Over 180 million people worldwide have diabetes, and if this health problem is not addressed, it is estimated that this number will more than double by 2030(1). The increasing prevalence of type 2 diabetes is associated with the obesity epidemic, and diagnosis is occuring at increasingly younger ages(Reference Silink2). The aim of diabetes management is to normalise blood glucose levels since improved blood glucose control is associated with a reduction in the development and progression of metabolic and other complications including retinopathy, nephropathy, neuropathy and CVD(Reference Stratton, Adler and Neil3). Nutritional factors affect blood glucose levels; however, there is currently no universally agreed approach to the dietary management of diabetes(4). Different carbohydrate (CHO) foods can be ranked by their overall effect on blood glucose levels using the glycaemic index (GI)(Reference Jenkins, Wolever and Taylor5). By contributing a gradual supply of glucose to the bloodstream, and hence stimulating lower, more sustained insulin release, low-GI foods such as lentils, beans and oats may contribute to improved glycaemic control compared with high-GI foods, such as white bread(Reference Jenkins, Wolever and Taylor5). Low-GI diets may also increase insulin sensitivity by minimising fluctuations in blood glucose levels and reducing the secretion of insulin over the day(Reference Crapo, Reaven and Olefsky6).
There is controversy about the utility of a low-GI diet in meal planning for people with diabetes. The authors of one Cochrane systematic review concluded that there were no high-quality data on the efficacy of diet alone for the treatment of type 2 diabetes(Reference Nield, Moore and Hooper7), but low-GI diets were not considered in that review. In another review, it was concluded that low-GI diets exert a small, but clinically useful, effect on medium-term glycaemic control in diabetes(Reference Brand-Miller, Hayne and Petocz8), and other reviews have addressed related health issues(Reference Anderson, Randles and Kendall9–Reference Opperman, Venter and Oosthuizen12).
The most recent position statement from the American Diabetes Association maintains that glycaemic control is best attained by monitoring total CHO intake via CHO counting, CHO exchange or experience-based estimation, and that use of a low-GI diet may provide only a modest secondary benefit above consideration of total CHO alone(4).
The aim of this systematic review was to assess the effects of low-GI diets primarily on glycaemic control (measured by glycated Hb (HbA1c), fructosamine or glycated serum albumin (GSA)) in people with diabetes. It is an updated version of a Cochrane review by the same authors(Reference Thomas and Elliott13), including a more recent large-scale randomised controlled trial (RCT).
Methods
Identification of studies
We conducted electronic searches of the Cochrane Library (issue 1, 2009), MEDLINE (1950 to March 2009), EMBASE (1988 to March 2009) and CINAHL (1982 to March 2009). We used a search strategy, with no restriction on language, that included the identification of any relevant systematic reviews and meta-analyses as well as the identification of eligible studies(Reference Thomas and Elliott13).
Studies eligible for inclusion were RCT with an intervention lasting 4 weeks or longer, which compared a low-GI diet with a higher GI diet for people with diabetes. Studies were excluded if the intervention was only a generalised recommendation to increase the proportion of low-GI foods in the diet without provision of explicit detail; if the intervention was either not directly supervised or well-documented, for example, through the use of food diaries or the provision of food; if there was a co-intervention in the experimental group that was not applied to the control group also; or if the diabetes was already optimally controlled at the start of the study (participants had HbA1c levels < 6·5 %).
Outcome measures
HbA1c was used as the main measure of glycaemic control (the primary outcome of interest) for studies where the intervention lasted more than 6 weeks. Fructosamine or GSA levels were used, when provided, as the measure of glycaemic control for studies if the intervention lasted 6 weeks or less, since in this time frame, fructosamine or GSA levels are more reliable indicators of glycaemic control than glycation of Hb(Reference Goldstein, Little and Lorenz14, Reference Winocour, Bhatnagar and Kalsi15). The turnover of human serum albumin is much shorter (half-life 14–20 d) than that of Hb (erythrocyte life span 120 d), so the degree of glycation of serum proteins (mostly albumin), indicated by fructosamine or GSA, better reflects the level of glycaemia over short time periods than does glycation of Hb(Reference Goldstein, Little and Lorenz14). Nevertheless, it has been reported that measurements of total glycated serum protein and GSA correlate well with one another and with measurements of HbA1c(Reference Goldstein, Little and Lorenz14).
