It is projected that the worldwide prevalence of diabetes is likely to increase to more than 300 million by the year 2025 (King et al. Reference King, Aubert and Herman1998). Diabetes not only afflicts prosperous nations, but often reaches its highest frequency in poor and disadvantaged communities that can least afford the heavy burden of treatment and long-term complications. Although sulphonylureas, biguanides, insulin sensitizers (thiazolidinediones) and other current drugs are valuable in the treatment of type 2 diabetes mellitus, their use is restricted by cost, limited pharmacokinetic properties, secondary failure rates and accompanying side-effects (Krentz & Bailey, Reference Krentz and Bailey2005). Moreover, these therapies only partially compensate for metabolic derangements seen in diabetes and do not correct the fundamental biochemical lesion (Taylor & Agius, Reference Taylor and Agius1988). Natural products have been a source of medicinal treatments for thousands of years, and plant-based systems continue to play an essential role in the primary health care of approximately 80 % of the world's underdeveloped and developing countries (King et al. Reference King, Aubert and Herman1998). Thus, it is prudent in the current context to look for new and efficacious approaches from the vast reserves of phytotherapy.
Trigonilla foenum-graecum (fenugreek) is native to the area from the Eastern Mediterranean to Central Asia and Ethiopia, and is much cultivated in India, Pakistan and China (Morton, Reference Morton1990). It has a long history of medical uses in Ayurvedic and Chinese medicine, and has been used for numerous indications, including labour induction, indigestion and as a general tonic to improve metabolism and health (Basch et al. Reference Basch, Ulbricht, Kuo, Szapary and Smith2003). In India, the seeds of fenugreek are commonly consumed by people suffering from diabetes. An antidiabetic effect of fenugreek seeds has been demonstrated in experimentally induced diabetes in dogs, rats and mice (Ribes et al. Reference Ribes, Sauvaire, Da Costa and Loubatieres-Mariani1986; Amin et al. Reference Amin, Abdul-Ghani and Suleiman1987; Swanston-Flatt et al. Reference Swanston-Flatt, Day, Flatt, Gould and Bailey1989; Grover et al. Reference Grover, Yadav and Vats2002). It has also been reported to exert antihyperglycaemic effects in human subjects with both type 1 and type 2 diabetes (Riyad et al. Reference Riyad, Abfdul-Salam and Mohammad1988; Sharma et al. Reference Sharma, Raghuram and Rao1990; Madar & Shomer, Reference Madar and Shomer1998).
An active component of T. fenugreek seeds has been found to be associated with a defatted fraction, rich in fibre containing steroidal saponins and proteins (Valette et al. Reference Valette, Sauvaire, Beccou and Ribes1984). A novel amino acid derivative, 4-hydroxyisoleucine, also extracted from fenugreek seeds, has been shown to stimulate glucose-dependent insulin release from isolated rat and human islets (Sauvaire et al. Reference Sauvaire, Petit, Broca, Manteghetti, Baissac, Fernandez-Alvarez, Gross, Roye, Leconte, Gomis and Ribes1998). It has been reported also that fenugreek acts by delaying glucose absorption and enhancing its utilization (Raghuram et al. Reference Raghuram, Sharma, Sivakumar and Sahay1994). Effects of fenugreek on glucose uptake and utilization have been noted in peripheral tissues (Sharma, Reference Sharma1986) and an antioxidant effect has also been described (Ravikumar & Anuradha, Reference Ravikumar and Anuradha1999).
Trigonella foenum-graecum appears to exhibit a range of actions of potential benefit for the treatment of diabetes. In the present study, the antidiabetic effects of methanol extract-free residue of the fenugreek seed were evaluated. Possible mechanisms of action were elucidated using animal models of diabetes and in vitro cellular systems. This included assessment of carbohydrate digestion, absorption, gastrointestinal (GI) motility, and both the secretion and action of insulin.
