High Na intake is a serious public health challenge in China and worldwide because of its contribution to the high prevalence and concomitant risks of elevated blood pressure and CVD( Reference Strazzullo, D'Elia and Kandala 1 , Reference Stamler, Rose and Stamler 2 ). Na intake has been reported to be higher in the Asian than in the Western population, with the intake being highest in the Chinese population( Reference Zhou, Stamler and Dennis 3 ). According to the 2010 Report on Chronic Disease Risk Factor Surveillance in China, the average salt intake per family member is estimated to be about 10·6 g/d. More worryingly, approximately 81 and 73 % of individuals have been reported to consume over 5 and 6 g/d, respectively( 4 ).
Previous studies have demonstrated that high Na intake was positively associated with blood pressure, and the risk of stroke and CVD( Reference Strazzullo, D'Elia and Kandala 1 , Reference Stamler, Rose and Stamler 2 , Reference O'Donnell, Yusuf and Mente 5 – Reference Pfister, Michels and Sharp 8 ). Although several studies, attempting to examine the relationship between Na intake and the risk of the metabolic syndrome (MetS), have been conducted globally( Reference Oh, Kim and Lee 9 – Reference Rhee, Kim and Kim 15 ), detailed analyses evaluating the association between the risk of high Na intake estimated by 24 h urinary Na excretion and the MetS have been limited( Reference Baudrand, Campino and Carvajal 13 – Reference Rhee, Kim and Kim 15 ). Meanwhile, none of the population-based studies has specifically examined the association of 24 h urinary Na excretion with the risk of the MetS among Chinese adults.
Therefore, in the present study, we analysed the data from 1906 Chinese adults to examine the association between 24 h urinary Na and K excretion and the risk of the MetS among Chinese adults.
Methods
Participants
In 2011, the Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) Project was conducted initially. Details of the study design and preliminary results have been published previously( Reference Bi 16 ). Briefly, the study used a four-stage stratified sampling method to select a provincially representative sample of the general adult population aged 18–69 years in China. A total of 2112 participants were randomly selected and invited to provide 24 h urine samples. Finally, 1906 participants were identified and retained for this analysis, after we excluded eighty-eight participants with incomplete 24 h urine collections, forty-nine with incomplete MetS data, and sixty-nine missing other variables of interest. The study was approved by the Ethics Committee of the Shandong Center for Disease Control and Prevention. All study participants gave written informed consent.
Data collection
Information on variables, including demographic characteristics, smoking habit, alcohol consumption, leisure-time physical activity, as well as previous diagnosis and treatment of hypertension and diabetes, was collected at local health stations, in community clinics, or during home visits by specially trained research staff using a standard questionnaire. High school education was defined as having ≥ 9 years of schooling. Alcohol consumption was defined as drinking alcohol at least twelve times during the past year. Smoking was defined as having smoked at least 100 cigarettes across lifetime.
Overall, three blood pressure measurements were obtained in the sitting position after at least 5 min of rest by trained investigators. Waist circumference was measured at 1 cm above the navel at minimal respiration. Body weight and height were measured with participants wearing light indoor clothing without shoes during clinical examination. BMI was calculated as weight (in kg) divided by height (in m2).
Overnight fasting blood specimens ( ≥ 10 h) were obtained at the examination centres and shipped to Jinan ADICON Clinical Laboratories where the measurements of fasting plasma glucose, TAG, as well as HDL-cholesterol concentrations were made. Plasma glucose concentration was measured using a modified hexokinase enzymatic method. Serum cholesterol and TAG levels were analysed enzymatically using commercially available reagents.
