INTRODUCTION
Leishmaniasis as a neglected tropical disease, affects vast populations in tropical and subtropical areas all over the world. According to the latest report, from the World Health Organization (WHO, 2016), 399 million people in 11 highly endemic countries are at risk for the cutaneous form of the disease [cutaneous leishmaniasis (CL)], and 556 million individuals are in danger of visceral leishmaniasis (VL) in the 12 most infected countries. Every year, 900 000 to 1·3 million new cases and 20 000 to 30 000 death, are reported in endemic areas (WHO, 2016). There is currently no approved vaccine against leishmaniasis for humans. The only available confirmed vaccines are for canine visceral leishmaniasis prevention, including Leishmune, Leishtec and CaniLeish (Jain and Jain, Reference Jain and Jain2015). The practice of leishmanization, which was the only truly effective approach against the cutaneous form, was terminated due to safety concerns (Savoia, Reference Savoia2015).
Current treatments for leishmaniasis include chemotherapy with antimonials for the cutaneous and mucocutaneous forms, and Amphotericin B (AmB) for VL. Other than cytotoxicity, drug resistance is the main obstacle for current therapy (Mohapatra, Reference Mohapatra2014). A long-term study on the mechanism of leishmaniasis and recovery, highlighted the role of Th1 cellular responses (Scott and Novais, Reference Scott and Novais2016), so researchers tried to apply cytokines, immunomodulators and immune cells as immunotherapeutic agents to trigger essential factors in the immune system for healing.
In this review, the roles of current chemotherapeutic agents and different immunotherapy approaches in treating leishmaniasis will be discussed. The importance of cytokines and immunomodulators alone and in combination with current therapies will be explored. Furthermore, live and killed leishmanial vaccines and cellular therapy will be discussed. The final section is dedicated to introducing a new approach for treatment using Leishmania tarentolae.
CURRENT CHEMOTHERAPY AGAINST LEISHMANIASIS, PROS AND CONS
Different chemical compounds have been found to be effective against leishmanisis; however, most are not safe and are difficult to use. Finding appropriate anti-leishmanial therapeutic solutions has been a priority for the health systems of endemic countries. The following section, is a brief summary of current chemotherapies used to treat leishmaniasis.
Antimonials
Antimonials, are the first line of anti-Leishmania drugs used all over the world. The original drug was first inactivated in the parasite, but reduction of the pentavalent to the trivalent form through the application of thiols by host macrophages and parasite cells, makes it an effective weapon against the parasite. The amastigote form of the parasite is sensitive to antimonials, as only it is able to conduct the necessary chemical reduction inside the host. Although it is the most commonly used medication against leishmaniasis, the mechanism of action of antimonials still unclear. However, in recent decades, resistance posed serious challenges in their usage for leishmaniasis treatment (Mohapatra, Reference Mohapatra2014). For example, 65% of the VL patients in the Indian subcontinent showed resistance to antimonials, which led to banning of the drug in Bihar, India (Haldar et al. Reference Haldar, Sen and Roy2011). Other than resistance, hepatic and renal toxicity can leave patients with lifelong health problems. Six decades of antimonial use have provided the parasite enough time to develop resistance mechanisms, including prevention of drug activation, decreased uptake into the parasite, increase drug efflux and high thiol burden in macrophages, which enhances oxidative stress in the host cell (Mohapatra, Reference Mohapatra2014).
Miltefosine
As an anticancer and anti-Leishmania drug, Miltefosine is the only oral medication available against VL and CL. Although the drug is easy to take, its long half-life increases teratogenicity and resistance potential. Additionally there have been reports of gastrointestinal discomfort (Keynan et al. Reference Keynan, Larios, Wiseman, Plourde, Ouellette and Rubinstein2008). Sensitivity to Miltefosine is different among parasitic species (Dorlo et al. Reference Dorlo, Balasegaram, Beijnen and de Vries2012). Miltefosine attacks Leishmania through three different mechanisms: protein kinase inhibition, which leads to apoptosis; immunomodulatory effect in macrophages; and changes in parasite plasma membrane structure (Vincent et al. Reference Vincent, Weidt, Rivas, Burgess, Smith and Ouellette2014).
Paromomycin
Paromomycin is categorized as a natural aminoglycoside. Aminoglycosides are effective against multiple bacterial species, and they are also being used orally against enteric parasites such as Amoeba, Giardia and Tapeworms. The parenteral form of the drug is known to be effective against VL, and either in its pure ointment form or in combination with Gentamicin (15% Paromomiycin + 0·5% Gentamicin), it is also indicated for CL treatment (Shalev et al. Reference Shalev, Rozenberg, Smolkin, Nasereddin, Kopelyanskiy, Belakhov, Schrepfer, Schacht, Jaffe and Adir2015). Several clinical trials have been performed to evaluate different formulations of Paromomycin on leishmaniasis (Guedri et al. Reference Guedri, Zaatour, Alaya, Bettaieb, Gharbi, Boukthir, Chlif, Abdelhamid, El Ahmadi and Louzir2013).
Paromomiycin can affect ribosomal activity, inhibiting protein synthesis and mitochondrial membrane potential, which deprives the parasite of energy (Chawla et al. Reference Chawla, Jhingran, Panigrahi, Stuart and Madhubala2011). It is worth to mention that the binding of Paromomycin to ribosomes is highly selective and limited to the parasite, which indicates its safety as anti-leishmanial drug (Fernández et al. Reference Fernández, Malchiodi and Algranati2011).
Amphotericin B
AmB is a polyen fungicide that has shown the most promise against VL. Its liposomal formulation, Ambisome, was used in India to overcome increasing numbers of VL cases. However, the drug is expensive and is generally only available through international health organizations such as WHO (Sundar and Chakravarty, Reference Sundar and Chakravarty2010).