Materials and methods
Plant materials and preparation of extract
Seeds of T. foenum-graecum were collected from a local market in Bangladesh and botanically authenticated with voucher specimens that were deposited in the National Herbarium, Bangladesh. Seeds were ground to fine powder by a cyclotec grinding machine. The powder (300 g) was extracted with aqueous 80 % ethanol (3 × 400 ml, 30 min each time) by refluxing and the supernatant was discarded. The remaining residue was further extracted with chloroform (3 × 750 ml, 30 min each time) at room temperature. From the remaining residue (100 g), methanol extract-free residue of soluble dietary fibre (SDF) was prepared (25·2 g) according to published methods (Theander & Westerlund, Reference Theander and Westerlund1986).
Animals and induction of type 1 and 2 diabetes
Male Long-Evans male rats were bred at BIRDEM (Dhaka, Bangladesh) and maintained on a 12 h light–dark cycle at 21 ± 2°C. A standard pellet diet and water were supplied ad libitum. The overall nutrient composition of the diet was 36·2 % carbohydrate, 20·9 % protein, 4·4 % fat and 38·5 % fibre, with metabolisable energy content of 11·8 MJ/kg (2820 kcal/kg). Induction of type 1 diabetes was performed by a single intraperitoneal injection of overnight fasted rats (180–220 g) with 65 mg streptozotocin/kg body weight freshly dissolved in 0·5 m-citrate buffer (pH 4·5). The blood glucose level was checked 7 d after streptozotocin administration. Animals having blood glucose levels >20 mm were considered to have diabetes. Type 2 diabetes was induced by a single intraperitoneal injection of 48-h-old rats with 90 mg streptozotocin/kg body weight (Bonner-Weir et al. Reference Bonner-Weir, Trend, Honey and Weir1981). Experiments were carried out on these animals 3 months after injection. Prior to the experiments reported, the diabetic rat models did not receive any therapy to control diabetes.
Acute and chronic effects of soluble dietary fibre of Trigonella foenum-graecum
To evaluate the acute effects on basal blood glucose, the SDF fraction was administered by gavage (0·5 g/kg body weight) to 12 h fasted non-diabetic, type 1 and type 2 diabetic rats. The control group received an equal volume of deionized water (10 ml/kg). In another set of experiments, the SDF fraction was similarly administered together to the three groups of rats with glucose (2·5 g/kg body weight). Controls received glucose only. To evaluate long-term effects of fenugreek on glucose homeostasis, SDF fraction (0·5 g/kg body weight) was administered to type 2 diabetic rats by gavage twice daily for 28 d. Control rats received an equal dose of water. Blood samples were collected from the cut tail tip at the times indicated in Table 1 and the figures. Terminal samples were collected from the aorta under pentobarbital anaesthesia. Serum was separated by centrifugation and stored at − 20°C until further analysis.
Table 1 Effects of 28 d treatment with soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on serum glucose and other parameters in type 2 diabetic rats†(Mean values and standard deviations, n 12)
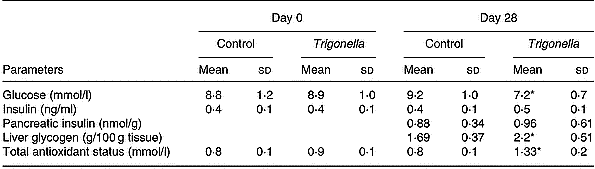
Mean values were significantly different from those of the Day 28 control group (unpaired t-test): *P < 0·05.
† Diabetes was induced by injection of neonatal rats with 90 mg streptozotocin/kg body weight 3 months previously. SDF extract of T. foenum-gracum seeds was administered by gavage (0·5 g/kg body weight) twice daily for 28 d. For details of procedures, see p. 516.