Secondary outcomes of interest included adverse effects, insulin action and quality of life.
Selection of studies
Two reviewers independently reviewed the abstracts from the literature searches to identify potentially eligible studies. Any study that did not fulfil the defined inclusion criteria was eliminated, i.e. it was not an RCT, did not involve people with diabetes, had no comparator, included a co-intervention in only one arm or had an intervention period of < 4 weeks (Fig. 1)(Reference Moher, Cook and Eastwood16).

Fig. 1 Adapted quality of reporting of meta-analysis flow chart of study selection. RCT, randomised controlled trials; HbA1c, glycated Hb.
Quality assessment
Two reviewers independently assessed the quality of each included trial based on specific criteria(Reference Jadad, Moore and Carroll17, Reference Schulz, Chalmers and Hayes18), namely minimisation of selection bias, attrition bias and detection bias. In dietary intervention studies, blinding of participants and investigators is generally difficult, hence blinding was not included as a quality criterion. Blinding of outcome assessors, where mentioned, was recorded.
We tested for heterogeneity between trial results using the standard χ2 test to examine whether any variation in study results could be due to the variation expected by chance alone, with significance level set at α = 0·1. Quantification of the effect of heterogeneity was assessed by means of I 2(Reference Higgins and Thompson19). Publication bias was assessed by examining funnel plot asymmetry(Reference Cooper and Hedges20, Reference Tang and Liu21).
Statistical analysis
All data were initially analysed using a fixed effect model. Meta-analysis of trial results was done when appropriate, that is if data were available from more than one trial, and results were sufficiently homogeneous and of sufficient quality. For dichotomous outcomes, we had planned to express effect size in terms of relative risk with 95 % CI, but no relevant dichotomous outcomes were reported in the included trials.
Results
Description of studies
From the initial search, 2944 records were identified. From the abstracts of these records, we identified thirty-three papers for examination of the full text. The other studies were excluded because they were not relevant to the question under study in this review; they were duplicate papers; some or all the participants did not have diabetes; they had no control group or no randomisation; they did not compare similar groups; there was a co-intervention that was not applied to both groups or the duration of the intervention was < 4 weeks. All twelve studies identified for inclusion in the review were RCT(Reference Brand, Colagiuri and Crossman22–Reference Jenkins, Kendall and McKeown-Eyssen33). They were conducted in Australia(Reference Brand, Colagiuri and Crossman22, Reference Collier, Giudici and Kalmusky23, Reference Gilbertson, Brand-Miller and Thorburn27, Reference Luscombe, Noakes and Clifton29), Canada(Reference Wolever, Jenkins and Vuksan32, Reference Jenkins, Kendall and McKeown-Eyssen33), France(Reference Fontvieille, Rizkalla and Penfornis24, Reference Rizkalla, Taghrid and Laromiguiere31), Italy(Reference Giacco, Parillo and Rivellese26), Mexico(Reference Jimenez-Cruz, Bacardi-Gascon and Turnbull28), Thailand(Reference Komindr, Ingsriswang and Lerdvuthisopon30) and UK(Reference Frost, Wilding and Beecham25). The duration of the dietary intervention ranged from 4(Reference Luscombe, Noakes and Clifton29–Reference Rizkalla, Taghrid and Laromiguiere31) to 52 weeks(Reference Gilbertson, Brand-Miller and Thorburn27).
Quality of studies
No trial included in the review reported any significant differences between characteristics of participants in the treatment groups at baseline. Although all the included trials were described as randomised, only two reported the method of randomisation(Reference Frost, Wilding and Beecham25, Reference Jenkins, Kendall and McKeown-Eyssen33). One study reported the method of allocation concealment(Reference Jenkins, Kendall and McKeown-Eyssen33). Eight studies were analysed as intention to treat(Reference Brand, Colagiuri and Crossman22–Reference Fontvieille, Rizkalla and Penfornis24, Reference Gilbertson, Brand-Miller and Thorburn27, Reference Komindr, Ingsriswang and Lerdvuthisopon30–Reference Jenkins, Kendall and McKeown-Eyssen33). Two studies reported that the assessors were blinded(Reference Gilbertson, Brand-Miller and Thorburn27, Reference Jenkins, Kendall and McKeown-Eyssen33). In studies that had participants lost to follow-up, reasons were given(Reference Frost, Wilding and Beecham25, Reference Gilbertson, Brand-Miller and Thorburn27–Reference Luscombe, Noakes and Clifton29, Reference Jenkins, Kendall and McKeown-Eyssen33), except in one study(Reference Giacco, Parillo and Rivellese26).