AmB controls Leishmania infections through two distinct mechanisms. The first includes, auto-oxidation of AmB, leading to the production of free radicals. The second mechanism requires the binding of AmB to sterols in the membrane of the parasites, which makes pores that cause an ion imbalance. Additionally, selective interaction of AmB with cholesterols in the macrophage membrane, blocks the parasite from entering uninfected cells, thus stopping further spread (Paila et al. Reference Paila, Saha and Chattopadhyay2010; Purkait et al. Reference Purkait, Kumar, Nandi, Sardar, Das, Kumar, Pandey, Ravidas, Kumar and De2012).
LEISHMANIA INTERACTIONS WITH HOST IMMUNE RESPONSES
Leishmania like many other parasites, have established systematic resistance against the host immune system. The long development of the unicellular organisms, has taught them how adopt to harsh situations in order to survive. Macrophages are the ultimate destination of Leishmania parasites in the mammalian host, as this is where the parasites can evade the immune system. Some escape mechanisms (Gupta et al. Reference Gupta, Oghumu and Satoskar2013) previously elucidated in Leishmania are listed below:
• Blocking complement system maturation by preventing C5–C9 membrane attack complex formation.
• Using Lipophosphoglycan to facilitate macrophage entrance receptors such as Fc and phosphatidylserine receptors.
• Altering the TLR2/TLR4 signalling pathway to turn off the cytokine cascade.
• Preventing phagosome to lysosome fusion inside macrophages.
• Controlling pH inside the phagosome by interrupting the V-ATPase pump.
• Employing the specific iron transporters to supply the parasite with iron.
• Reducing expression of B7 and CD40 as essential factors for the T-cell antiparasitic response.
• Preventing cytokine activation signalling in macrophages through inhibition the JAK/STAT pathway.
• Changing expression levels of cytokines and chemokines.
It is easy to see that Leishmania parasites can have tremendous effects on the host immune system. The parasite takes advantage of manipulating different immune mechanisms to survive in the host. Thus, treatment with immune system factors is an alternative approach to combat the infection. Increasing knowledge of the nature of the Leishmania infection helps to discover more reliable and effective treatments. Many attempts have been made to treat better the infection in order for faster recovery. Some studies focus on applying a special cytokine in the treatment protocol, while others try to block a specific undesirable pathway or utilize specific cells to help alleviate the infection. Some researchers have tried combining chemo- and immunotherapy to achieve better results. In the next section, we will provide a summary of these approaches (Fig. 1).
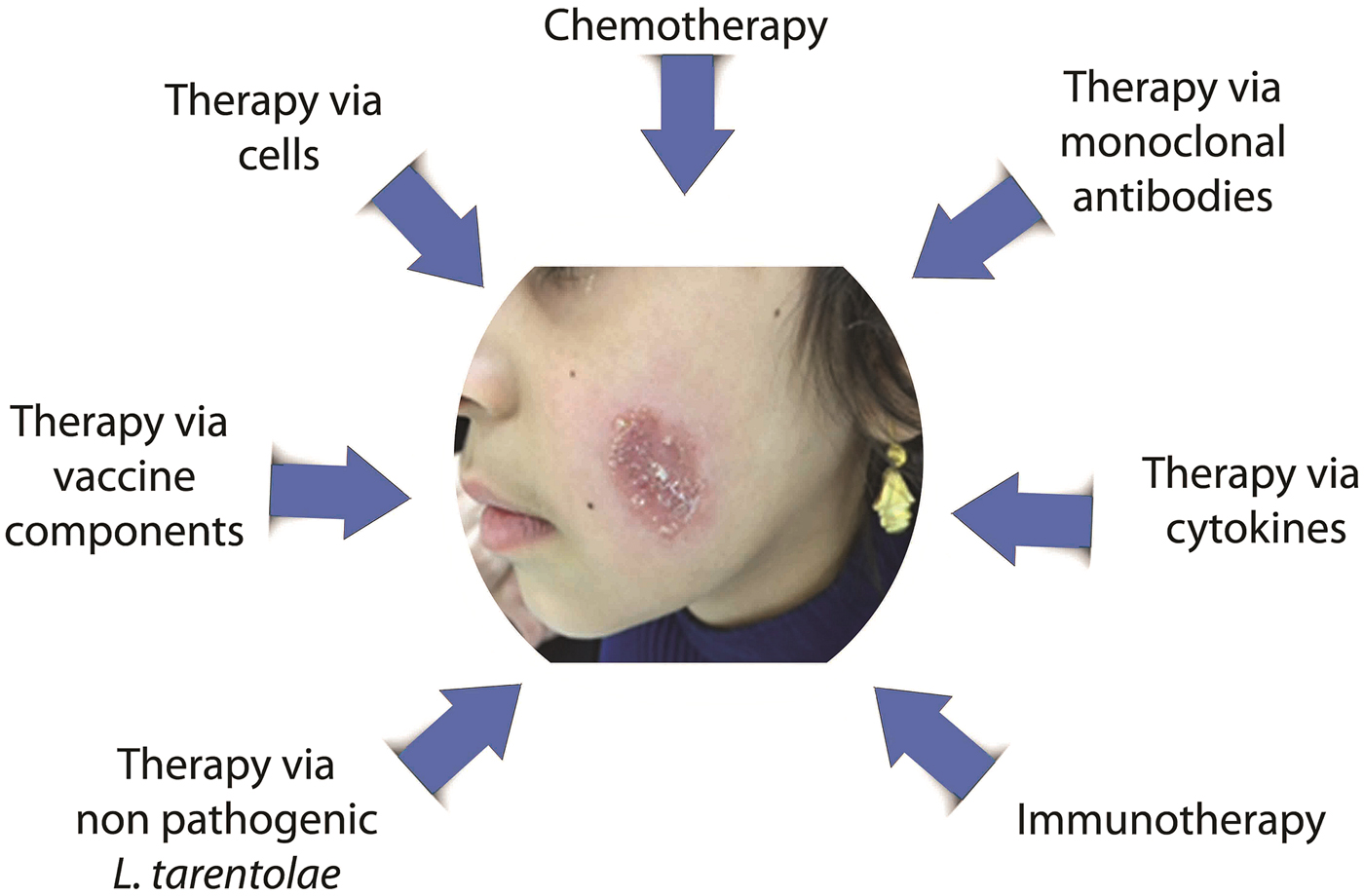
Fig. 1. Different treatment approaches against leishmaniasis.
