Bone mineral content at older age is determined both by peak bone mass attained in young adulthood and by adult bone loss( Reference Davies, Evans and Gregory 1 ). While the clinical consequences of adverse bone health are seen predominantly in older age, accumulating evidence indicates that many predisposing factors arise in childhood and adolescence( Reference Davies, Evans and Gregory 1 ). Therefore, interventions designed to maximize bone health should start at an early age and continue through adolescence.
While peak bone mass is mainly genetically determined( Reference Heaney, Abrams and Dawson-Hughes 2 ), lifestyle – specifically dietary interventions promoting Ca and vitamin D (VitD) intakes during childhood and adolescence – play an important role, especially when sun exposure is not frequent( Reference Davies, Evans and Gregory 1 , Reference Julian-Almarcegui, Gomez-Cabello and Huybrechts 3 , Reference Julian, Gonzalez-Gross and Breidenassel 4 ).
Although there are some areas of debate, there is general agreement that prevention of VitD and Ca deficiencies is a public health priority. Health benefits from adequate VitD and Ca intakes include contribution to the maintenance of normal bones and teeth, growth and development of bone, absorption or utilization of Ca and P, blood Ca concentrations and maintenance of normal muscle function( Reference Golden and Abrams 5 ). For instance, the authors of a recent systematic review and meta-analyses about nutrient and food intakes on fracture risk concluded that adverse bone health events such as fractures may occur in children and adolescents not meeting Ca dietary recommendations( Reference Handel, Heitmann and Abrahamsen 6 ).
Unfortunately, previous findings on Ca and VitD intakes in European and US children and adolescents suggest very low compliance with Ca and VitD recommendations: approximately 95 % of adolescents do not meet the recommendations( Reference Huybrechts, Lin and De Keyzer 7 , Reference Mouratidou, Vicente-Rodriguez and Gracia-Marco 8 ). Natural VitD sources most notably are oil-rich fish and eggs, although small amounts provided by meat can be important in some countries( Reference Uusitalo, Kronberg-Kippila and Aronsson 9 ). Identifying both the food sources contributing most to daily Ca and VitD intakes and the families with children and adolescents with low Ca and VitD intakes will support the formation of targeted public health interventions when aiming to increase Ca and VitD consumption in European adolescents.
Previous regional studies with smaller sample sizes in children and adolescents have found associations of sociodemographic status and lifestyle factors with Ca and VitD intakes. A higher prevalence of inadequate Ca intake was reported in low-income Greek and Flemish children and pre-school children, as compared with their higher socio-economic counterparts( Reference Huybrechts, Lin and De Keyzer 7 , Reference Manios, Moschonis and Mavrogianni 10 ). Similarly, lower VitD and Ca intakes were related to family affluence in Brazilian adolescents( Reference Martini, Verly and Marchioni 11 ).
Lifestyle-related factors including physical activity (PA) and sedentary behaviours contribute in the variation in peak bone mass; however, its relationship with dietary patterns in children and adolescents has been scarcely studied( Reference Martini, Verly and Marchioni 11 , Reference Ramos, Costa and Araujo 12 ). Television (TV) viewing, for example, especially during high school, might have long-term effects on eating choices and contribute to poor eating habits in young adulthood( Reference Barr-Anderson, Larson and Nelson 13 ). However, studies investigating the relationship between TV viewing and Ca and VitD intakes specifically in European adolescents are still lacking.
Unfortunately, the aforementioned studies cannot be extrapolated to the whole European adolescent population and therefore it seems timely to investigate dietary sources of Ca and VitD intakes, and their associated sociodemographic and lifestyle factors, among European adolescents as a basis for future targeted public health strategies.
