INTRODUCTION
Q fever is a zoonosis caused by the Gram-negative, obligate intracellular bacterium Coxiella burnetii. The disease has been known since the 1930s and occurs worldwide, except Antarctica and possibly New Zealand [Reference Rodolakis1–Reference Raoult, Marrie and Mege3]. Q fever is a notifiable disease in only a few countries including Germany. The diagnosis is often overlooked due to its non-specific presentation in both humans and animals. As a result the true worldwide incidence is not known.
C. burnetii appears in three different morphological forms: large cell variants (LCV), small cell variants (SCV), and small dense cells (SDC), which can be differentiated by their morphology, physical and chemical resistance as well as antigenic and metabolic characteristics [Reference Arricau-Bouvery and Rodolakis4, Reference Heinzen, Hackstadt and Samuel5]. The SDC and the SCV in particular are considered to be the persistent forms in the host [Reference Arricau-Bouvery and Rodolakis4] and are responsible for the high resistance of C. burnetii to environmental stress. They possess high levels of resistance to UV radiation, heat, desiccation, sonication, and pressure as well as osmotic and oxidative stress. Due to these facts these bacteria are able to survive extracellularly as infectious particles for at least 150 days [Reference Arricau-Bouvery and Rodolakis4, Reference Welsh6].
Furthermore, C. burnetii displays antigenic variation by appearing in two different infectious forms, phase I and a less infectious phase II [Reference Porten7]. This variation is related to changes in the lipopolysaccharide (LPS) layer [Reference Wiebe, Shankel and Burton8–Reference Stoker and Fiset10]. Bacteria with a complete LPS (phase I LPS), are highly virulent, whereas bacteria with phase II LPS have an atrophied LPS and show lower virulence [Reference Woldehiwet9, Reference Runge and Ganter11]. C. burnetii infects several host species such as arthropods, birds, pets, domestic and wild mammals and humans [Reference Maurin and Raoult12], but ruminant livestock such as cattle, and in particular goats and sheep are identified as the most common sources of human infections [Reference Berri, Laroucau and Rodolakis13, Reference Baca and Paretsky14].
C. burnetii infections in goats and sheep are usually asymptomatic during the non-lambing period. The most common clinical manifestations appear during late pregnancy until lambing, i.e. abortion, stillbirth, and the delivery of weak offspring [Reference Tissot-Dupont and Raoult15, Reference Palmer16]. Other symptoms such as pneumonia, conjunctivitis and hepatitis occur rarely in infected animals [Reference Berri17]. Infected female animals shed large quantities of bacteria into the environment in the course of abortion or normal delivery, not only through the birth fluids, placenta, and fetal membranes, but also urine and faeces [Reference Berri17–Reference Arricau-Bouvery19].
Inhaling aerosols, which have been contaminated with these parturition products or urine and faeces of infected animals, respectively, is the most important route of human C. burnetii infections [Reference Berri, Laroucau and Rodolakis13, Reference Tissot-Dupont and Raoult15]. C. burnetii can also be transmitted, less commonly, through the consumption of raw milk and dairy products [Reference Tissot-Dupont and Raoult15, Reference Fishbein and Raoult20]. The diagnosis of acute Q fever in humans is frequently not made due to the non-specific nature of the illness. Features include fever, pneumonia, headache, and weakness. Chronic infection in humans can result in severe valvular endocarditis, granulomatous hepatitis, and rarely in osteomyelitis [Reference Berri17, Reference Fournier, Marrie and Raoult21].
Within the framework of a national Q fever research project, experiments were performed at the Research Station for Animal Husbandry, Animal Breeding and Small Animal Breeding, University of Hohenheim on the Swabian Alb. The research station consists of two farms, Lower Lindenhof, where pigs, cattle, and poultry are kept, at an altitude of 489 m, about 1 km away from the next village, and Upper Lindenhof about 2 km from Lower Lindenhof. Upper Lindenhof is situated in a solitary location on a plateau 720 m above sea level surrounded mainly by forest with the next village about 3 km away.