Participants
The twelve included studies involved a total of 612 participants. Three studies had participants with type 1 diabetes(Reference Collier, Giudici and Kalmusky23, Reference Giacco, Parillo and Rivellese26, Reference Gilbertson, Brand-Miller and Thorburn27), eight studies had participants with type 2 diabetes(Reference Brand, Colagiuri and Crossman22, Reference Frost, Wilding and Beecham25, Reference Jimenez-Cruz, Bacardi-Gascon and Turnbull28–Reference Jenkins, Kendall and McKeown-Eyssen33), and one study had participants with either type 1 or type 2 diabetes(Reference Fontvieille, Rizkalla and Penfornis24). Two studies involved children, all of whom had type 1 diabetes(Reference Collier, Giudici and Kalmusky23, Reference Gilbertson, Brand-Miller and Thorburn27).
Interventions
Ten studies compared the low-GI diet to a higher-GI diet(Reference Brand, Colagiuri and Crossman22–Reference Giacco, Parillo and Rivellese26, Reference Jimenez-Cruz, Bacardi-Gascon and Turnbull28–Reference Wolever, Jenkins and Vuksan32). In one study, the control diet was a measured CHO exchange diet(Reference Gilbertson, Brand-Miller and Thorburn27), and in another study, the control diet was a high-cereal fibre diet (Table 1)(Reference Jenkins, Kendall and McKeown-Eyssen33).
Table 1 Glycaemic index (GI) of intervention and control diets in the included studies
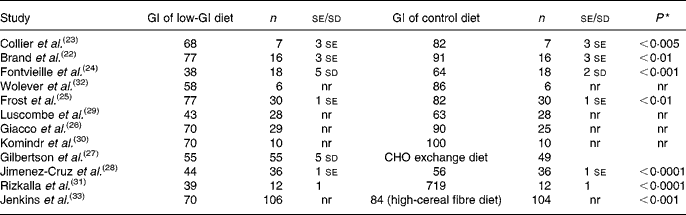
n, Number of participants in group; nr, not reported, CHO, carbohydrate.
* P-value relates to the difference in GI between the two diets.
Glycaemic control
Data were pooled from the seven studies that reported on HbA1c, the primary outcome of interest, in participants whose HbA1c was not optimised at baseline (n 457)(Reference Brand, Colagiuri and Crossman22, Reference Giacco, Parillo and Rivellese26–Reference Jimenez-Cruz, Bacardi-Gascon and Turnbull28, Reference Komindr, Ingsriswang and Lerdvuthisopon30, Reference Rizkalla, Taghrid and Laromiguiere31, Reference Jenkins, Kendall and McKeown-Eyssen33) (Fig. 2). Compared with people who received higher GI diets, there was a significant decrease in % HbA1c levels in people who received low-GI diets, indicating improved glycaemic control in the low-GI group (WMD − 0·4 % HbA1c, 95 % CI − 0·7, − 0·2, P = 0·001) (Fig. 2). In the study that compared a low-GI diet with a CHO exchange diet, the mean HbA1c level had decreased significantly by 12 months in the low-GI group than in the CHO exchange group (P = 0·05)(Reference Gilbertson, Brand-Miller and Thorburn27). Twice as many participants in the low-GI group (45 %) attained acceptable HbA1c levels than participants in the CHO exchange group (22 %; P = 0·02 after adjustment for baseline values).