Methods
Study design
The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Cross-Sectional Study is a population-based multi-centre investigation of the nutritional and lifestyle status of adolescents, carried out in ten European cities (Vienna in Austria, Ghent in Belgium, Lille in France, Dortmund in Germany, Athens and Heraklion in Greece, Pécs in Hungary, Rome in Italy, Zaragoza in Spain and Stockholm in Sweden) between October 2005 and December 2008. A detailed description of its design and sampling procedures is published elsewhere( Reference Moreno, De Henauw and Gonzalez-Gross 14 ). The study population comprised adolescents aged 12·5–17·5 years. Adolescents were excluded from the database a posteriori if they met any of the following exclusion criteria: no measurement of weight and/or height; participated simultaneously in another clinical trial; or suffered from an acute infection the week prior to the examination. The total HELENA population consisted of 3528 eligible adolescents (52·3 % females). Participants from Heraklion and Pécs were excluded from the current analyses, as no nutrient intake information was available due to logistical problems. For the purpose of the current study, only adolescents who provided data on two non-consecutive 24 h dietary recalls were included (n 2330). Energy under-reporters (calculated using Goldberg’s cut-offs) were further excluded (n 526)( Reference Black 15 ) because the results of descriptive analyses showed that Ca and VitD intakes were significantly higher (P<0·05) in under-reporters compared with normal reporters. After exclusion, the final sample was 1804 participants (949 females).
The study was performed following the ethical guidelines of the Declaration of Helsinki 1964, the Good Clinical Practice rules and the legislation about clinical research in humans in each of the participating countries. All study participants and their parents provided a signed informed consent form. The protocol was approved by the Human Research Review Committees of the institutions involved.
Physical examination
Body weight (in kilograms) was measured using an electronic scale (type SECA 861; UK), precision 100 g and range 0–150 kg. Standing height (to the nearest centimetre) was measured on a stadiometer (type Seca 225; UK), precision 0·1 cm and range 70–200 cm. The triceps and subscapular skinfold thickness was measured on the left side of the body. A Holtain calliper (Crymych, UK) was used to measure skinfold thickness to the nearest 0·2 mm, and a non-elastic tape was used to measure circumference to the nearest 0·1 cm. Adolescents were classified on sexual maturation using Tanner’s five-stage scale following physical examination performed by a physician (stages I, II, III, IV and V)( Reference Tanner and Whitehouse 16 ).
Dietary assessment
Dietary intake was assessed by two non-consecutive computerized 24 h recalls, including weekdays and weekend days, and the mean of these two days (adjusted for within-person variability) was included as daily intake. The 24 h recalls were collected using the HELENA-Dietary Intake Assessment Tool (HELENA-DIAT) (17) . Trained dietitians assisted the adolescents to complete the 24 h recalls when needed. Adolescents autonomously selected all consumed foods and beverages from a food list in the HELENA-DIAT.
Foods and recipes recorded by the adolescents were converted into nutrients with a food composition database (German Nutrient Database, BLS) that contains a large number of nutrients and food items (approximately 12 000 coded foods). It has been demonstrated to be good at estimating nutrient intakes in European adolescents( Reference Julian-Almarcegui, Bel-Serrat and Kersting 18 ). The contribution of each of forty-four food groups to Ca and VitD intake was computed as the sum of the amount of Ca or VitD provided by the food divided by the total intake of that nutrient from all foods for the entire study population. Food and beverage consumption was expressed as grams and millilitres per day, respectively.
Based on the 24 h recalls, a validated diet quality index (DQI) was designed. This is composed of the five pillars guidelines presented in percentages: dietary quality, dietary diversity, dietary equilibrium, a meal index and a PA score( Reference Vyncke, Cruz Fernandez and Fajo-Pascual 19 ). For the purpose of the current analysis, the mean of these components was calculated in 1804 adolescents; as such, the DQI ranged from −33 to 100 %, with higher scores reflecting a higher diet quality. The score was calculated for each day and a mean of the daily scores was taken as global index score of the individual.
Adolescents were asked about taking any micronutrient supplements. Nevertheless, we did not find any difference in either Ca or VitD intake between consumers of supplements and non-consumers (data not shown).
Sociodemographic factors (socio-economic status and educational level)
A self-reported questionnaire was used to collect data on living conditions, family structure, employment status, and parental occupation and education level( Reference Iliescu, Beghin and Maes 20 ). The Family Affluence Scale (FAS) was used as an indicator of the adolescents’ material affluence (reflecting family expenditure and consumption). The scale ranged from 0 to 8; it was re-coded and dichotomized as ‘low familial wealth’ (score=0–4) and ‘high familial wealth’ (score=5–8). Parental educational level was also recorded (primary education, lower secondary education, higher secondary education or university degree) and later dichotomized into ‘lower education’ (primary and lower secondary education) and ‘higher education’ (higher secondary education and university degree).