Additionally, a single 24 h urine sample was collected from each participant. All participants were instructed orally on the collection of 24 h urine samples. Each participant was provided with two urine collection plastic containers, with boric acid (about 1 g) being used as a preservative in each container. Participants were asked to discard the first specimen in the morning, and from then on to collect all specimens for up to 24 h. Trained investigators were responsible for recording the starting and ending time of each specimen collection and interviewing about completeness using a standard questionnaire. All samples were shipped to the certified clinical laboratory (ADICON Clinical Laboratory, Inc.) for the analysis of Na and K concentrations using the ion-selective electrode method with an Olympus AU680 autoanalyser (CV: 1·5 % for Na and 2·5 % for K). Creatinine was determined using the picric acid method with the Olympus AU640 analyser (CV 3·0 %). The completeness of urine collections was validated by urine volume and urinary creatinine excretion. Collections with urine volume ≥ 500 ml and urinary creatinine within sex-specific mean (2 sd; 1·91–18·27 mmol for men and 1·36–14·28 mmol for women) were considered complete.
Definition of the metabolic syndrome
The MetS was defined according to the harmonised criteria as the presence of three or more of the following risk factors( Reference Alberti, Eckel and Grundy 17 ): (1) central obesity – waist circumference ≥ 90 cm for Chinese men and ≥ 80 cm for Chinese women; (2) elevated blood pressure – systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg, or antihypertensive drug treatment for patient with hypertension; (3) elevated TAG – fasting plasma TAG level ≥ 1·7 mmol/l or drug treatment for elevated TAG level; (4) reduced HDL-cholesterol – fasting HDL-cholesterol < 1·0 mmol/l in men and < 1·3 mmol/l in women, or drug treatment for reduced HDL-cholesterol level; (5) elevated fasting glucose – fasting glucose level ≥ 5·6 mmol/l or drug treatment for elevated glucose level.
Statistical analysis
Data are expressed as either means and standard deviations for continuous variables or percentages for categorical variables. Study participants were classified into three categories according to their 24 h urinary Na excretion ( < 195·4, 195·4–252·0 and ≥ 252·0 mmol) and 24 h urinary K excretion ( < 30·7, 30·7–45·5 and ≥ 45·5 mmol), separately.
Logistic regression models were applied to estimate OR and 95 % CI for the odds of the MetS according to 24 h urinary Na or K excretion, adjusted for age, sex, education level (less than high school or high school graduate), smoking habit, leisure-time physical activity, alcohol consumption, hypertension, as well as urbanisation (urban v. rural). Participants with 24 h urinary excretion of Na < 195·4 mmol or K < 30·7 mmol were used as reference groups for those analyses estimating OR and 95 % CI. The presence of a linear trend was tested by using the medians of the average 24 h urinary Na excretion in each group treated as a continuous variable in the logistic regression models( Reference Gu, Kelly and Wu 18 ).
All statistical analyses were conducted using SAS statistical software version 9.3 (SAS Institute, Inc.). All tests were two-sided, and a P value of < 0·05 was considered statistically significant.
Results
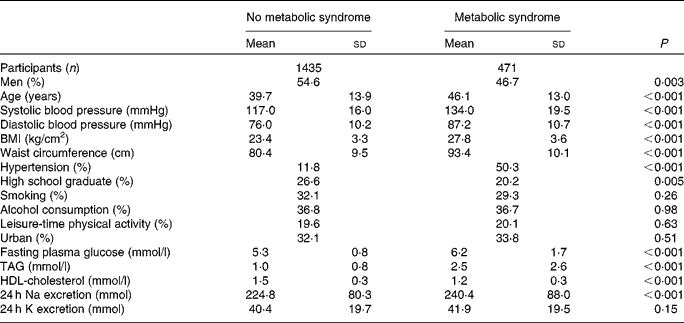
On average, 1003 (52·6 %) of the study participants were men and 903 (47·4 %) were women. Table 1 shows the characteristics of the study participants by MetS status. Of the 1906 participants, 471 (24·7 %) had the MetS. Participants with the MetS were older and more likely to have higher mean systolic and diastolic blood pressure, waist circumference, BMI, fasting glucose, TAG and 24 h Na excretion compared with their counterparts without the MetS. However, high school education and HDL-cholesterol were more prevalent in participants without the MetS (Table 1).