Effects of soluble dietary fibre of Trigonella foenum-graecum on sucrose absorption from the gut
The effects of fenugreek on sucrose absorption from the gut were determined by measurement of unabsorbed sucrose content after an oral sucrose load. Non-diabetic and type 2 diabetic rats, fasted for 12 h, received a 50 % sucrose solution by gavage (2·5 g/kg body weight) with or without SDF fraction of T. foenum-graecum (0·5 g/kg body weight). Blood samples were obtained from the tail vein before and 30, 60, 120 and 240 min after sucrose load for the determination of glucose. Some of the rats were killed at the same timings for determination of unabsorbed sucrose contents of the GI tract. The GI tract was excised and divided into six segments: the stomach, the upper 20 cm, middle and lower 20 cm of the small intestine, the caecum and the large intestine. Each segment was washed out with acidified ice-cold saline and centrifuged at 3000 rpm (1000 g) for 10 min. The resulting supernatant was boiled for 2 h to hydrolyse the sucrose following neutralization of the solution with NaOH. Blood glucose concentrations and the amount of glucose liberated from residual sucrose in the GI tract were measured. The GI sucrose content was calculated from the amount of liberated glucose (Goto et al. Reference Goto, Yamada, Ohyama, Matsuo, Odaka and Ikeda1995).
Effects of soluble dietary fibre of Trigonella foenum-graecum on intestinal glucose absorption
An intestinal perfusion technique (Swintosky & Pogonowskawala, Reference Swintosky and Pogonowskawala1982) was used to study the effect of fenugreek on intestinal absorption of glucose in 36 h fasted non-diabetic rats anaesthetized with sodium pentobarbital (50 g/kg body weight). The SDF fraction of T. foenum-graecum (10 mg/ml), equivalent to 0·5 g/kg, suspended in Krebs Ringer buffer, supplemented with glucose (54 g/l), was passed through the pyloris and the perfusate was collected from a catheter inserted at the end of the ileum. The control group received glucose alone. Perfusion was carried out at a constant rate of 0·5 ml/min for 30 min at 37°C. The results are expressed as a percentage of absorbed glucose, calculated from the amount of glucose in solution before and after the perfusion.
Effects of soluble dietary fibre of Trigonella foenum-graecum on intestinal disaccharidase activity and gastrointestinal motility
The SDF fraction of T. foenum-graecum was administered by a gavage (0·5 g/kg body weight) to 20 h fasted non-diabetic rats. After 60 min, the rats were killed and the small intestine was isolated, cut longitudinally, rinsed with ice-cold saline and homogenized in 10 ml saline (0·9 % NaCl). Aliquots of homogenate were then incubated with 40 mm-sucrose at 37°C for 60 min. Disaccharidase activity was calculated from the glucose concentration converted from sucrose as μmol/mg protein per h. For determination of GI motility, the experiment was carried out according to the method previously described by Chatterjee (Reference Chatterjee1993). After 60 min, BaSO4 milk (10 % w/v in 0·5 % sodium carboxymethyl cellulose) suspension was similarly administered and 15 min later, the rats were killed and the GI tract was excised. The visible distance traversed by BaSO4 milk was measured and expressed as a percentage of the total length of small intestine.
Effects of soluble dietary fibre of Trigonella foenum-graecum on insulin secretion
Long-Evans rats (180–250 g) were used for the studies on insulin secretion from the perfused pancreas and isolated islets. Pancreatic perfusion studies were carried out at 37°C according to the method of Giroux et al. (Reference Giroux, Portha, Kergoat, Bailbe and Picon1983). Pancreatic islets were isolated by collagenase digestion (Moskalewski, Reference Moskalewski1969). In both of these studies, SDF fraction of T. foenum-graecum was tested at 30 μg/ml. BRIN-BD11 cells were used to evaluate the effects on insulin secretion in a pure population of insulin-producing cells over a concentration range of 8–5000 μg/ml. The production and characteristics of this clonal cell line have been described in detail elsewhere (McClenaghan et al. Reference McClenaghan, Barnett, Ah-Sing, Abdel-Wahab, O'Harte, Yoon, Swanston-Flatt and Flatt1996).