APPLICATION OF CYTOKINES OR OTHER IMMUNOMODULATORS TO BOOST HOST IMMUNE RESPONSE AGAINST LEISHMANIASIS
After discovering the importance of the Th1/Th2 balance in the outcome of leishmaniasis (Scott and Novais, Reference Scott and Novais2016), researchers try to apply key cytokines to influence this balance. Interleukin (IL)-12 and interferon (IFN)-γ are two cytokines in the centre of many immunotherapy approaches. Additionally, inhibition of Th2 cytokines (like IL-10 or IL-4) or their production pathways were tested simultaneously and in separate experiments. In addition to cytokines and monoclonal antibodies for blocking and promoting certain pathways, some immunomodulators have proven ideal partners for using in treatment protocols.
Application of cytokines or monoclonal antibodies in leishmaniasis treatment
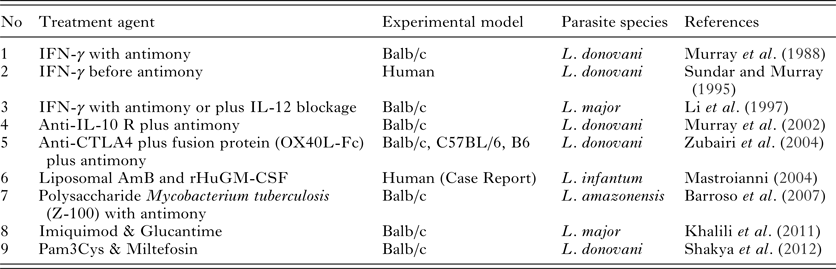
Reed et al. first used lymphokines collected from murine spleen cell culture supernatant encapsulated in liposomes for the treatment of VL. Treated mice had a lower parasite burden in their livers compare with control animals, which demonstrated the positive effect of lymphokines in leishmaniasis treatment (Reed et al. Reference Reed, Barral-Netto and Inverso1984). In another study, Murray et al. tried to treat the visceral form of the disease through the administration of recombinant Th1 cytokines, such as rIFN-γ and rIL-2, after challenge with Leishmania donovani (Murray et al. Reference Murray, Stern, Welte, Rubin, Carriero and Nathan1987). Administration of anti-IL-4 and rIFN-γ to control the Th2 response in leishmaniasis was also tested against Leishmania major in Balb/c mice. Treatment started after infectious challenge, and 85% of the animals that received anti-IL-4, resolved the disease (Sadick et al. Reference Sadick, Heinzel, Holaday, Pu, Dawkins and Locksley1990). In another study for the treatment of VL in C57BL/6 and Balb/c mice, rIFN-γ and muramyl tripeptide (MTP-PE) were packaged in liposomes to decrease adverse side-effects. Liposomes were applied as several intravenous (i.v.) injections in varying doses. Treated mice had a decreased parasite burden in the spleen (Hockertz et al. Reference Hockertz, Franke, Paulini and Lohmann-matthes1991).
Furthermore, Murray et al. (Reference Murray, Lu, DeVecchio, Matsuhashi, Ma and Heinzel2003) studied the effect of inhibiting the IL-10 receptor (IL-10R) in the treatment of VL in L. donovani-infected C57BL/6 and Balb/c mice. The results showed that mice treated with anti-IL-10R could control parasite load in the liver, increased level of IFN-γ in serum and iNOS production in macrophages. This indicated the positive effect of IL-10 receptor inhibition in reducing fatality of L. donovani infection (Murray et al. Reference Murray, Lu, DeVecchio, Matsuhashi, Ma and Heinzel2003).
To inhibit the suppressor cytokine IL-10, Bodas et al. studied the treatment efficacy of anti-IL-2, anti-IL-2R and anti-IL-10 antibodies. It is known that in initial phase of VL, IL-2 production is necessary to induce IL-10 as a suppressor cytokine and IFN-γ as inducer of Th1 response. Mice were challenged and injected intraperitoneally (i.p.) with the aforementioned antibodies at different time points after challenge. The group which received anti-IL-2, anti-IL-10 and anti-IL-2R antibodies together easily limited the parasite growth in the spleen and controlled disease progression (Bodas et al. Reference Bodas, Jain, Awasthi, Martin, Loka, Dandekar, Mitra and Saha2006).
Castellano et al. blocked IL-10 production through administration of a human monoclonal antibody (anti-hIL-10) to promote a Th1 response in CL patients diagnosed with Leishmania amazonensis infection. Patients showed decreased IL-10, IL-4 and TNF-α levels. Although patients with active lesions showed TNF-α production after treatment, this strategy did not alter the CXCL10 production, which is an IFN-γ dependent chemokine (Castellano et al. Reference Castellano, Argiro, Dessein, Dessein, da Silva, Correia and Rodrigues2015). In a recent experiment, the therapeutic efficacies of anti-IL-10R and anti-GITR (glucocorticoid-induced TNF receptor-related protein) were examined in C57BL/6 mouse model against L. donovani infection. The results indicated that blocking IL-10 can control the parasite burden in mice, but combination therapy with both mAb did not inhibit parasite proliferation in the liver and spleen, even in a low-dose challenge programme. Treatment with both antibodies increased IFN-γ and TNF-α significantly higher than using either alone (Faleiro et al. Reference Faleiro, Kumar, Bunn, Singh, Chauhan, Sheel, Amante, de Oca, Edwards and Ng2016) (Table 1).