On this farm only goats and sheep are kept and a small number of houses and flats are let to employees and other residents.
In April 2009 the abortion rate in goats and sheep increased at Upper Lindenhof. The Chemical and Veterinary Investigation Office (CVUA) in Stuttgart conducted pathological examinations on three aborted kid foetuses and parts of one placenta confirming infection with C. burnetii in April 2009. Four people, living or working on the farm, were also diagnosed with Q fever infections in March, May and June 2009. They presented with non-specific clinical symptoms such as raised temperature, fatigue, headache, muscle pain and breast pain. In three cases Q fever was diagnosed by the family doctor, based on serological investigations. In the other case polymerase chain reaction (PCR) from a laryngeal lavage confirmed the suspicion.
In May 2009, all animals of the research station were examined for C. burnetii infection using enzyme-linked immunosorbent assay (ELISA) and PCR. ELISA and indirect immunofluorescence assay (IIFA) testing was performed on humans working or living on the farm in July 2010. Due to the isolated location of Upper Lindenhof this outbreak provided an opportunity to investigate vertical and horizontal transmission routes within sheep in the following lambing seasons and to follow-up the infectious situation of the flock until the end of 2012.
METHODS
Examinations were performed on 263 Merino land sheep and 165 Dahlem Cashmere goats (a triple purpose breed) as well as eight farm employees and 15 farm residents. Sheep and goats were kept separately in different barns, but grazed on the same pastures and were handled by the same shepherds. Delivery of 222 ewes occurred during February and March 2009, and of 72 goats during April and May 2009 with a further eight ewes during June and July 2009. Four of the infected employees were shepherds with direct contact with the animals. The other four infected employees were plant breeders, but they worked in close proximity to the animals as their premises and the fields were located between the different stables and pastures (Fig. 1). The residents' houses were located in the immediate vicinity of one of the three stables and some range land (Fig. 1).
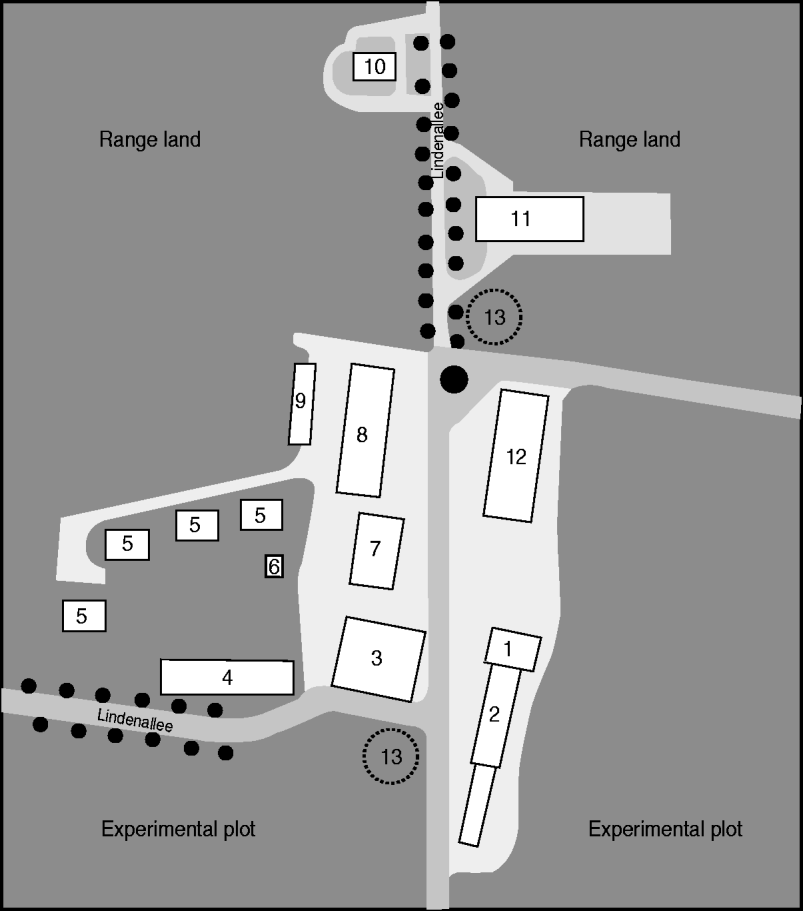
Fig. 1. Map of Upper Lindenhof. 1, Administration; 2, dairy goats barn and kid barn; 3, storage room; 4, laboratory – plant breeding; 5, residents' houses; 6, transformer station; 7, factory; 8, goat barn; 9, garages; 10, silo; 11, sheep barn; 12, machinery hall; 13, underground water tank.