Effects of soluble dietary fibre of Trigonella foenum-graecum on glucose uptake and insulin action
3T3-L1 cells (ATCC, Manassas, VA, USA) were seeded in twelve-well plates at a density of 100 000 cells per well (Orange Scientific, Sunnyvale, CA, USA) and fed every 2 d with Dulbecco's Modified Eagle's Medium supplemented with penicillin (50 U/ml), streptomycin (50 μl/ml) and fetal bovine serum (10 %, v/v). Cells were maintained at 37 ± 2°C and 5 % CO2. At confluency, adipocyte differentiation was initiated with 1 μg/ml insulin, 0·5 mm-3-isobutyl-1-methylxanthine and 0·25 μm-dexamethosone. Cells were maintained in this medium for 3 d followed by a further 3 d in medium supplemented with insulin (1 μg/ml) alone. Cells were returned to original medium for 1–2 d followed by incubation in serum-free medium for 2–3 h to establish basal glucose uptake. Cell monolayers were washed and then incubated for 15 min at 37°C in Krebs Ringer buffer supplemented with SDF fraction and insulin as indicated in the figures. After 15 min, glucose uptake was initiated, according to the established protocol of Frost & Lane (Reference Frost and Lane1985), by the addition of 50 μl tritiated 2-deoxyglucose (0·5 μCi/well) plus glucose (50 mm final concentration). This high glucose level was chosen to enable a good signal response to be recorded. The experiment was terminated after 5 min by three rapid washes with ice-cold buffer, after which cells were detached and lysed with 0·1 % SDS and subsequently lysed. Radioactivity was measured on a Wallac 1409 scintillation counter (Wallac, Turke, Finland) and glucose uptake was expressed as dpm (Frost & Lane, Reference Frost and Lane1985).
Analysis
Glucose was measured by the glucose–oxidase method, using kits from Sera Pak (Berkeley, CA, USA). Hepatic glycogen was estimated by using the anthrone method (Vander Vries, Reference Vander Vries1954). Protein contents were determined using the detergent compatible protein kit (Bio-Rad, Hercules, CA, USA). Total antioxidant status (Cobbaert et al. Reference Cobbaert, Arentsen, Mulder, Hoogerbrugge and Lindemans1997) was determined using ABTS® substrate assay kit according to manufacturer's instructions (Sera Pak). Insulin was measured by ELISA using kits supplied by Crystal Chem. Inc. (Downers Grove, IL, USA) or RIA (Flatt & Bailey, Reference Flatt and Bailey1981). Results are presented as means and standard deviations. Groups of data were compared using unpaired Student's t test and Mann–Whitney U test where appropriate. Where data were collected over a number of time-points, analysis was based on repeated measures ANOVA, with Bonferroni adjustment to ensure an overall error rate of 5 %. One-way ANOVA was performed and pair-wise comparisons to the control group made using Dunnett's test to preserve an overall error rate of 5 %. Differences were considered significant at P < 0·05.
Results
Acute and chronic effects of soluble dietary fibre of Trigonella foenum-graecum on glucose homeostasis
Administration of SDF fraction in the fasting state to either non-diabetic or diabetic (type 1 and type 2) rats did not exert any hypoglycaemic or glucose-lowering action (Fig. 1(A)). When administered by gavage simultaneously with an oral glucose load, the SDF fraction significantly countered the rise of serum glucose at 75 min (P < 0·01) in non-diabetic rats, and at 30 min (P < 0·05) in type 1 and type 2 diabetic rats (Fig. 1(B)). Oral administration of sucrose caused a rapid elevation in blood glucose with a peak at 30 min both in non-diabetic and type 2 diabetic rats (Fig. 2). This rise in blood glucose was significantly suppressed at 30 and 60 min by administration of the SDF fraction in all groups of rats (P < 0·05). Administration of plant extract twice daily for 28 d to type 2 diabetic rats significantly lowered (P < 0·05) serum glucose levels (Table 1). This was accompanied by 1·3–1·5-fold increases in total antioxidant status and hepatic glycogen, respectively (P < 0·05). Serum and pancreatic insulin did not differ between the extract fed and control groups (Table 1).