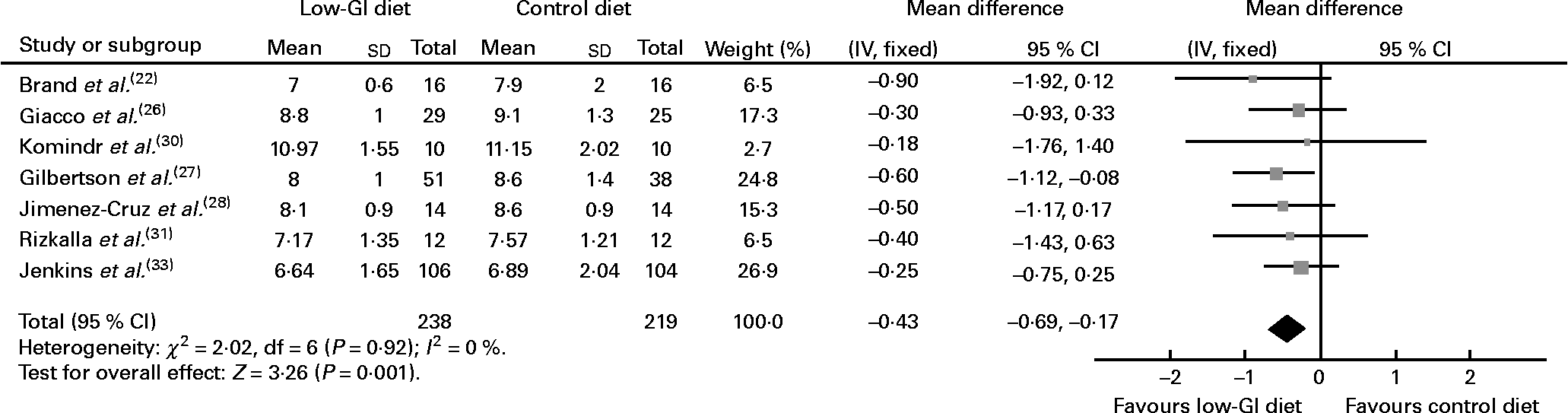
Fig. 2 Change in glycated Hb (% HbA1c) in people with diabetes on low-glycaemic index (GI) diet compared with that in people with diabetes on high-GI or other diet.
In the four studies reporting the results for glycaemic control as fructosamine (n 141), three were 6 weeks or less in duration(Reference Fontvieille, Rizkalla and Penfornis24, Reference Frost, Wilding and Beecham25, Reference Luscombe, Noakes and Clifton29, Reference Wolever, Jenkins and Vuksan32). Pooled data showed a decrease in fructosamine (WMD − 0·23 mmol/l, 95 % CI − 0·47, 0·00, P = 0·05) with a low-GI diet than with a high-GI diet (Fig. 3)(Reference Fontvieille, Rizkalla and Penfornis24, Reference Frost, Wilding and Beecham25, Reference Luscombe, Noakes and Clifton29, Reference Wolever, Jenkins and Vuksan32).
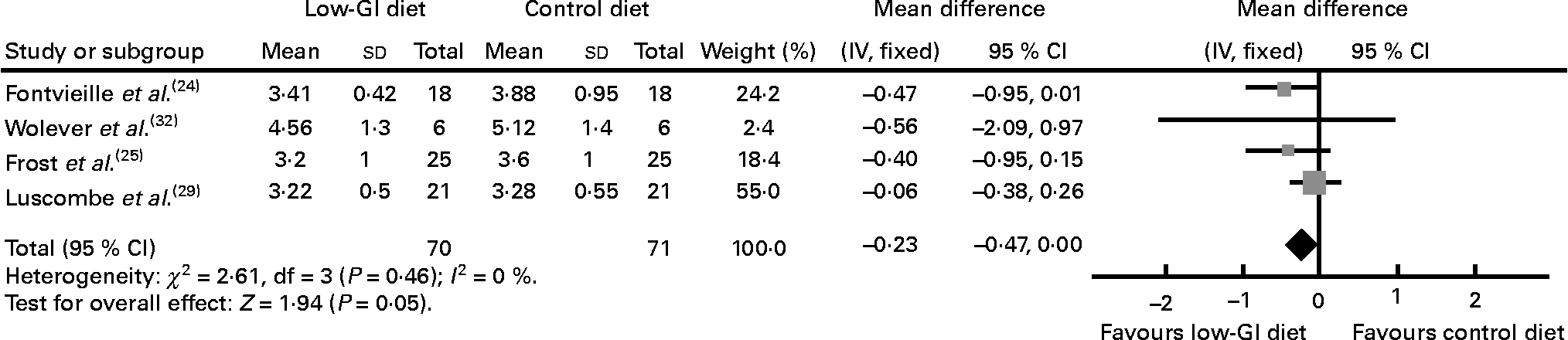
Fig. 3 Change in fructosamine (mmol/l) in people with diabetes on a low-glycaemic index (GI) diet compared with that in people with diabetes on a high-GI or other diet.