Table 1. Application of cytokines and immunomodulators for leishmaniasis treatment

Immunomodulators as an alternative approach to control leishmaniasis
Immunomodulators can be many different types of substances, from chemical materials to natural products that have immune system activity. These substances can either boost or down regulate the immune system response, based on their properties. By applying immunomodulators, it is possible to revert the Leishmania masked immune system in a way to control the infection.
For over 10 years, immunostimulatory CpG oligodeoxynucleotides (ODNs) have been utilized as Toll-like receptor 9 (TLR9)-dependent innate immune activators and vaccine adjuvants. In 1999, Walker et al. pioneered the application of CpG and non-CpG motif as therapies against L. major infection in wild-type and IFN-γ deficient Balb/c mice. Almost all (95%) of Balb/c mice in the group treated with CpG ODNs survived 10 weeks after challenge, and administration of CpG ODNs as a local injection at the infected site or at a distant site had the same effect, indicating their systemic effects against the infection (Walker et al. Reference Walker, Scharton-Kersten, Krieg, Love-Homan, Rowton, Udey and Vogel1999). In a recent experiment, acetyl salicylic acid (ASA) was used orally as immunomodulator to resolve an L. major infection in Balb/c mice. ASA reduced the lesion size and declined visceralization of L. major in Balb/c mice. ASA unspecifically increased the nitric oxide production and decreased the amastigote proliferation in macrophages (Nahrevanian et al. Reference Nahrevanian, Jalalian, Farahmand, Assmar, Rastaghi and Sayyah2012).
Faezi et al. studied the efficacy of applying L-argenine in Balb/c mice for the treatment of CL. L-argenine strengthens the nitric oxide production pathway in macrophages and can limit the infection when orally administered (Faezi, Reference Faezi2015).
In another approach, chitin and chitosan, were used as immunomodultors. Chitin is a homopolymer extracted from shrimp shells and chitosan is its more acetylated form. Treatment efficacy of each polymer was determined against L. major infection in Balb/c mice. Both chitin- and chitosan-treated mice showed smaller lesions and reduced parasite load in the lymph nodes compare with controls. Although chitin was a more efficient therapeutic agent, it stimulated the production of IL-10 and TNF-α compare with chitosan (Hoseini et al. Reference Hoseini, Moradi, Alimohammadian, Shahgoli, Darabi and Rostami2016) (Table 1).
COMBINATION APPROACH USING IMMUNOTHERAPY AND CHEMOTHERAPY
There are efforts to potentiate chemotherapeutic agents with various immunomodulators as a multidisciplinary treatment of leishmaniasis. The following section describes several different protocols in this direction.
In VL mouse model, the efficacy of applying IFN-γ with antimony was among the first experiments in this direction. The results indicated that the antimony dosage required to inhibit parasite growth decreased by 4–10-fold with the use of IFN-γ (Murray et al. Reference Murray, Berman and Wright1988). In 1995, a study was conducted in India on the administration of IFN-γ before antimony therapy in VL. After 20 days of treatment with IFN-γ, four out of nine patients recovered completely. The remainder, showed decreased amount of parasitaemia in their spleen aspirates (Sundar and Murray, Reference Sundar and Murray1995).
In another study, the combination of antimony with IFN-γ was tested in Balb/c mice infected with L. major. Neither antimony nor IFN-γ alone promoted recovery, but the combination was effective. Using an antibody to inhibit IL-12, decreased recovery indicating the process requires IL-12. Studying the cytokine profile in leishmaniasis lesions showed that, in combination therapy, IL-10 and IL-4 expression were reduced in the lesion. In addition iNOS and the p40 chain of IL-12 were over expressed in the lesion (Li et al. Reference Li, Sutterwala and Farrell1997).
To determine the role of IL-10 in combination therapy, Murray et al. evaluated of IL-10R inhibition in wild-type, IL-10 deficient and IL-10 over expressing mice. After challenge with L. donovani, they started treatment with antimony and anti-IL-10R monoclonal antibody. In the IL-10 knockout mouse, the infection period in the liver was very short and the parasite cleared completely in four weeks. In the IL-10-overexpressing animals, the parasite burden was much higher than wild-type. Blocking IL-10R in normal mice also reduced the time of infection and cleared the liver completely. Granuloma formation was higher in the IL-10-knockout mice compared to the wild-type and IL-10-overexpressing mice. This indicates that IL-10 inhibition combined with antimony treatment can increase the rate of recovery from VL (Murray et al. Reference Murray, Lu, Mauze, Freeman, Moreira, Kaplan and Coffman2002).
Furthermore, Murray et al. studied the effect of using Anti-CD40 and anti-CTLA4 in combination with antimony (Sbv) in Balb/c and C57BL/6 mice against L. donovani infection. The binding of CD40 and its ligand is an essential step for T cell activation. On the other hand, CTLA-4 can reduce T cell activation through decreased B7-CD28 binding. In this study, anti-CD-40 acted as an agonist of CD40 ligand, which triggered IL-12 production, and activating T cells. Anti-CTLA-4 worked to block the negative regulation of T cell activation. Thus, these approaches can increase IFN-γ and recruit mononuclear cells to the site of infection (Santos et al. Reference Santos, Aguiar, de Souza, de Lima, Palatnik and Palatnik-de-Sousa2003). Zubairi et al. proposed that the costimulatory pathways of the chimeric fusion protein OX40L-Fc; a T cells stimulator through OX40; and a CTL-4 blocker monoclonal antibody, which has receptors that inhibit T cells, killed the Leishmania parasite by both improving the granuloma maturation rate, CD4+ T cell proliferation, and finally killing the Leishmania parasite. This treatment had no significant effect on necrotic or fibrotic reactions or the levels of endogenous anti-inflammatory cytokines such as IL-10 and TGF-β (Zubairi et al. Reference Zubairi, Sanos, Hill and Kaye2004).