Fig. 1 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on (A) fasting serum glucose and (B) glucose tolerance in non-diabetic (upper panels), type 1 (middle panels) and type 2 (lower panels) diabetic rats. Fasted rats were given SDF fraction by gavage (0·5 g/kg body weight) with or without glucose (2·5 g/kg body weight). For details of procedures, see p. 515. Values are means with their standard deviations depicted by vertical bars (n 6). Mean values were significantly different from those of the control rats: *P < 0·05. •, Control; ◯, SDF fraction of T. foenum-graecum.
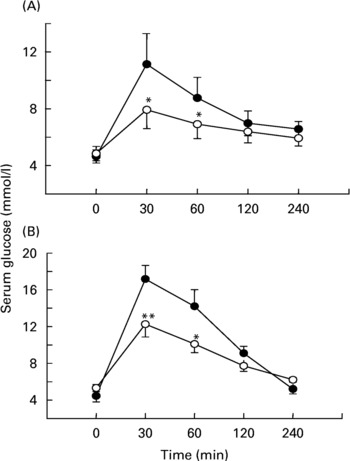
Fig. 2 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on serum glucose after sucrose load in (A) non-diabetic and (B) type 2 diabetic rats. Rats were fasted for 20 h and administered a sucrose solution (2·5 g/kg body weight) by gavage with or without SDF fraction (0·5 g/kg body weight). For details of procedures, see p. 515. Values are means with their standard deviations depicted by vertical bars (n 6). Significances are derived from repeated measures ANOVA and adjusted using a Bonferroni correction. Mean values were significantly different from those of the control rats: *P < 0·05; **P < 0·01. •, Control; ◯, SDF fraction of T. foenum-graecum.
Effects of soluble dietary fibre of Trigonella foenum-graecum on unabsorbed sucrose content of the gut
After oral administration of sucrose to non-diabetic rats, sucrose was detected at 30 min in the stomach (37 (sd 6) mg), and the upper (11 (sd 4) mg) and middle (9 (sd 3) mg) small intestine. At 1 h, sucrose was present in the stomach (10 (sd 3) mg) and the upper small intestine (2 (sd 0·9) mg). By 2 h, amounts of sucrose had decreased in the stomach (3 (sd 1) mg) and in the upper small intestine (1 (sd 0·7) mg). When the SDF fraction (0·5 g/kg body weight) was administered simultaneously with the sucrose load, the residual total sucrose content remaining in the GI tract was increased significantly (P < 0·05) compared to control (Fig. 3(A)). Similar effects of fenugreek (P < 0·05) were observed in type 2 diabetic rats after a sucrose load (Fig. 3(B)).

Fig. 3 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on gastrointestinal sucrose content after oral sucrose loading in (A) non-diabetic and (B) type 2 diabetic rats. Rats were fasted for 20 h prior to administration of a sucrose solution (2·5 g/kg body weight) by gavage with or without SDF fraction (0·5 g/kg body weight). For details of procedures, see p. 515. Values are means with their standard deviations depicted by vertical bars (n 6). Mean values were significantly different from those of the control rats: *P < 0·05. ■, Control;, SDF fraction of T. foenum-graecum.
Effects of soluble dietary fibre of Trigonella foenum-graecum on intestinal glucose absorption
As shown in Fig. 4, intestinal glucose absorption in non-diabetic rats was almost constant during 30 min of perfusion. Addition of T. foenum-graecum to the glucose perfusate resulted in a substantial decrease in intestinal glucose absorption during the whole perfusion period (P < 0·05).