Lifestyle factors (physical activity and television viewing)
PA records were obtained by means of two different tools: an adolescent-adapted version of the International Physical Activity Questionnaire (IPAQ-A) and accelerometers.
The original valid and reliable questionnaire, which was developed for adults aged 18–65 years, assessed PA of the last 7 d in four different domains (work; transport; house and garden; leisure time)( Reference Craig, Marshall and Sjostrom 21 ). To adapt the questionnaire to an adolescent study population, questions about PA at work were replaced by questions about PA at school, namely physical education, walking and moderate and vigorous PA at school. As in previous studies, moderate and vigorous PA were summed and combined into a single moderate-to-vigorous PA (MVPA) variable (expressed in min/week)( Reference De Cocker, Ottevaere and Sjostrom 22 ).
Uniaxial accelerometers (Actigraph MTI, model GT1M; Manufacturing Technology Inc., Fort Walton Beach, FL, USA) were used to objectively measure PA. Adolescents were asked to wear the accelerometer for seven consecutive days during all waking hours, except for water-based activities. At least three days of recording, with a minimum of 8 h registration per day, was set as an inclusion criterion. The time sampling interval was set at 15 s and bouts of ≥20 min of consecutive zero counts were deleted from the data sets. Total PA was expressed as total counts recorded divided by total daily registered time (counts/min).
A standardized self-reported sedentary behaviour questionnaire was administered to assess TV watching( Reference Rey-Lopez, Vicente-Rodriguez and Ortega 23 ). Adolescents reported the frequency of specified sedentary behaviours using predefined response categories separately for weekdays and weekends. The reliability (one-week test–retest) of the questionnaire has been studied previously in a similar group of adolescents, with the majority of the variables showing an almost perfect agreement (Cohen’s weighted κ value of 0·81 and 0·96 for weekdays and weekend days, respectively)( Reference Rey-Lopez, Vicente-Rodriguez and Ortega 23 , Reference Rey-Lopez, Ruiz and Ortega 24 ).
Statistical analysis
All analyses were carried out with the statistical software package IBM SPSS Statistics Version 20.0. Kolmogorov–Smirnov tests were carried out to test the normality of the studied variables. The Multiple Source Method was used to estimate usual intakes of nutrients and foods. This statistical modelling technique takes into account within-person and between-person variability and calculates usual intakes corrected for age, sex and study centre( Reference Haubrock, Nothlings and Volatier 25 ). The population proportion formula was used to determine the percentage contribution of each food group to Ca and VitD intakes. As mentioned before, this includes summing the amount of the nutrient provided by the food and then dividing by the total intake of that nutrient from all foods for the entire study population. Dietary sources were equally distributed for boys and girls, and consequently they are presented together.
Sex-specific mixed-model linear regression analyses were then used to verify associations between Ca and VitD intakes and parental education, FAS, PA and TV watching. Variables were included separately in the model. Study centre was included as the random intercept. Age, Tanner stage and energy intakes were entered as covariates in a first model (model 1) and the DQI entered afterwards in a second model (model 2).
Results
Table 1 shows descriptive characteristics of the participants. Mean Ca and VitD intakes were 833·9 (sd 372·2) mg/d and 1·8 (sd 0·9) µg/d, respectively. Boys had higher VitD and Ca intakes than girls. In addition, boys were more active than girls independently of the method used to measure PA (all P<0·05).
Table 1 Characteristics of the study participants; adolescents (n 1804) aged 12·5–17·5 years from eight European cities, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study
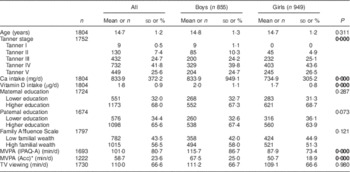
MVPA, moderate-to-vigorous physical activity; IPAQ-A, adolescent-adapted International Physical Activity Questionnaire; Acc, accelerometer; TV, television.
Data are presented as means and standard deviations for continuous variables, or as numbers and percentages for categorical variables.
Significant differences between genders are indicated in bold font (P<0·05).