In a case report on the treatment of a male diagnosed with AIDS and VL in Italy, physicians applied liposomal AmB and rHuGM-CSF (recombinant human granulocyte macrophage colony-stimulating factor). Investigations showed that his spleen size reduced after treatment and clinical symptoms of VL disappeared (Mastroianni, Reference Mastroianni2004).
Barroso et al. examined the potential of a polysaccharide from Mycobacterium tuberculosis named as Z-100 with antimony to treat L. amazonensis in Balb/c mice. However, this combination therapy showed no significant difference when compared to antimony alone (Barroso et al. Reference Barroso, Marco, Calvopina, Kato, Korenaga and Hashiguchi2007).
Khalili et al. applied Imiquimod and Glucantime to study recovery of Balb/c mice against L. major infection. They found that Imiquimod plus Glucantime treated controlled foot pad swelling and parasite burden in the lymph nodes more than either treatment alone (Khalili et al. Reference Khalili, Dobakhti, Niknam, Khaze and Partovi2011). In 2012, Shakya et al. determined the effect of a lower dose of the anti-leishmanial drug Miltefosine in combination with a single dose of an immunomodulator, Pam3Cys (tripalmytoil-Cysteine) on Balb/c mice infected by L. donovani. They showed that this complex significantly promoted treatment due to increases in the levels of Th1/Th2 cytokines and ROS, RNS and H2O2 production (Shakya et al. Reference Shakya, Sane, Vishwakarma and Gupta2012) (Table 2).
Table 2. Combination of chemo and immunotherapy against leishmaniasis
CELLS AS THERAPEUTIC TOOLS
Using cells as therapeutic agents is another approach to overcome infectious diseases and cancer. Dendritic cells are the most important antigen-presenting cells at the interface of innate and adaptive immunity, and they initiate immune responses in the body. Dendritic cells suppress the early secretion of IL-10, which helps to spread the parasite and so some researchers have tried a cell therapy protocol to facilitate recovery from leishmaniasis (Schwarz et al. Reference Schwarz, Remer, Nahrendorf, Masic, Siewe, Müller, Roers and Moll2013).
To achieve the best treatment outcome, a combination of cell and chemotherapy may be recommended. In a study, the potential of bone marrow-derived dendritic cells (BMDDCs) pulsed with soluble L. donovani antigen and treated with antimony was examined for treatment of VL. The combination treatment resulted in complete clearance of the parasite in the liver and spleen, indicating the effectiveness of dual treatment (Ghosh et al. Reference Ghosh, Pal, Ray, Maitra, Mandal and Bandyopadhyay2003). In another study, BMDDCs were pulsed with L. amazonensis antigen and injected into mice infected with L. amazonensis. They found that IL-12 production significantly increased, but this response was not sufficient to promote the healing process in the animals (Vanloubbeeck et al. Reference Vanloubbeeck, Ramer, Jie and Jones2004). Altogether, using DCs for leishmaniasis treatment had significant effect in the reduction of parasites and in increasing the levels of Th1 cytokines in animal models (de Castro and Pereira, Reference de Castro and Pereira2014).
It has been shown that regulatory T cells (Tregs) are important for controlling infection, and Ehrlich et al. investigated whether increasing the amount of Tregs could be used as an immunotherapeutic treatment. They treated L. donovani infected mice with a combination of rIL-2/anti-IL-2 Ab to expand Tregs. This treatment reduced the parasite load, healed the lesions and reduced the cytokines by increasing the number of Tregs (in draining lymph nodes and spleen) (Ehrlich et al. Reference Ehrlich, Castilho, Goldsmith-Pestana, Chae, Bothwell, Sparwasser and McMahon-Pratt2014) (Table 3).
Table 3. Cellular therapy in leishmaniasis

VACCINE COMPONENTS AS IMMUNOTHERAPEUTIC AGENTS
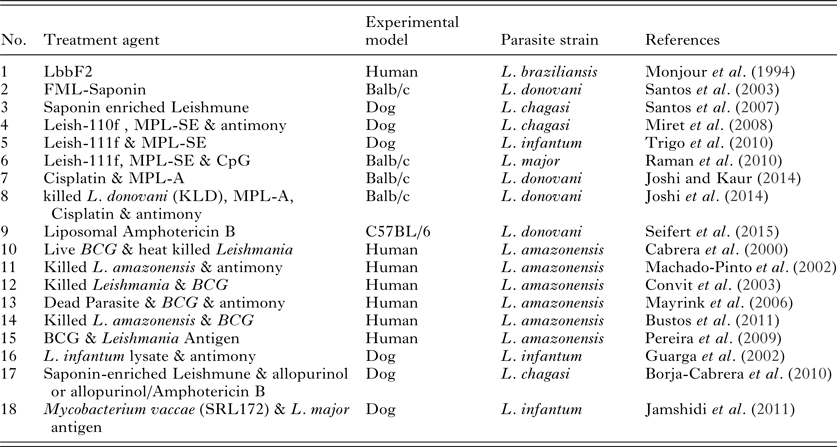
There are various studies in which different components of vaccine materials, including a specific leishmanial component, live and killed parasites, were used as immunotherapeutic tools. Among the first studies, Mojour et al. tested the effect of parasite-derived antigen Fraction 2 (LbbF2, 94-67KD) on 25 patients with American Cutaneous Leishmaniasis (ACL) caused by Leishmania braziliensis and compared it with antimony therapy. They demonstrated that both treatments had the same results and that the antigen could stimulate T helper cells in associated with the production of key cytokines at the lesion site (Monjour et al. Reference Monjour, Neogy, Vouldoukis, Silva, Boisnic, Brito, Lesot, Vignot, Martins and Jardim1994).
Santos et al. investigated the immunotheraputic effect of Fucose Manose Ligand (FML) L. donovani -Saponin in a murine VL model caused by L. donovani. They indicated that this therapy had an effect on the modulation of infection, leading to a decrease in the parasitic load in the liver and overall disease symptoms disease (Santos et al. Reference Santos, Aguiar, de Souza, de Lima, Palatnik and Palatnik-de-Sousa2003).