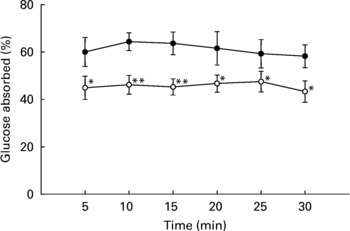
Fig. 4 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on intestinal glucose absorption in non-diabetic rats. Rats were fasted for 36 h and intestine was perfused with glucose (54 g/l) with or without SDF (10 mg/ml). For details of procedures, see p. 516. Values are means with their standard deviations depicted by vertical bars (n 6). Significances are derived from repeated measures ANOVA and adjusted using a Bonferroni correction. Mean values were significantly different from those of the control rats: *P < 0·05; **P < 0·01. •, Control; ◯, SDF fraction of T. foenum-graecum.
Effects of soluble dietary fibre of Trigonella foenum-graecum on intestinal disaccharidase activity and gastrointestinal motility
Oral administration of SDF fraction to non-diabetic rats significantly inhibited (P < 0·01) disaccharidase (sucrase) activity (Fig. 5(A)). When effects on GI motility were assessed in non-diabetic rats, the percentage traverse of BaSO4 was increased significantly (P < 0·01) by the SDF fraction compared to the control group (Fig. 5(B)).

Fig. 5 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on (A) intestinal disaccharidase activity and (B) gastrointestinal motility (by BaSO4 traversed) in non-diabetic rats. Rats were fasted for 12–20 h prior to administration of SDF fraction by gavage (0·5 g/kg body weight). Enzyme activity was determined and BaSO4 administered at 60 min. Motility was measured over the following 15 min. For details of procedures, see p. 516. Values are means with their standard deviations depicted by vertical bars (n 12). Mean values were significantly different from those of the control rats: **P < 0·001.
Effects of soluble dietary fibre of Trigonella foenum-graecum on insulin secretion
SDF fraction did not affect insulin secretion from perfused pancreas (Fig. 6(A)) or isolated islets when tested at 30 μg/ml. Insulin secretion from islets (ng/mg protein), expressed as median (range, n 8), was 2·99 (2·65–4·27) and 5·41 (4·91–9·27) at 3 and 11 mm-glucose, respectively (P < 0·01). Values were not significantly different in the presence of 30 μg/ml SDF fraction at 3·2 (2·18–3·42) and 5·52 (4·81–6·12) ng insulin/mg protein, respectively. Similar observations were noted when clonal BRIN-BD11 cells were tested at a range of concentrations from 8 to 5000 μg/ml (Fig. 6(B)). Alanine (10 mm) was used as positive control.

Fig. 6 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum on insulin release from perfused pancreas (A) and clonal BRIN-BD11 cells (B). Pancreas was perfused with SDF at 5 mg/ml with basal glucose of 2·8 mm. For details of procedures, see p. 516. Values are means with their standard deviations depicted by vertical bars (A, n 4; B, n 8). One-way ANOVA was performed and pairwise comparisons to control performed using Dunnett's test. Mean values were significantly different compared to 5·6 mm glucose: **P < 0·001. Ala, 10 mm alanine; G11mM, 11 mm glucose; Fr, soluble dietary fibre fraction of fenugreek.
Effects of soluble dietary fibre of Trigonella foenum-graecum on glucose uptake
SDF fraction significantly increased (P < 0·05) glucose uptake in 3T3 adipocytes compared to control (Fig. 7). The stimulatory effects were enhanced significantly in the presence of 10− 9m-insulin.

Fig. 7 Effects of soluble dietary fibre (SDF) fraction of Trigonella foenum-graecum (1000 mg/ml) on glucose uptake by 3T3-L1 adipocytes. For details of procedures, see p. 516. Values are means with their standard deviations depicted by vertical bars (n 6). One-way ANOVA was performed and pairwise comparisons were made using Dunnett's test to preserve an overall error rate of 5 %. Mean values were significantly different from those of the no insulin group: *P < 0·05; ***P < 0·001. Mean values were significantly different from those of the 10− 9 m-insulin alone group: ††P < 0·01; †††P < 0·001. ■, No insulin;, 10− 9 m-insulin; □, 10− 6 m-insulin.