Table 1 Baseline characteristics of the study participants according to metabolic syndrome status (Mean values and standard deviations)
For all participants, the respective mean 24 h urinary Na and K excretion was 228·7 and 40·8 mmol. The 24 h urinary Na excretion was significantly higher among participants with central obesity, elevated blood pressure or elevated TAG than among those without these risk factors. Similarly, 24 h urinary K excretion was statistically significantly associated with central obesity (Table 2). In addition, mean 24 h urinary Na excretion for participants with none (n 498), one (n 511), two (n 426), three (n 302), and four or five (n 169) of the metabolic risk factors was 214·9, 227·2, 233·5, 241·3 and 238·7 mmol, respectively. The corresponding 24 h urinary K excretion was 39·0, 40·7, 41·7, 42·3 and 41·2 mmol, respectively. In aggregate, mean 24 h urinary Na but not K excretion significantly increased with the number of metabolic risk factors (P for linear trend < 0·001 and P for linear trend = 0·12, respectively; Fig. 1).
Table 2 Mean 24 h urinary excretion of sodium and potassium among the participants with and without each component of the metabolic syndrome (Mean values and standard deviations)
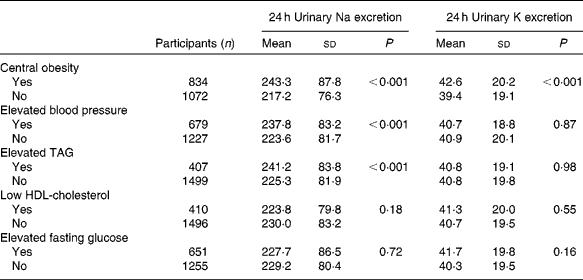
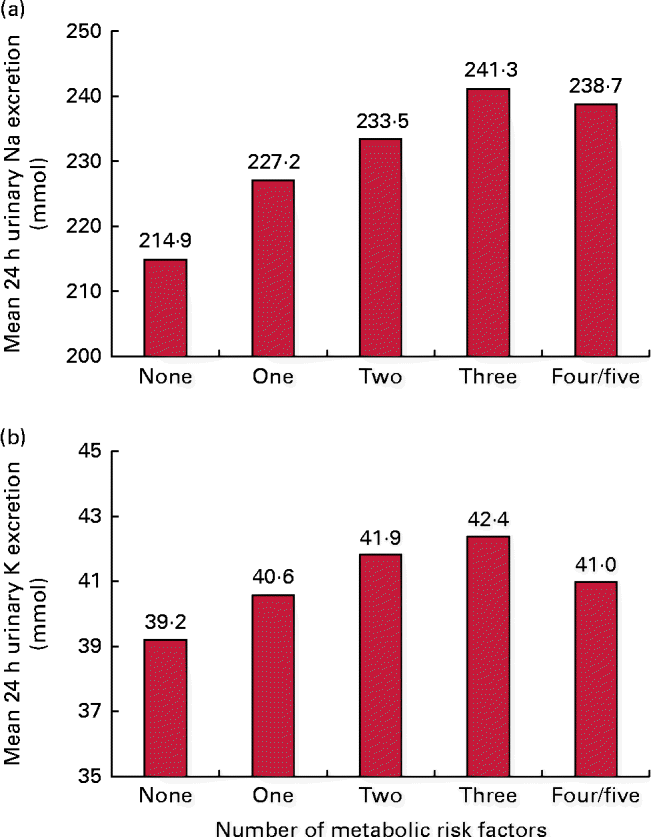
Fig. 1 Mean 24 h urinary (a) sodium and (b) potassium excretion according to the number of metabolic risk factors. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
As expected, 24 h urinary Na excretion was a strong independent predictor of the risk of the MetS. The respective multivariate-adjusted OR for the MetS compared with participants with 24 h urinary Na excretion < 195·4 mmol were 1·40 (95 % CI 1·05, 1·87) and 1·54 (95 % CI 1·16, 2·06) for participants with 24 h urinary Na excretion 195·4–252·0 and ≥ 252·0 mmol. A statistically significant dose–response relationship between 24 h Na excretion and the odds of the MetS was documented (P for linear trend < 0·05; Table 3). Increases in 24 h urinary Na excretion were associated with significantly increased odds of the MetS. In general, an increase in 24 h urinary Na of 100 mmol was significantly associated with a 29 % increased odds of the MetS (OR 1·29, 95 % CI 1·12, 1·48; Table 4). However, no significantly positive association was observed between 24 h urinary K excretion and the risk of the MetS (Table 3).