Glycosylated albumin levels decreased significantly with the low-GI intervention, but not with the high-GI comparison, in the one study that reported this outcome (glycosylated albumin with the low-GI diet was 13·2 (sem 1·5) to 10·7 (sem 2·2) %, P < 0·05; and with the high-GI diet was 13·1 (sem 2·3) to 14·6 (sem 1·9) %, NS)(Reference Collier, Giudici and Kalmusky23).
Adverse effects
Two included trials with participants with type 1 diabetes reported adverse effects(Reference Giacco, Parillo and Rivellese26, Reference Gilbertson, Brand-Miller and Thorburn27). In the meta-analysis for episodes of hypoglycaemia, there was heterogeneity (I 2 = 50·8 %; P < 0·05), possibly due to differing comparison diets, one of which was a high-GI diet(Reference Giacco, Parillo and Rivellese26), while the other was a best practice measured CHO exchange diet(Reference Gilbertson, Brand-Miller and Thorburn27), and so the results for the two studies have been reported separately. In one study with participants with type 1 diabetes, where the control diet was a higher-GI diet, episodes of hypoglycaemia were significantly fewer with the low-GI diet than with the control diet ( − 0·8 episodes, 95 % CI − 1·3, − 0·3, P < 0·01)(Reference Giacco, Parillo and Rivellese26). In the second study conducted in children with type 1 diabetes, where the control diet was a measured CHO exchange diet, there was no difference in hypoglycaemic episodes(Reference Gilbertson, Brand-Miller and Thorburn27).
However, the proportion of participants who reported more than fifteen episodes of hyperglycaemia per month was significantly lower for the low-GI diet group than for the measured CHO exchange group (35 v. 66 %, P = 0·006 after adjustment for baseline values) at 12 months(Reference Gilbertson, Brand-Miller and Thorburn27).
Insulin sensitivity
One included study measured whole body peripheral insulin sensitivity, using an euglycaemic hyperinsulinaemic clamp, and reported insulin sensitivity to be significantly higher after the consumption of the low-GI diet than after that of the high-GI diet(Reference Rizkalla, Taghrid and Laromiguiere31).
Quality of life
One trial that was conducted in children reported on quality of life, and found that it was significantly influenced by the type of diet(Reference Gilbertson, Brand-Miller and Thorburn27). In this trial, twice as many parents of those in the low-GI group than of those in the high-GI group stated that their children had no difficulties in selecting their own meals at the 12-month time point (51 v. 24 %, P = 0·01). Also, almost twice as many parents of those in the low-GI group than of those in the high-GI group reported that diabetes never limited the type of family activities pursued (53 v. 27 %, P = 0·02).
Follow-up
In the study that reported results at 12-month follow-up, there was a significant decrease in % HbA1c in the low-GI diet group compared with the comparison group who were on measured CHO exchange diets (8·0 (sem 1·0) v. 8·6 (sem 1·4) % HbA1c, P < 0·05)(Reference Gilbertson, Brand-Miller and Thorburn27).
Discussion
This review provides evidence that low-GI diets can significantly improve diabetic control in less than optimally controlled people with diabetes. Low-GI diets lower % HbA1c levels by 0·4 % compared with comparison diets. This decrease is clinically significant, and is comparable to the decrease achieved through medications for newly diagnosed type 2 diabetes(Reference Holman, Cull and Turner34, 35). In the industry guide on diabetes drug development, the US Department of Health(36) states that a 0·3 % reduction in HbA1c is clinically meaningful. Improvements of this size have been associated with a reduction in the risk of microvascular complications(Reference Stratton, Adler and Neil3). The UK Prospective Diabetes Study Group found that any reduction in HbA1c was likely to reduce the risk of complications, and that each 1 % reduction in HbA1c was associated with a reduction in risk of 21 % (95 % CI 17, 24 %, P < 0·0001) for any end point related to diabetes and a reduction in risk of 37 % (95 % CI 33, 41 %, P < 0·0001) for microvascular complications(Reference Stratton, Adler and Neil3).