Discussion
The present study was designed to investigate the antidiabetic actions of fenugreek seeds and the possible mechanisms of action of the SDF fraction. When administered orally, the SDF fractions improved glucose tolerance in normal, type 1 and type 2 diabetic rats. Longer-term administration to type 2 diabetic rats over 28 d also decreased fasting serum glucose. These observations accord with the growing body of evidence that seeds of T. foenum-graecum include natural products exhibiting a range of reproducible antidiabetic effects (Riyad et al. Reference Riyad, Abfdul-Salam and Mohammad1988; Alarcon-Aguilara et al. Reference Alarcon-Aguilara, Roman-Ramos, Perez-Gutierrez, Aguilar-Contreras, Contreras-Weber and Flores-Saenz1998).
It has been shown previously that the SDF fraction did not stimulate insulin secretion during a simple feeding study (Rokeya et al. Reference Rokeya, Hannan, Haque, Ali, Nahar, Mosihuzzaman and Azad Khan1998). This has been further confirmed by the present longer-term administration studies in type 2 diabetic rats and experiments using the perfused pancreas, isolated islets and clonal β cells. Thus 4-hydroxyisoleucine previously isolated as an insulinotropic agent from fenugreek seeds (Sauvaire et al. Reference Sauvaire, Petit, Broca, Manteghetti, Baissac, Fernandez-Alvarez, Gross, Roye, Leconte, Gomis and Ribes1998) will have been excluded from the SDF fraction by the earlier extraction procedure. Interestingly, the SDF fraction increased the glycogen content significantly in the liver of type 2 diabetic rats after 28 d treatment. Since hepatic glycogen is markedly decreased in diabetes and normalized by insulin treatment (Prasannan & Subrahmanyam, Reference Prasannan and Subrahmanyam1965; Welihinda & Karunanayake, Reference Welihinda and Karunanayake1986; Grover et al. Reference Grover, Yadav and Vats2002), this suggests that administration of the SDF fraction may have increased insulin action. Indeed, the present work indicates that fenugreek significantly enhanced glucose transport in 3T3-L1 adipocytes in the absence and presence of insulin. Such an effect was observed using a high glucose concentration in the buffer which may increase basal glucose uptake and even make the effect of insulin less apparent. Effects of fenugreek on glucose uptake and utilization have been also reported in peripheral tissues (Sharma et al. Reference Sharma, Sarkar, Hazra, Mishra, Singh, Sharma, Maheshwari and Maheshwari1996). The present findings may be broadly comparable with the actions of metformin, which include effects on glucose transporters, non-oxidative glucose metabolism and hepatic glucose output (Davidson & Peters, Reference Davidson and Peters1997).