Ethics statement
The experimental procedures on the animals were performed in accordance with the principles outlined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Sampling of the animals was performed by veterinarians of the University of Veterinary Medicine Hannover Foundation. Human blood samples were obtained within the framework of occupational investigations concerning the Q fever outbreak and taken by the Company Medical Office of the University of Hohenheim.
Samples
Goats and sheep
In May 2009, serum samples were obtained from 261 sheep and 146 goats. The animals were bled by venepuncture of the vena cava cranialis [Reference Ganter, Herrmann and Waibl22], the blood was centrifuged (10 000 g), and the supernatant was stored at 4°C.
Additionally, EDTA blood samples were acquired from 261 sheep and 142 goats. Vaginal swabs were taken from five ewes and 100 goats. Milk samples were taken from all nine ewes that were still lactating, and 64 goats. Before taking the milk samples, the udders were disinfected with a cloth moistened with alcohol (Desco Wipes, Dr. Schumacher GmbH, Germany). Rectal swabs were taken from nine sheep. The EDTA blood and milk samples, as well as the vaginal and rectal swabs were stored at 4°C and analysed within 24 h.
Humans
In July 2010, the 23 employees and residents had serum samples taken and completed a questionnaire compiled to obtain relevant information regarding age, gender, occupation, contact with the animals, pre-existing diseases, clinical symptoms, risk factors for Q fever, medical consultations and treatments.
PCR
Goats and sheep
DNA was extracted from swabs and milk samples with the Qiagen Mini kit (Qiagen, Germany) and the Nucleospin Tissue kit (Macherey-Nagel, Germany) according to the manufacturers' instructions. DNA extraction and PCR analyses of blood samples was performed temporally and spatially separated from milk samples and swabs with the Nucleospin Virus kit (Macherey-Nagel) using a Hamilton MicrolabStar robot (Hamilton, Germany).
The PCR for detecting C. burnetii was based on the method originally described by Willems et al. [Reference Willems23], which amplifies a 687-bp sequence of the IS1111 gene. In our investigations we used the LightCycler 2.0TM instrument (Roche Diagnostics, Germany). After the initial denaturation at 95°C for 10 min, 5 cycles were performed with denaturation at 95°C for 4 s, primer annealing at 75, 73, 71, 69 and 67°C for 8 s each and elongation at 77°C for 15 s. After this ‘touch-down’ 35 cycles were run, denaturating at 95°C for 4 s, annealing at 65°C for 8 s, and elongating at 72°C for 15 s. Amplification of specific DNA fragments resulted in a product with an average T m of 87·8°C (range 87·7–88·4°C). Negative controls to identify DNA carry-over and positive controls were included in each PCR run. Blood and tissue samples from uninfected sheep, which were negative in C. burnetii PCR in former investigations, were included in the DNA extraction procedure as negative controls and distilled water used as a no-template control. Placenta samples from a sheep infected with C. burnetii were used as a positive control.
ELISA and IIFA
Goats and sheep
Serum samples were analysed for the occurrence of antibodies against mixed phase I and phase II antigens of C. burnetii with a commercial ELISA (CHEKIT Q fever, IDEXX Laboratories, Germany) according to the manufacturer's instructions. The optical density (OD) of the positive control and the OD of the samples were corrected by subtracting the OD of the negative control. As recommended by the manufacturer, serum samples were considered to be positive by ELISA if they had a corrected OD value with a percentage of ⩾40%. They were considered negative if the percentage was <30%, and doubtful if it was between 30% and 40% compared to the positive control serum.