Table 3 Adjusted OR of the metabolic syndrome and its components according to tertiles of 24 h urinary sodium and potassium excretion (Odds ratios and 95 % confidence intervals)
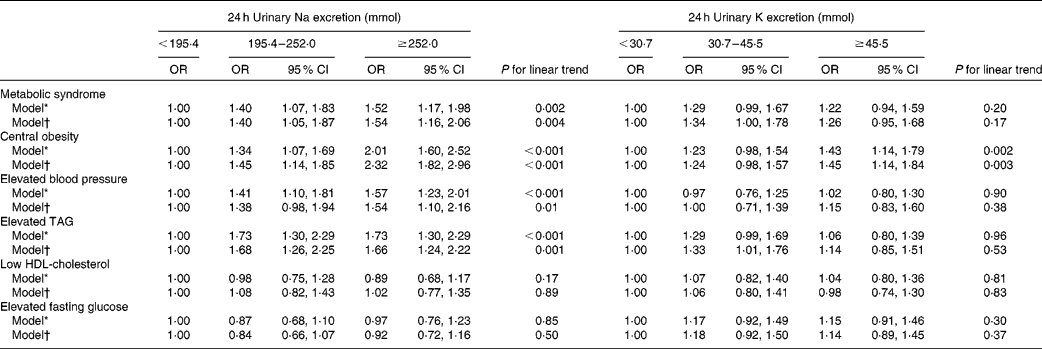
* Adjusted for age.
† Adjusted for age, sex, high school education, urbanisation, leisure-time physical activity, alcohol consumption, smoking habit and hypertension.
Table 4 Adjusted OR of the metabolic syndrome and its components associated with a 100 mmol increase in 24 h urinary sodium excretion (Odds ratios and 95 % confidence intervals)
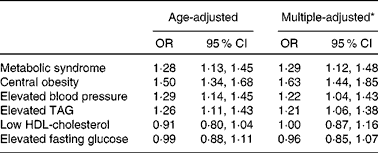
* Adjusted for age, sex, high school education, urbanisation, leisure-time physical activity, alcohol consumption, smoking habit and hypertension.
As for the components of the MetS, the odds of central obesity, elevated blood pressure and elevated TAG significantly increased across the increasing tertiles of 24 h urinary Na excretion (1·00, 1·45 and 2·32, respectively, for central obesity; 1·00, 1·38 and 1·54, respectively, for elevated blood pressure; 1·00, 1·68 and 1·66, respectively, for elevated TAG; P for linear trend < 0·05 for all). Overall, for each increment of 100 mmol 24 h urinary Na excretion, the odds of central obesity, elevated blood pressure and elevated TAG was 63, 22 and 21 % higher, respectively (Table 4). Nevertheless, 24 h urinary K excretion was not significantly associated with the components of the MetS apart from central obesity (Table 3).
Sensitivity analysis
We conducted a sensitivity analysis by applying creatinine coefficients, calculated as urinary creatinine excretion (in mg/d) divided by weight (in kg), to assess the completeness of 24 h urine collections. Creatinine coefficients of 14·4–33·6 in men and 10·8–25·2 in women were categorised as an acceptable 24 h urine collection( Reference Liu, Katsumi and Yukio 19 ). After exclusion of the participants with incomplete 24 h urine samples, a total of 1371 participants were retained for this analysis. All results were similar to those reported in the main analysis. For example, compared with participants with 24 h urinary Na excretion < 195·4 mmol, the multiple-adjusted OR for those with 24 h urinary Na excretion 195·4–252·0 and ≥ 252·0 mmol were 1·77 (95 % CI 1·26, 2·49) and 1·77 (95 % CI 1·26, 2·48) for the MetS (P for linear trend = 0·002), respectively. Similarly, compared with the participants with 24 h urinary K excretion < 30·7 mmol, the multiple-adjusted OR for those with 24 h urinary K excretion 30·7–45·5 and ≥ 45·5 mmol were 1·36 (95 % CI 0·96, 1·93) and 1·38 (95 % CI 0·98, 1·94) for the MetS, respectively.