In 2007, Santos and his colleagues administrated the Leishmune vaccine (FML-Saponin) in a dog model as a therapeutic agent. They found that Leishmune combined with an increased concentration of Saponin may improve the immunotherapeutic effect on seropositive and symptomatic dogs infected by Leishmania chagasi. This well-designed vaccine (enriched-Leishmune-vaccine) could significantly reduce the clinical symptoms and parasite load in the liver, spleen, bone marrow, and blood (Santos et al. Reference Santos, Borja-Cabrera, Miyashiro, Grechi, Reis, Moreira, Martins Filho, Luvizotto, Menz and Pessôa2007).
The combination of vaccines, Leish-110f, and MPL-se (Monophosphoril Lipid A) as an adjuvant, with antimony was tested against VL in a dog model. In this experiment, vaccine plus antimony or vaccine alone, both reduced the mortality and increased survival in dogs. Additionally, the cellular responses in these two groups were higher compared to chemotherapy alone or control (Miret et al. Reference Miret, Nascimento, Sampaio, França, Fujiwara, Vale, Dias, Vieira, da Costa and Mayrink2008). Trigo et al. examined another candidate of human trial, Leish-111f + MPL-SE, in two separate experiments on naturally infected dogs, and compared these results with Glucantime treatment alone or in combination with the vaccine. Their results indicated that Leish-111f + MPL-SE was effective for mild cases of canine VL and also reduced the symptoms of severe canine VL but Glucantime alone failed to treat most of the cases (Trigo et al. Reference Trigo, Abbehusen, Netto, Nakatani, Pedral-Sampaio, de Jesus, Goto, Guderian, Howard and Reed2010).
Raman et al. applied the same formulation (Leish-111f + MPL-SE) plus CpG ODNs as a treatment against L. major infection in Balb/c mice. Their experiment showed that the group which had received Leish-111f with MPL-SE and CpG ODNs, could induce an effective T cell response. MPL-SE plays important role as an agonist of TLR9. The CD4 population and IL-12p70 production increased when Leish-111f was used in combination with both adjuvants (Raman et al. Reference Raman, Bhatia, Picone, Whittle, Bailor, O'Donnell, Pattabhi, Guderian, Mohamath and Duthie2010).
In 2014, Joshi et al., investigated the effect of immunochemotherapy containing a Leishmania-specific 78 kDa antigen accompanied by cisplatin (platinum-based anti-cancerous drug) added to adjuvant, MPL-A on L. donovani infected Balb/c mice. This treatment approach increased levels of Th1 cytokine (IFN-γ and IL-2) and decreased levels of Th2 cytokines (IL-4 and IL-10), suggesting a potential treatment combination (Joshi and Kaur, Reference Joshi and Kaur2014). In another study, they compared chemotherapy, immunotherapy, and immunochemotharpy in Balb/c mice harbouring an L. donovani infection. They applied killed L. donovani, (KLD) parasite, MPL-A (monophosphoryl lipid A), cisplatin, and antimony for treatment. The immunotherapy group treated with KLD and MPL-A, the chemotherapy group treated with antimony and cisplatin, and the immunochemotherapy group treated with a combination of all treatments at different time points. They found that, KLD plus Antimony reduced the parasite burden and IgG1 levels and increased the DTH and IgG2 response in comparison to either treatment alone. Immunochemotherapy with KLD, MPL-A, and antimony was revealed the most effective protocol, with 98% parasite burden reduction, and produced high levels of IFN-γ and reduced levels of IL-10 and IL-4 (Joshi et al. Reference Joshi, Malla and Kaur2014).
Recently, LEISHDNAVAX; a DNA vaccine mixture of five independent MIDGE-Th1 (Modified to foster Th1-type immune responses) vectors encoding different antigens conserved among Leishmania species (KMP11, TSA, CPA, CPB and P74) was used in the treatment of C57BL/6 mice infected with L. donovani. LEISHDNAVAX showed significant antileishmanial efficacy when coadministered with a single dose of liposomal AmB, but not when used as a monotherapy (Seifert et al. Reference Seifert, Juhls, Salguero and Croft2015).
Cabrera et al. applied heat killed promastigotes of L. amazonensis with live Mycobacterium bovis BCG and indicated that, this approach shifted the T cell response towards Th1 and increased production of IFN-γ. Their results indicated that, this therapy is safe, inexpensive, and effective for ACL patients (Cabrera et al. Reference Cabrera, Castes, Trujillo, Convit and Shaw2000).
The application of antimony with killed-L. amazonensis-vaccine was tested against ACL. A total of 102 patients were diagnosed with ACL were treated with either antimony or Killed L. amazonensis plus antimony. All patients in the test group recovered completely. In control group only 8% of the patients responded to treatment, indicating that the combination therapy was more effective (Machado-Pinto et al. Reference Machado-Pinto, Pinto, Da Costa, Genaro, Marques, Modabber and Mayrink2002).
In Venezuela, 11 532 patients diagnosed with ACL over 9 years (from 1990 to 1999) were treated with heat killed Leishmania plus BCG of which 5341 cases were studied after treatment. In 95·7% of cases, clinical healing was achieved. Mild side-effects were seen in patients who received BCG alone and immunotherapy was unsuccessful in 143 patients. Their treatment protocol proceeded with combination therapy instead (Convit et al. Reference Convit, Ulrich, Zerpa, Borges, Aranzazu, Valera, Villarroel, Zapata and Tomedes2003).
In a comprehensive study in South America, chemotherapy was compared with immunotherapy and immunochemotharapy. In this study, 542 patients diagnosed with ACL were treated either with antimony, dead parasite as vaccine, BCG, or a combination. The rate of recovery in antimony and the vaccine/antimony combination were the same; the combination reduced the recovery time from 87 to 62 days and the patients reported fewer side-effects. Other protocols using combination therapies did not show any significant changes from antimony administration alone (Mayrink et al. Reference Mayrink, Botelho, Magalhães, Batista, Lima, Genaro, Costa, Melo, Michalick and Williams2006).