Humans
ELISA (Virion/Serion, Germany) was used as a screening test to detect C. burnetii antibodies (phase II IgG, phase II IgM) in human serum samples in accordance with the manufacturer's instructions. Seropositive samples were confirmed by an investigation with an IIFA, detecting phase II IgG and phase II IgM as well as phase I IgG and phase I IgM (Bios, Focus Diagnostics, USA). The sera were tested in different dilutions (1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024). A titre ⩾1:16 for the IgM and IgG response to phase II was considered as a diagnostic titre for Q fever.
RESULTS
Goats and sheep
Clinical signs
In February and March 2009, an abnormally high number (eight) of abortions in sheep in the late pregnancy stage was observed at Upper Lindenhof. At first this incident was associated with a sheep-shearing workshop which had taken place on the farm in January. No further investigations were made at this stage, even though an increase in the number of weak offspring of up to 16% was also observed.
In April 2009, eight abortions in the late pregnancy stage in goats and a high number of weak offspring (11%) within the goat flock prompted further investigation. C. burnetii infection was diagnosed in the aborted kid foetuses and a goat's placenta by CVUA.
During this season losses for goats amounted up to 25% (abortions, weak offspring) (Fig. 2); 14% losses were due to abortions and an additional 11% of offspring died within the first week due to weakness. In sheep the loss rate was up to 18%; 2% losses due to abortions and 16% losses due to weak offspring. Five percent of the lambs died within the first week and 11% between the second and tenth week postnatal. No other clinical signs were observed within the sheep and goat flocks.
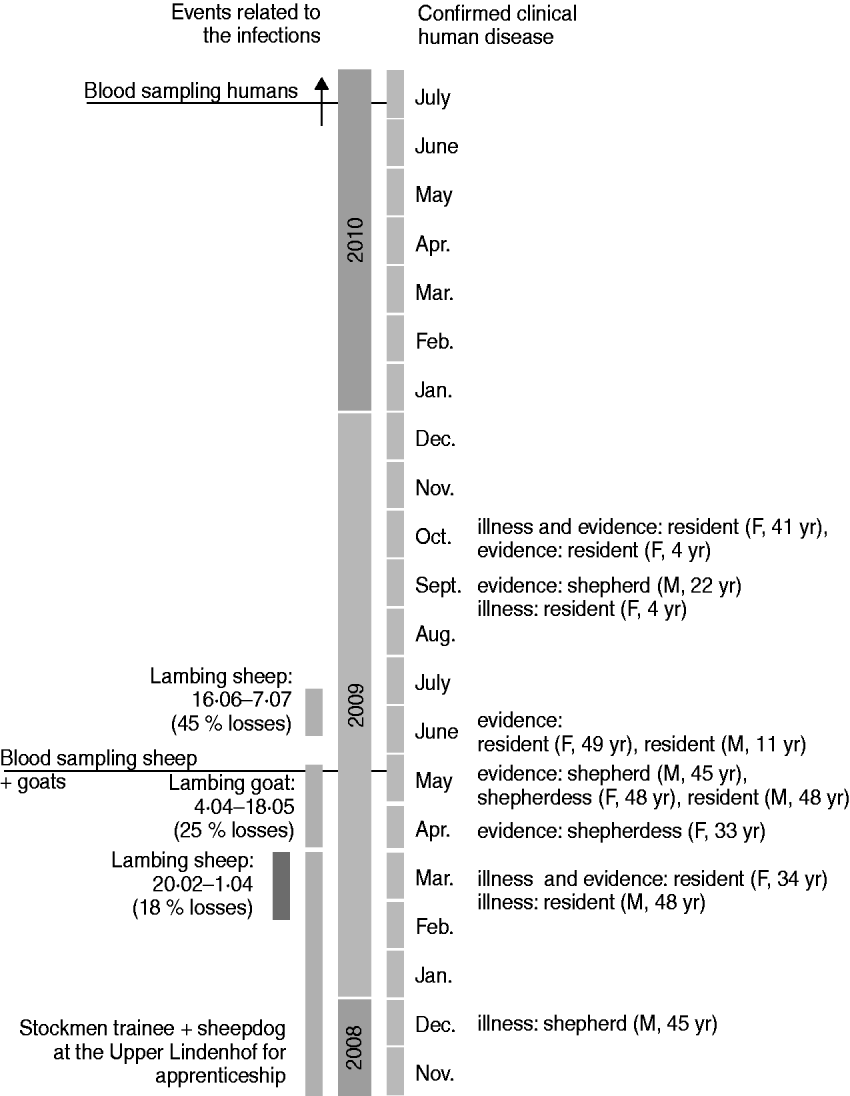
Fig. 2. Time scale 2008–2010.