Discussion
Data from the present study identified that 24 h urinary Na but not K excretion significantly increased with number of metabolic risk factors. As expected, we found a significant dose–response relationship between 24 h urinary Na excretion and the risk of the MetS, even after adjustment for multiple risk factors. It indicates that high Na intake is a stronger independent predictor of the risk of the MetS. Moreover, high Na intake was significantly associated with the risk of MetS components, including central obesity, elevated blood pressure and elevated TAG. However, there was no significant association between K intake and the risk of the MetS. Our findings clearly illustrate the public health importance of the reduction of Na intake for preventing the MetS.
To our knowledge, this was the first study to report the association of Na and K intake assessed by 24 h urine sample with the risk of the MetS in China. It has important clinical and public health implications because high Na intake and the risk of the MetS are becoming common in the Chinese adult population( 4 , Reference Gu, Reynolds and Wu 20 ). Accordingly, these findings contribute to the existing literature and provide new and important information in relation to the relationship between Na intake and the risk of the MetS in the Chinese general adult population, and suggest that the reduction of Na intake should be an important priority for reducing the prevalence of the MetS in China.
We observed that Na intake significantly increased with the number of metabolic risk factors, which is in line with previous studies( Reference Raisanen, Silaste and Kesaniemi 11 , Reference Hoffmann and Cubeddu 14 , Reference Rhee, Kim and Kim 15 ), but inconsistent with the finding of a cross-sectional study conducted in 781 normotensive Brazilian adults aged 25–64 years( Reference Rodrigues, Baldo and de Sa Cunha 12 ). This inconsistency could be, in part, due to the estimation of Na intake in 12 h nocturnal urine samples in the Brazilian study.
In most but not all studies, high Na intake has been identified as a risk factor of the MetS( Reference Teramoto, Kawamori and Miyazaki 10 – Reference Rhee, Kim and Kim 15 ). To date, only two previous studies have attempted to quantify the association between Na intake and the risk of the MetS( Reference Raisanen, Silaste and Kesaniemi 11 , Reference Baudrand, Campino and Carvajal 13 ). Baudrand et al. ( Reference Baudrand, Campino and Carvajal 13 ) reported that compared with participants having urinary Na excretion ≤ 150 mEq/24 h, those with urinary Na excretion >150 mEq/24 h were significantly associated with the elevated risk of the MetS (OR 3·98, 95 % CI 2·15, 7·36) among 370 participants aged 18–85 years recruited from low- and middle-income primary care centres in Santiago, Chile. The study by Raisanen et al. ( Reference Raisanen, Silaste and Kesaniemi 11 ) has suggested that participants in the third tertile of Na intake, estimated by 7 d food records, were at a higher odds of the MetS compared with those in the first tertile of Na intake (OR 1·84, 95 % CI 1·23, 3·05). Similarly, in the present study, we examined the association between daily Na intake and the risk of the MetS by dividing the participants into three groups according to the tertiles of 24 h urinary Na excretion. The present study indicated that high Na intake was significantly associated with the risk of the MetS. Moreover, we documented a strong dose–response association between Na intake and the risk of the MetS. This might be due to the participants with high Na intake consuming more energy and unhealthy food( Reference Navia, Aparicio and Perea 21 ). In addition, in the present study, K intake was not a statistically significant predictor of the MetS. However, it has been reported that low K intake is significantly associated with a higher prevalence of the MetS in hypertensive Japanese women( Reference Teramoto, Kawamori and Miyazaki 10 ).