Although a possible in vivo effect of the SDF fraction on insulin action in type 2 diabetic rats may be possible, it is less likely to explain the acute improvement of oral glucose tolerance in the severely insulin-deficient type 1 diabetic model. To evaluate the effects of T. foenum-graecum on carbohydrate absorption in the gut, an intestinal perfusion technique was employed. The SDF fraction significantly inhibited intestinal glucose absorption and suppressed the rise of blood glucose when administered simultaneously with oral sucrose. Consistent with this, sucrose was detected in the middle and lower part of the intestine in SDF-treated rats whereas the unabsorbed sucrose content of the whole gut was almost negligible in the control group by 4 h. Based on clinical studies, Sharma (Reference Sharma1986) proposed that fenugreek fibre-enriched diets induced a delay in the absorption of carbohydrate, thereby reducing insulin requirements. The findings from our studies and others (Al-Habori & Rahman, Reference Al-Habori and Rahman1998; Madar & Shomer, Reference Madar and Shomer1998) indicate that the antihyperglycaemic effects of T. foenum-graecum are mediated, at least partly, by decreasing intestinal glucose absorption. The observation that the SDF fraction increased sucrose content of the stomach in non-diabetic and type 2 diabetic rats at 30–60 min supports the notion that fenugreek also reduces gastric emptying (Nahar et al. Reference Nahar, Rokeya, Ali, Hassan, Nur-e-Alam, Chowdhury, Azad Khan and Mosihuzzaman2000). Dietary fibres such as guar gum similarly delay gastric emptying and are well known to reduce postprandial glycaemia (Jenkins et al. Reference Jenkins, Leeds, Gassull, Cohet and Alberti1977, Reference Jenkins, Jenkins, Kendall, Le Roith, Taylor and Olefsky2000).
In the present study, the SDF fraction also exerted in vivo inhibitory effects on the digestive enzymes, which is consistent with other reports (Wong et al. Reference Wong, Traianedes and O'Dea1985; Edwards et al. Reference Edwards, Johnson and Read1988; Riyad et al. Reference Riyad, Abfdul-Salam and Mohammad1988). The present findings suggest that reduction of sucrose absorption may be related to the inhibition of enzyme activity in the gut. Amin et al. (Reference Amin, Abdul-Ghani and Suleiman1987) suggested that a relatively low molecular mass fraction of aqueous extract of T. foenum-graecum was responsible for inhibition of carbohydrate-degrading enzymes. Such effects can be compared with the actions of acarbose, an α-glucosidase inhibitor, that effectively reduces postprandial hyperglycaemia in diabetic patients (Bressler & Johnson, Reference Bressler and Johnson1992).
GI disturbances are often disabling complications of diabetes. Metabolic abnormalities such as hyperglycaemia and electrolyte imbalance play a contributory role in the disruption of GI motility in diabetic patients. It has been well demonstrated that acute hyperglycaemia inhibits GI motility (Feldman & Schiller, Reference Feldman and Schiller1983). In the present study, the SDF fraction of fenugreek increased GI motility in non-diabetic rats. The present findings suggest that the inhibition of intestinal glucose absorption by the extract may partly reflect an increase in GI motility. Retardation of gastric emptying is one strategy employed for the development of antidiabetic drugs and accounts for part of the therapeutic actions of Byetta (Exenatide), stable peptide analogue of glucagon-like peptide 1 (7–36) amide and Pramlintide, stable analogue of Islet Amyloid Polypeptide, respectively (Green et al. Reference Green, Irwin, Gault, O'Harte and Flatt2005).
Although current treatment regimes decrease the risk of complications, oxidative stress associated with metabolic dysregulation continues to be a significant source of tissue damage (Genet et al. Reference Genet, Kale and Baquer2002). Administration of SDF fraction of T. foenum-gracum seeds to type 2 diabetic rats for 28 d resulted in an increase in total antioxidant status. Such antioxidant properties of the extract may be valuable in the prevention and possible reversal of diabetic complications as highlighted by others (Anuradha & Ravikumar, Reference Anuradha and Ravikumar2001; Genet et al. Reference Genet, Kale and Baquer2002).
In conclusion, the present study has demonstrated that SDF fraction of Trigonella foenum-graecum significantly improved glucose homeostasis in type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing or mimicking insulin action. Fenugreek is widely distributed and the seeds are commonly used as an edible item, implying a relative lack of toxicity. Fenugreek therefore represents a potentially useful dietary adjunct for the treatment of diabetes and a potential source for the discovery of new orally active antidiabetic agent(s).
Acknowledgements
The study was supported in part by the University of Ulster Research Strategy Fund and the Department of Pharmacology, Biomedical Research Group, BIRDEM, Dhaka, Bangladesh.