Main Ca and VitD sources were the same for boys and girls. Hence, Table 2 shows the main Ca and VitD sources according to different food groups for boys and girls combined. Milk and cheese were the main Ca sources (23 and 19 %, respectively). Fish products were the main VitD sources (30 %). Cakes, pies and biscuits were the second main source of VitD in our population (16 %); these products are made mainly with butter and eggs, important VitD sources, and are consumed in high amounts.
Table 2 Dietary sources of calcium and vitamin DFootnote * among adolescents (n 1804) aged 12·5–17·5 years from eight European cities, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study
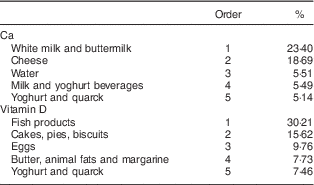
* Population proportion formula: sum of the amount of the nutrient provided by the food divided by the total intake of that nutrient from all foods for the entire study population (n 1804).
Table 3 presents the correlations of Ca and VitD intakes with sociodemographic and lifestyle factors. For boys, significant positive correlations were found between Ca intake and father’s education. In contrast, significant negative correlations were found between VitD intake and MVPA and TV viewing (all P<0·05). For girls, significant positive correlations were found between Ca intake and parental education (P<0·05; Table 3).
Table 3 Crude Spearman rank correlations of calcium (mg/d) and vitamin D (µg/d) intakes with sociodemographic and lifestyle factors among adolescents (n 1804) aged 12·5–17·5 years from eight European cities, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study
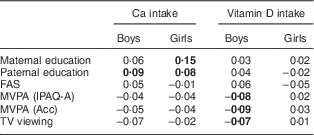
FAS, Family Affluence Scale; MVPA, moderate-to-vigorous physical activity; IPAQ-A, adolescent-adapted International Physical Activity Questionnaire; Acc, accelerometer.
Significant values are indicated in bold font (P<0·05).
Table 4 presents the results of the mixed-model linear regression analyses for Ca and VitD intakes and sociodemographic and lifestyle factors. The models were first adjusted for age, Tanner stage and energy intake (model 1) and further for diet quality (model 2). Ca intake was positively associated with maternal education (β=56·41; 95 % CI 1·98, 110·82) and negatively associated with TV viewing in boys (β=–0·43; 95 % CI −0·79, −0·07); however, these associations lost significance upon further adjustment for diet quality. In girls, Ca intake was positively associated with mother’s (β=73·08; 95 % CI 34·41, 111·74) and father’s education (β=43·29; 95 % CI 5·44, 81·14) and FAS (β=37·45; 95 % CI 2·25, 72·65). The association between Ca intake and mother’s education remained significant after further adjustment for diet quality (β=41·66; 95 % CI 0·94, 82·38).
Table 4 Mixed-model analyses between calcium (mg/d) and vitamin D (µg/d) intakes and sociodemographic and lifestyle factors among adolescents (n 1804) aged 12·5–17·5 years from eight European cities, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study

FAS, Family Affluence Scale; MVPA, moderate-to-vigorous physical activity; IPAQ-A, adolescent-adapted International Physical Activity Questionnaire; Acc, accelerometer.
* Model 1: adjusted for age, Tanner stage and energy intake; model 2: adjusted for model 1 and diet quality index.
Significant values are indicated in bold font (P<0·05).
Discussion
Main results
In this large European, population-based, cross-sectional study in adolescents, milk and cheese were the main food sources of Ca, and fish, cakes, pies and biscuits the main food sources of VitD. Ca intake was positively associated with parental education and FAS in girls, although only maternal education remained significant after adjusting for the DQI; and positively associated with mother’s education and negatively associated with TV viewing in boys, although both associations disappeared after adjusting for the DQI. To the best of our knowledge, the present study is the largest that has assessed dietary sources of Ca and VitD intakes and their determinants in European adolescents.