Several studies have examined the association of Na intake with waist circumference, changes in blood pressure, the risk of incident hypertension, and the risk of incident diabetes and CVD mortality( Reference Cook, Obarzanek and Cutler 6 , Reference Larsen, Angquist and Sorensen 22 – Reference Hu, Jousilahti and Peltonen 24 ). It has been documented that waist circumference significantly increased with Na intake( Reference Navia, Aparicio and Perea 21 , Reference Larsen, Angquist and Sorensen 22 ). However, these estimates were based on a small sample size( Reference Larsen, Angquist and Sorensen 22 ). A recent multi-ethnic cohort study has found that the Na:K ratio was an independent predictor of the risk of obesity( Reference Jain, Minhajuddin and Neeland 25 ). Interestingly, we observed that both high Na and K intakes were significantly associated with the increased risk of central obesity. The INTERSALT study (an international study of electrolyte excretion and blood pressure) conducted in fifty-two centres from thirty-two countries has suggested that a 100 mmol increase in 24 h urinary Na excretion was associated with at least 2·2 mmHg increase in systolic blood pressure( Reference Stamler, Rose and Stamler 2 ). Consistently, in the present study, the odds of elevated blood pressure increased across the tertiles of 24 h urinary Na excretion. However, Stolarz-Skrzypek et al. ( Reference Stolarz-Skrzypek, Kuznetsova and Thijs 23 ) found that there was no significant relationship between 24 h urinary Na excretion and the risk of incident hypertension. Additionally, in a prospective cohort study of 1935 Finnish aged 35–64 years, high Na intake has been significantly associated with the increased risk of diabetes. However, we found that there was no significant association between Na intake and elevated fasting glucose levels.
Some potential limitations of the present study should be noted. The cross-sectional nature of the present study makes it hard to establish any casual relationship. In addition, the Na intake estimated by a single 24 h urine collection might have overestimated or underestimated the actual Na intake in the Chinese adult population. This random measurement error, owing to day-to-day variation in Na intake in individuals, would possibly bias the association towards zero. Given that Na intake habits would potentially be altered after participants are diagnosed with any component of the MetS, OR might have been slightly underestimated due to the lack of data on changes in Na intake habits over time. Furthermore, Na intake is associated with several dietary factors including energy intake as well as the amount of food consumption, and it cannot be ruled out that without adjustment for these dietary factors, the effect of Na intake on the MetS could be overestimated. Finally, lack of information on drug treatment for lipid abnormality (elevated TAG and reduced HDL-cholesterol) in the present study might result in a slight misclassification of the MetS. However, awareness, treatment and control rates of hypercholesterolaemia were extremely low in China. For example, the control rate was less than 2·0 % in the Chinese population( Reference He, Gu and Reynolds 26 ). Nonetheless, the strengths of the present study include its stringent quality-control procedures, large sample size, use of 24 h urine collection considered as the ‘gold’ standard method for the estimation of Na intake, and careful measures of outcome variables.
In summary, the present study reported an independent and dose–response association between 24 h urinary Na excretion and the odds of the MetS. However, no significant association was observed between 24 h urinary K excretion and the odds of the MetS. These findings suggest that the reduction of Na intake should be a potential approach for reducing the risk of the MetS and its societal burden in China.
Acknowledgements
The authors acknowledge the contributions of the investigators and participants to this study.
The present study was supported by a grant from the Chinese Center for Disease Control and Prevention, Beijing, China and a provincial technical development plan grant (2012GSF11828) from the People's Government of Shandong Province, Jinan.
The sponsors had no role in the design and conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript.
The authors' contributions are as follows: X. G. and J. M. designed the study; X. C., J. T., L. Y., J. R., J. Z., Z. L., J. D., J. X., X. C. and H. L. conducted the research; Z. G. analysed the data and wrote the first draft of the manuscript; X. G. and J. M. revised the paper. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