Two studies were conducted in children, all of whom had type 1 diabetes. Studies conducted in children with longer follow-up periods would be useful to determine the impact of low-GI diets on both overall quality of life and long-term glycaemic control.
Although all the included studies were RCT, some had methodological limitations including failure to report on allocation concealment and lack of outcome assessor blinding. Participants in the included trials were both adults and children with diabetes, suggesting that the results would be relevant to a broad spectrum of age groups in other similar communities. Studies included people with either type 1 or type 2 diabetes, or both, and hence the results of the review have relevance to both types of diabetes. None of the trials were conducted in developing countries.
Two studies included in the meta-analysis compared a low-GI diet to other treatment diets: a measured CHO exchange diet(Reference Gilbertson, Brand-Miller and Thorburn27) and a high-cereal fibre diet(Reference Jenkins, Kendall and McKeown-Eyssen33). Even with these studies included, the meta-analysis showed that the low-GI diet improved HbA1c levels compared with comparison diets. In the study that compared a low-GI diet with a measured CHO exchange diet, involving children with type 1 diabetes(Reference Gilbertson, Brand-Miller and Thorburn27), twice as many participants in the low-GI group than those in the CHO exchange group exhibited acceptable HbA1c levels at 12 months without any increase in the rate of hypoglycaemic occurrences. Hence, even when compared to a measured CHO exchange diet, the low-GI diet resulted in greater improvement in glycaemic control.
Although insulin is the mainstay of treatment for type 1 diabetes, our review suggests that a low-GI diet can be a useful adjunctive treatment, as it improved HbA1c levels in both the studies conducted in children with type 1 diabetes(Reference Collier, Giudici and Kalmusky23, Reference Gilbertson, Brand-Miller and Thorburn27).
In type 2 diabetes, insulin sensitivity was affected by the GI of the diet, significantly increasing in the low-GI group than in the high-GI group(Reference Rizkalla, Taghrid and Laromiguiere31). This improvement may benefit patients with diabetes by lessening, or even avoiding, their requirement for medication. In a study where medications were adjusted as necessary, significantly less diabetic medication was required in people with type 2 diabetes on the low-GI diet than in those on the American Diabetes Association-recommended diet to achieve equivalent control of HbA1c levels(Reference Ma, Olendzki and Merriam37).
Low-GI diets have also been reported as causing greater weight loss in overweight or obese people compared with control diets, as well as improving lipid profiles. Low-GI diets resulted in significant decreases in body mass, total fat mass, BMI, total cholesterol and LDL-cholesterol compared with control diets(Reference Thomas and Elliott38).
Further research should investigate the effect of incorporating low-GI diets into the lifestyles of people with diabetes, because there is some indication that these diets may improve quality of life(Reference Gilbertson, Brand-Miller and Thorburn27). There is evidence to support the use of a low-GI diet as a long-term maintenance diet. A recent study conducted in participants with type 2 diabetes and optimised HbA1c levels at baseline showed that maintenance of steady HbA1c levels and sustained reductions in both postprandial glucose and C-reactive protein were more often achieved in participants on a low-GI diet than in controls at 12 months(Reference Wolever, Gibbs and Mehling39). Studies with longer follow-up periods are required to determine the feasibility of incorporating a low-GI diet as part of a lifestyle, and the potential benefits for quality of life and long-term glycaemic control.
Lowering the GI of the diet appears to be an effective method to improve glycaemic control in diabetes, and should be considered as part of the overall strategy of diabetes management.
Acknowledgements
E. J. E. is supported by an Australian National Health and Medical Research Council Practitioner Fellowship (ID 457084). We thank Professor Chris Cowell, Head of Endocrinology, the Children's Hospital at Westmead, for expert clinical advice; Samantha Clarke, Acting Head Diabetes Dietitian, the Children's Hospital at Westmead, for assistance in the trial search and Sunita Chauhan for development of the search strategy. There is no known potential conflict of interest. Both authors have contributed to the writing of the manuscript. This paper is based on a Cochrane review by the authors published in the Cochrane Library (www.thecochranelibrary.com), and includes more recent evidence. Cochrane reviews are regularly updated as new evidence emerges and in response to feedback.