Similarly to our study, milk and cheese have been shown to be the main Ca sources in Flemish pre-school children( Reference Huybrechts, Lin and De Keyzer 7 ), younger Spanish children( Reference Royo-Bordonada, Gorgojo and de Oya 26 ) and US children( Reference Keast, Fulgoni and Nicklas 27 ); and fish the main VitD source in Spanish children( Reference Royo-Bordonada, Gorgojo and de Oya 26 ). Cakes, pies and biscuits were the second main source of VitD in our population; these products are made mainly with butter and eggs, important VitD sources, and are consumed in high amounts. In the USA and Canada, milk and ready-to eat cereals are usually the main VitD sources( Reference Keast, Fulgoni and Nicklas 27 , Reference Vatanparast, Calvo and Green 28 ); this may be due to either differences between European and American fortification policies or the fact that fish consumption is higher in Europe than in the USA or Canada( 29 ). In adults, in the USA, VitD-fortified milk makes the highest contribution to VitD intake and in Scandinavian countries oil-rich fish consumption is relatively high, although both fortification and supplementation policies have also been implemented( Reference Spiro and Buttriss 30 ). In the HELENA study, we use the German food composition database and therefore VitD contents in foods are representative of Germany, where the emphasis is on encouraging more outdoor sun exposure rather than on supplementation or fortification.
We observed associations between Ca intake and parental education in boys and girls, but they remained significant only for female adolescents when adjusting for diet quality. These results are in agreement with previous studies of Brazilian( Reference Lopes, Sichieri and Salles-Costa 31 ) and Mexican( Reference Rodriguez-Ramirez, Mundo-Rosas and Shamah-Levy 32 ) adolescents in whom Ca intake was shown to be inversely associated with socio-economic status. Female European adolescents usually present healthier and varied dietary patterns( Reference Richter, Heidemann and Schulze 33 ) compared with their male counterparts, which could explain differences by sex.
We did not find any association between TV watching and Ca or VitD intake in girls. TV viewing was a modifiable factor negatively associated with Ca intake only in boys, but this association disappeared when adjusting for diet quality. We failed to find an association between PA and VitD intake although previous studies have suggested that playing more outside and watching less TV can lead to higher levels of VitD in adolescents( Reference Absoud, Cummins and Lim 34 ); however, VitD status and intakes are not positively associated in the HELENA study sample( Reference Julian, Gonzalez-Gross and Breidenassel 4 ).
Strengths and limitations
The HELENA study has several strengths. The sampling procedure and the strict standardization of the fieldwork among the countries involved in the study avoided to a great extent the kind of confounding bias due to inconsistent protocols that makes comparing results from isolated studies difficult. In addition, the study includes important sets of confounders, i.e. age, diet quality and PA, which is crucial to analyse the association.
In contrast, our study has some of the inherited limitations of cross-sectional studies, mainly that the results cannot be interpreted in terms of cause-and-effect relationships. Assessing the diets of younger age groups is considered to be challenging, because their diets are highly variable from day to day. Thus, although adolescents are able to report, reports may be less accurate compared with adults( Reference Thompson and Subar 35 ).
Therefore, limitations in relation to the use of recalls as a method of assessment should be considered. For instance, recalled intakes are prone to under-reporting, rely on memory, require many days to capture an individual’s usual intake, are affected by within-person variation and provide imprecise estimation of servings. Although two days have been recommended as the minimum number of recalls required to correct for within-person variability or day-to-day variation, more than two measurements are still better when considering infrequently consumed foods such as fish for estimating usual intakes. Also, to compensate for the effect of within-person variation we used the Multiple Source Method( Reference Haubrock, Nothlings and Volatier 25 ). It should be noted as well that because of regular patterns of white milk consumption, Ca intakes generally have a relatively low ratio of intra- to inter-individual variance( Reference Moore, Bradlee and Gao 36 ). Furthermore, under-reporters were excluded in our sample to avoid unreliable dietary intakes( Reference Bel-Serrat, Julián-Almárcegui and González-Gross 37 ).
In addition, VitD intake calculated from the German Nutrient Database (BLS) could have been a rough estimate of the dietary VitD intake. The BLS is based on German, American, Swedish, Danish and Dutch food composition tables and thus on different analytical values. Meat and meat products have recently been shown to be better sources of VitD than previously thought( Reference Uusitalo, Kronberg-Kippila and Aronsson 9 ) because what is present in meat is mostly the intermediary metabolite, calcidiol, which on a molar basis is many times more potent than cholecalciferol( Reference Ovesen, Brot and Jakobsen 38 ). Two different updates of this food composition database have been released since the HELENA study was performed, in 2010 the German BLS 3·01 and in 2014 the German BLS 3·02( Reference Hartmann, Heuer and Hoffmann 39 ), and therefore VitD estimation may be more appropriate now than it was before. However, it is worth noting that the ideal approach would be to compare different nutrient data of different food composition databases especially when aiming to estimate the nutrient intakes in Europe.
