of the economy to provide current levels of formal care support in the future.
The effects of ageing on an age-related disorder such as dementia are likely to increase health and personal social service costs, but the level of such an increase is related to a wide range of demographic and economic factors. Given the current level of uncertainty, it would be unwise to make apocalyptic judgements regarding the effects of ageing in general, and dementia in particular, on health-care expenditure.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• For all individuals currently aged over 65 years, average life expectancy with dementia ranges from 0.7-0.9 years for men compared with 1.3-1.8 years for women.
-
• The use of health and social care resources is associated significantly with the severity of mental and physical functioning.
-
• The future workload of health-care professionals who care for individuals with dementia is likely to depend upon changes in risk factors for dementia and the development of therapies that alter the natural history of disease.
LIMITATIONS
-
• The cost model assumes that the impact of policy changes that affects the future demand and supply of services for people with dementia is cost neutral.
-
• As it is difficult to estimate the magnitude of risk factors among future cohorts and the extent to which new therapies alter disease history, predictions about future costs should be interpreted with caution.
-
• To understand the future sustainability of public funding for health and social care services for frail older people, further work is required to assess the impact of demographic changes over the wider economy.
Acknowledgements
The authors thank the Medical Research Council and Department of Health for their support and financial assistance in this study and the older people and their informal carers who took the time and trouble to participate. In addition, our gratitude goes to Cherie McCracken, National Coordinator of CFAS, and to the study fieldworkers (Pat Clark, Linda DeFries, Janet Hornsby, Shirley Martin and Katharine Wilton). We would also like to thank Barbara Gregson, Claire Bamford, Ken Wright, Carol Brayne, Paul Fenn, Tony Johnson and two anonymous referees for helpful comments on earlier versions of this paper. Any errors and omissions remain the sole responsibility of the authors.







eLetters
No eLetters have been published for this article.