In a case report from Argentina, a patient diagnosed with CL was treated with heat killed L. amazonensis plus BCG. After receiving two doses in a 7-week interval, the lesion was completely cured. The patient's CD4 and CD8 populations from different time points were analysed. It was demonstrated that cells that are CD45RA+, or naive T cells had stable count during the study; however, CD45RO+ cells (which is the gold standard for memory T cells) were increased a year after treatment. The results were compared to 12 healthy volunteers from the same area (Bustos et al. Reference Bustos, Barrio, Ramoneda, Ramos, Mora, Convit and Basombrío2011).
In another study a monthly immunotherapy regimen of the monovalent L. amazonensis (PH8 vaccine) and L. braziliensis (M2903 vaccine), together with BCG was administrated to patients. All wounds showed temporary healing and Leishmania skin tests were negative. IFN-γ was not found in mononuclear cell cultures treated with Leishmania antigens. No relationship was observed between increasing frequency of the immunotherapy and wound healing. Furthermore, they suggested that this immunotherapy schedule decreased the parasite load and activated the monocytes and natural killer cells (Pereira et al. Reference Pereira, Dorta, Pereira, Bastos, Oliveira, Pinto, Galdino, Mayrink, Barcelos and Toledo2009).
There have been multiple attempts to control VL in dogs. In one experimental study, dogs were infected with Leishmania infantum, and treatment was performed through the administration of antimony and L. infantum lysate, which was prepared by continuous freezing and thawing. Due to the outbreed nature of the animals, there was some controversy in evaluating the efficacy of the treatment. While, some animals showed a period of clearance, they eventually infection relapsed. The treated animals did show elevated levels of T lymphocytes, but the infection remained in the lymph nodes and the parasite did not clear completely (Guarga et al. Reference Guarga, Moreno, Lucientes, Gracia, Peribáñez and Castillo2002).
In 2007, Santos et al. used Leishmune vaccine (FML-Saponin) as a therapeutic agent in a dog model. They found that Leishmune combined with an increased concentration of Saponin improved the immunotherapeutic effect on seropositive and symptomatic dogs infected by L. chagasi. The vaccine (enriched-Leishmune-vaccine) could reduce the clinical symptoms and parasite load in the liver, spleen, bone marrow and blood significantly (Santos et al. Reference Santos, Borja-Cabrera, Miyashiro, Grechi, Reis, Moreira, Martins Filho, Luvizotto, Menz and Pessôa2007).
Borja-Cabrera and Santos continued their study to test the enriched-Leishmune-vaccine on dogs naturally infected with L. donovani and compared it with Immunochemotherapy (enriched-Leishmune-vaccine in combination with Allopurinol or AmB/Allopurinol). They followed up the animals' symptoms until 4·5 years after treatments. They concluded that immunochemotherapy not only abolished all the disease symptoms but also reduced infection and survival of the infected dogs (Borja-Cabrera et al. Reference Borja-Cabrera, Santos, Santos, Trivellato, Kawasaki, Costa, Castro, Nogueira, Moreira, Luvizotto, Palatnik and Palatnik-de-Sousa2010).
Furthermore, Jamshidi et al. studied the efficacy of autoclaved L. major with heat-killed Mycobacterium vaccae (SRL172) plus antimony to treat L. infantum-infected dogs. Although treatment with antimony alone cleared the parasite relapses in infection were seen in this group. Treatment with SRL172 alone was slower than antimony. The combination therapy also showed relapse in some dogs (Jamshidi et al. Reference Jamshidi, Avizeh, Mohebali and Bokaie2011) (Table 4).
Table 4. Vaccines as chemotherapeutic agents against leishmaniasis
POSSIBLE RECOMMENDATIONS TO USE NON PATHOGENIC L. TARENTOLAE AS IMMUNOTHERAPEUTIC TOOL
Leishmania tarentolae, which has never been associated with any human leishmaniasis, was first tested by Breton et al. as a vaccine candidate against leishmaniasis L. tarentolae can infect antigen-presenting cells such as macrophages and dendritic cells and can differentiate into amastigote-like forms, but it is unable to survive within macrophages or cause any clinical symptoms of the disease in hamsters or immunocompromised SCID mouse models (Breton et al. Reference Breton, Tremblay, Ouellette and Papadopoulou2005). Genome sequence analysis has revealed that L. tarentolae is syntenic to the three pathogenic Leishmania species (L. major, L. infantum and L. braziliensis) and more than 90% of the ~8200 parasite genes are shared by all Leishmania species. Nevertheless, some of the genes that were shown either to be important for pathogenesis or were preferentially expressed in the intracellular amastigote stage in the pathogenic species are absent in L. tarentolae or present in low copy numbers. This could explain the reduced capacity of L. tarentolae to live as an intracellular parasite and its diminished pathogenic potential in humans. Genetic manipulation and engineering of this non-pathogenic Leishmania strain could further improve its immunogenic potential as a live vaccine and induce a protective immunity against several Leishmania species, thus rendering this live vector one of the most promising attempts towards the development of an effective and safer anti-Leishmania vaccine. In our first attempt, the A2 gene, which is believed to contribute to the viscerotropic nature of L. donovani and L. infantum, was expressed in L. tarentolae and used as a vaccine against L. infantum infection in Balb/c mice. A protective response was associated with high levels of IFN-γ and low levels of IL-5 (Mizbani et al. Reference Mizbani, Taheri, Zahedifard, Taslimi, Azizi, Azadmanesh, Papadopoulou and Rafati2009). Other studies have tested the combination of live and DNA vaccination alone or together as a potent approach to immunize mice. In our recent work, recombinant L. tarentolae harbouring CPA/CPB along with salivary protein PpSP15 on a DNA plasmid was used as an experimental vaccine in C57BL/6 and Balb/c