Food fortification policy varies throughout Europe. Mandatory VitD fortification of margarine was introduced in some countries in Europe as a way of preventing VitD deficiency in children and today many breakfast cereals are also fortified with VitD. Finland and Sweden began to fortify their milk with VitD in the 1990s but, overall, fortification of milk is not widely practised in Europe. Contributions from supplements were not taken into account due to supplementation differences between countries. Nevertheless, we did not find any difference in either Ca or VitD intake between consumers of supplements and non-consumers (data not shown).
Although these data are now 5–8 years old, to date the HELENA study is the only large-scale study among European adolescents and thus provides a valuable resource and benchmark for studying trends in Europe.
Recommendations
According to previous literature, European adolescents present insufficient serum VitD concentrations( Reference Gonzalez-Gross, Valtuena and Breidenassel 40 ) and they do not comply with VitD and Ca intake recommendations. Sun exposure is the main VitD source; unfortunately, during wintertime and especially in north European countries sun exposure is not enough to achieve optimal serum VitD levels.
As food fortification policy varies throughout Europe, the promotion of Ca and VitD dietary sources seems to be the most appropriate option. In this regard, enhancing the consumption of fish and milk could importantly contribute to higher VitD and Ca intakes, respectively, particularly among lower socio-economic classes.
Future policy development and implementation needs to target the main sources of dietary Ca and VitD in various populations, including low-educated families. In addition to the promotion of fish and milk consumption, government and manufacturers should encourage children and adolescents to eat healthier to support bone health.
Conclusions
Milk and cheese were the main food sources of Ca intake and fish was the most important source of VitD. Differences were observed by sex; female adolescents with high-educated parents had higher intakes of Ca. Low-educated families with poor diet quality should be the main target when strategizing health promotion programmes to enhance dietary Ca and VitD intakes.
Acknowledgements
Acknowledgements: The authors would like to thank the participants and their families for the collaboration. Financial support: The authors gratefully acknowledge the financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). C.J. received a grant from the Ministerio de Educación, Cultura y Deporte (grant number FPU13/00421). Additional support was received from the Spanish Instituto de Salud Carlos III (CIBERObn, grant number CB12/03/30038). The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that they have no conflicts of interest that may affect the contents of this work. Authorship: L.A.M. coordinated the HELENA project at the international level. L.A.M., F.G., Y.M., K.W., D.M., A.K., M.S. and S.D.H. were involved in the design of the HELENA project and locally coordinated the HELENA projects. G.V.-R., L.G.-M., M.G.-G, J.V. and I.H. organized the fieldwork and performed the data collection locally. I.H. and M.K. were responsible for the database management. C.J., L.A.M. and I.H. worked on the conceptualization of the study. C.J., T.M. and I.H. were involved in manuscript drafting and statistical analysis. All authors reviewed the manuscript, gave input and made significant improvements. Ethics of human subject participation: The study was performed following the ethical guidelines of the Declaration of Helsinki 1964, the Good Clinical Practice rules and the legislation about clinical research in humans in each of the participating countries. All study participants and their parents provided a signed informed consent form. The protocol was approved by the Human Research Review Committees of the institutions involved.