Serological response
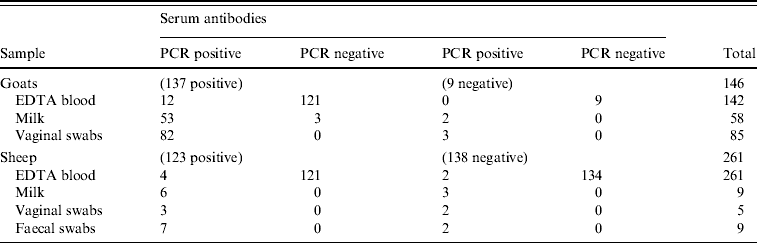
Antibodies against C. burnetii were detected by an ELISA in 123/261 sheep (47%) and 137/146 goats (94%). C. burnetii-specific DNA sequences were detected in 7/261 sheep blood samples (3%) and in 12/142 goat blood samples (8%) by PCR (Fig. 3). All vaginal swabs investigated (five sheep, 100 goats) were positive in the C. burnetii PCR (Fig. 3). Three of these sheep and 82 of these goats were also serologically positive (Table 1). All nine faecal swabs from sheep were positive. C. burnetii could be detected in 62/64 (97%) goat milk samples as well as in 7/9 (78%) sheep milk samples (Fig. 3). Table 1 shows the evaluation of the different sampling types in relation to the serum sample results.

Fig. 3. PCR results of blood, milk, vaginal and faecal swabs of sheep and goats of Upper Lindenhof in May 2009.
Table 1. Evaluation of the PCR results in different sampling types in relation to the serological results
Humans
Serology
Serological ELISA tests were performed in July 2010. All four stockmen, 2/4 of the plant breeders and 9/15 residents (60%) tested positive (Fig. 4). In total 15/23 humans were seropositive by ELISA and IIFA. There were no differences in age or gender distribution of the positive individuals (Table 2). All human cases had IgM antibody in low concentrations and phase I IgG antibodies with titres between 1:16 and 1:1024. Phase II IgG antibodies were present (between 1:64 and 1:1024) in all but one resident (age 50 years, male; 14/15 cases).

Fig. 4. Seroprevalence (IgG ELISA) within the three human groups living or working at Upper Lindenhof.