mouse models. The best results were obtained with priming with PpSP15 DNA followed by live recombinant L. tarentolae parasites expressing CPA/CPB and PpSP15 DNA as a booster regimen (Zahedifard et al. Reference Zahedifard, Gholami, Taheri, Taslimi, Doustdari, Seyed, Torkashvand, Meneses, Papadopoulou and Kamhawi2014). In another attempt, recombinant L. tarentolae expressing the tri-fused gene A2–CPA–CPB−CTE (CPB without C-terminal) were used as a new live vaccine strategy against VL. Two modalities, namely DNA/live and live/live vaccination, were administered to Balb/c mice, followed by L. infantum infectious challenge. We showed that an immunization with prime-boost DNA/live vaccination strategy elicited a promising immunization against a high-dose L. infantum challenge (Saljoughian et al. Reference Saljoughian, Taheri, Zahedifard, Taslimi, Doustdari, Bolhassani, Doroud, Azizi, Heidari and Vasei2013). Furthermore, we vaccinated outbreed dogs with a prime-boost regimen based on recombinant L. tarentolae expressing the tri-fused gene the A2–CPA–CPB and evaluated its immunogenicity and protective immunity against L. infantum infectious challenge. We showed that vaccinated animals developed partial protection with significantly higher levels of IgG2, but not IgG1, as well as IFN-γ and TNF-α, before and after challenge as compared to control animals. IL-10 levels were lower in the vaccinated animals after challenge (Shahbazi et al. Reference Shahbazi, Zahedifard, Taheri, Taslimi, Jamshidi, Shirian, Mahdavi, Hassankhani, Daneshbod and Zarkesh-Esfahani2015). Recently, we generated a recombinant non-pathogenic L. tarentolae-PpSP15 parasite and administered it along with CpG ODNs as a novel vaccine strategy against L. major infection in Balb/c mice. We observed high levels of IFN-γ and IL-17 production both pre- and post-challenge against L. major. This is the first report showing the efficacy and applicability of live non-pathogenic Leishmania secreting a sand fly salivary protein in the presence of CpG ODNs (Katebi et al. Reference Katebi, Gholami, Taheri, Zahedifard, Habibzadeh, Taslimi, Shokri, Papadopoulou, Kamhawi and Valenzuela2015).
Similar to previous studies where pathogenic strains of Leishmania have been utilized as immunotherapeutic agents, we highly recommended the use of L. tarentolae for this purpose. Combination of live L. tarentolae with different immunopotentioators such as CpG ODNs could be tested. The capacity to prepare different recombinant forms of the parasite with different genes, such as anti-microbial genes, cytokines, or chemokines, can create different opportunities for further investigation as immunotherapeutic tools by using live non-pathogenic L. tarentolae.
Several genetically-modified attenuated strains of L. donovani have been described in the past with similar potential to be used as immunotherapeutic tools (El-On, Reference El-On2009). Few examples include live attenuated strains of L. donovani lacking genes associated with virulence, such as the centrin 1 gene (Selvapandiyan et al. Reference Selvapandiyan, Debrabant, Duncan, Muller, Salotra, Sreenivas, Salisbury and Nakhasi2004), a growth regulating gene (Ldcen1 −/-) and p27 gene (Ldp27 −/-), an essential component of cytochrome c oxidase complex (Dey et al. Reference Dey, Meneses, Salotra, Kamhawi, Nakhasi and Duncan2010). These strains can be easily propagated as promastigotes but has limited replication as amastigotes. Recent clinical trials using animal models have been encouraging and confirm the safety, immunogenicity and efficacy of such genetically modified strains as vaccine candidates (Gannavaram et al. Reference Gannavaram, Dey, Avishek, Selvapandiyan, Salotra and Nakhasi2015).
DISCUSSION AND CONCLUSION
Most leishmaniasis treatments confront different obstacles from the complexity of the parasite nature to the negligence of pharmaceutical companies in designing suitable drugs due to the poverty of endemic countries. Only one drug is currently designed specifically for leishmaniasis treatment (Pentavalent antimony), and it causes hepatotoxicity in patients and resistance in parasite species over time (No, Reference No2016). Other medications, such as Miltefosine, AmB, Paromomycin and Pentamidine, also have different safety, side effect and cost problems, which makes them inapplicable for patients in endemic areas (Singh et al. Reference Singh, Singh, Chakravarty and Sundar2016).
Other than new attempts in leishmaniasis drug development with support from the Drug for Neglected Disease Initiative and WHO (Balasegaram et al. Reference Balasegaram, Ritmeijer, Lima, Burza, Ortiz Genovese, Milani, Gaspani, Potet and Chappuis2012), researchers have tried to potentiate routine treatments and alleviate side-effects by applying different approaches.
Through the tight interaction of the Leishmania parasite with the host immune system, the parasite tries to take advantage by suppressing cytokines and hiding in immune cells to persist in a mammalian host for an extended period of time (Ritter et al. Reference Ritter, Frischknecht and van Zandbergen2009). However, activation of the immune response through immunotherapy along with application of anti-leishmanial drugs can resolve the infection more easily. Immunotherapy also provides better opportunities for recovery in patients with non-healing Leishmania infections.
Besides cytokines, immune cells and vaccine candidates, non-pathogenic L. tarentolae is a new tool to modulate the immune response towards eliminating the infection. Recombinant L. tarentolae alone or in combination with other drugs could offer a novel mechanism to get rid of persistent infection in non-healing and immunocompromised patients who did not respond to regular therapies.
ACKNOWLEDGEMENTS
The authors would like to thank technical assistance of S. Alizadeh (Pasteur Institute of Iran, Immunotherapy and Leishmania Vaccine Research Department).
FINANCIAL SUPPORT
This project was supported by Iran National Science Foundation (grant number 43723) and Pasteur Institute of Iran (grant number 705) to S. R.