HELENA Study Group. Co-ordinator: Luis A. Moreno. Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, Chantal Gilbert. Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Sara Castelló, Fréderic Gottrand, Mathilde Kersting, Michael Sjöstrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, Luca Scalfi. Project Manager: Pilar Meléndez. Universidad de Zaragoza (Spain): Luis A. Moreno, Jesús Fleta, José A. Casajús, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Fernández Alvira, Gloria Bueno, Aurora Lázaro, Olga Bueno, Juan F. León, Jesús Mª Garagorri, Idoia Labayen, Iris Iglesia, Silvia Bel, Luis A. Gracia Marco, Theodora Mouratidou, Alba Santaliestra-Pasías, Iris Iglesia, Esther González-Gil, Pilar De Miguel-Etayo, Cristina Julian, Mary Miguel-Berges, Isabel Iguacel. Consejo Superior de Investigaciones Científicas (Spain): Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Ligia Esperanza Díaz, Javier Romeo, Ana Veses, Belén Zapatera, Tamara Pozo, David Martínez. Université de Lille 2 (France): Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, Juliana Von Berlepsch. Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany): Mathilde Kersting, Wolfgang Sichert-Hellert, Ellen Koeppen. Pécsi Tudományegyetem (University of Pécs) (Hungary): Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs Angster, Enikö Nagy, Orsolya Kovács, Judit Répasi. University of Crete School of Medicine (Greece): Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas, Manolis Sbokos, Eva Protoyeraki, Maria Fasoulaki. Institut für Ernährungs- und Lebensmittelwissenschaften – Ernährungphysiologie, Rheinische Friedrich Wilhelms Universität (Germany): Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, Petra Pickert. University of Granada (Spain): Manuel J. Castillo, Ángel Gutiérrez, Francisco B. Ortega, Jonatan R. Ruiz, Enrique G. Artero, Vanesa España, David Jiménez-Pavón, Palma Chillón, Cristóbal Sánchez-Muñoz, Magdalena Cuenca. Instituto Nazionalen di Ricerca per gli Alimenti e la Nutrizione (Italy): Davide Arcella, Elena Azzini, Emma Barrison, Noemi Bevilacqua, Pasquale Buonocore, Giovina Catasta, Laura Censi, Donatella Ciarapica, Paola D’Acapito, Marika Ferrari, Myriam Galfo, Cinzia Le Donne, Catherine Leclercq, Giuseppe Maiani, Beatrice Mauro, Lorenza Mistura, Antonella Pasquali, Raffaela Piccinelli, Angela Polito, Romana Roccaldo, Raffaella Spada, Stefania Sette, Maria Zaccaria. University of Napoli ‘Federico II’ Dept of Food Science (Italy): Luca Scalfi, Paola Vitaglione, Concetta Montagnese. Ghent University (Belgium): Ilse De Bourdeaudhuij, Stefaan De Henauw, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere, Inge Huybrechts. Medical University of Vienna (Austria): Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit Kubelka, Marion Boriss-Riedl. Harokopio University (Greece): Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Stamatoula Tsikrika, Christos Karaiskos. Institut Pasteur de Lille (France): Jean Dallongeville, Aline Meirhaeghe. Karolinska Institutet (Sweden): Michael Sjöstrom, Jonatan R. Ruiz, Francisco B. Ortega, María Hagströmer, Anita Hurtig Wennlöf, Lena Hallström, Emma Patterson, Lydia Kwak, Julia Wärnberg, Nico Rizzo. Asociación de Investigación de la Industria Agroalimentaria (Spain): Jackie Sánchez-Molero, Sara Castelló, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez. Campden BRI (UK): Chantal Gilbert, Sarah Thomas, Elaine Allchurch, Peter Burgess. SIK – Institutet foer Livsmedel och Bioteknik (Sweden): Gunnar Hall, Annika Astrom, Anna Sverkén, Agneta Broberg. Meurice Recherche & Development asbl (Belgium): Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, Laurence Fontaine. Campden & Chorleywood Food Development Institute (Hungary): Andras Sebok, Tunde Kuti, Adrienn Hegyi. Productos Aditivos SA (Spain): Cristina Maldonado, Ana Llorente. Cárnicas Serrano SL (Spain): Emilio García. Cederroth International AB (Sweden): Holger von Fircks, Marianne Lilja Hallberg, Maria Messerer. Lantmännen Food R&D (Sweden): Mats Larsson, Helena Fredriksson, Viola Adamsson, Ingmar Börjesson. European Food Information Council (Belgium): Laura Fernández, Laura Smillie, Josephine Wills. Universidad Politécnica de Madrid (Spain): Marcela González-Gross, Jara Valtueña, David Jiménez-Pavón, Ulrike Albers, Raquel Pedrero, Agustín Meléndez, Pedro J. Benito, Juan José Gómez Lorente, David Cañada, Alejandro Urzanqui, Rosa María Torres, Paloma Navarro.