Table 2. First evidence of Q fever, evaluation and symptoms as well as age, gender and occupation – distribution of people living or working at Upper Lindenhof and serum titres of the C. burnetii-positive individuals in July 2010
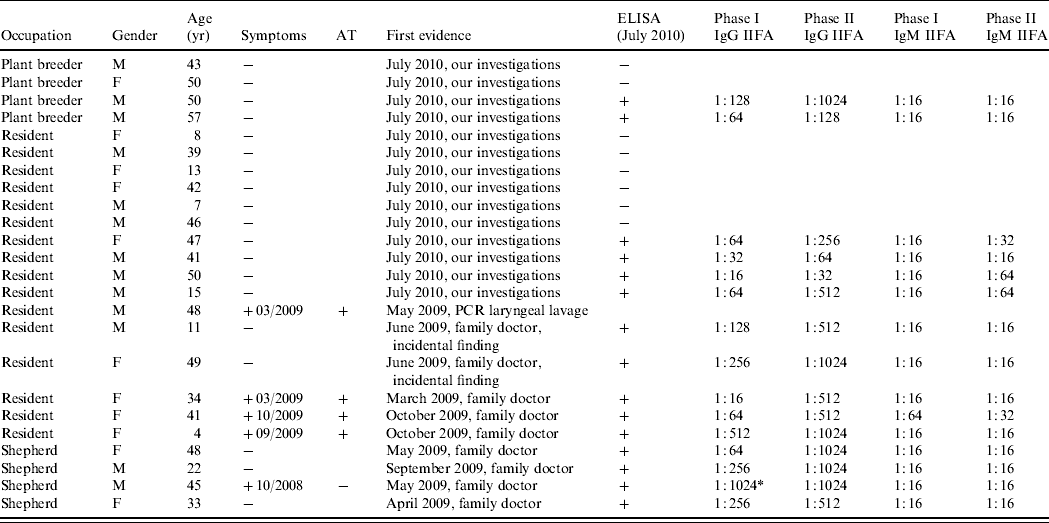
AT, Antibiotic treatment; +, positive; −, negative; M, male; F, female.
* Possible chronic manifestation.
Clinical signs in humans
Four residents reported clinical signs including severe headache, high temperature, fatigue, and muscle pain. Two residents had already been diseased in March 2009, the other two in September and October 2009, respectively (Fig. 2). Three of these residents had been diagnosed with Q fever by their family doctor based on clinical signs and serological results (no details available). The fourth patient had to be admitted to hospital because of severe pneumonia in March 2009. In May 2009 his laryngeal lavage was positive by PCR for C. burnetii. Unfortunately, he did not take part in the blood examinations in July 2010 and therefore no serological data are available.
The four stockmen on the farm were diagnosed by their family doctors after Q fever was recognized in the flocks. Only one of them showed any clinical symptoms typical for Q fever, i.e. severe headache, fatigue, muscle pain and chest pain, in December 2008 (Fig. 2).
In June 2009 two residents consulted their family doctor for other matters and were found incidentally to have antibodies to Q fever. The other ten residents and the four plant breeders did not know their serological status until our investigations took place in July 2010. In the serum samples of four residents and two plant breeders, antibodies against C. burnetii were found, but they could not remember any clinical symptoms within the previous 2 years (Table 2).
DISCUSSION
This case study describes the situation on an animal farm where an outbreak of Q fever occurred in humans which was temporally related to infection of a goat and sheep flock at an isolated site.
Animal losses within the sheep and goat flock were more frequently due to weak offspring than abortions in this outbreak. In general, in a Q fever-positive flock of small ruminants the abortion rate can vary from 3% to 80% [Reference Arricau-Bouvery and Rodolakis4, Reference Palmer16]. Q fever should therefore be considered as a potential aetiological agent in flocks with increased losses due to weak offspring, regardless of the frequency of abortions.
In this study goats were more severely affected as they suffered more abortions and delivered more weak offspring than sheep. This study also shows that animals (particularly sheep) can carry and shed C. burnetii, without displaying significant clinical signs of illness [Reference Berri17]. According to our results, goats as well as sheep shed C. burnetii in their vaginal mucus, milk or faeces. This fact corroborates earlier studies where infected dairy animals shed a high number of C. burnetii not only with the products of conception [Reference Abinanti18, Reference Hatchette24], but also with urine, faeces, and milk, for up to several months after parturition [Reference Arricau-Bouvery19, Reference Astobiza25]. The study demonstrates that during an acute outbreak of Q fever bacteria were excreted through all possible shedding routes by ewes and through milk and vaginal mucus by goats, although the small numbers of samples mean that statistical analysis was not possible. Goats showed a considerably higher seroprevalence compared to sheep. The different seroprevalences may not be a result of different susceptibility to the infection as we did not know the precise time of entry of C. burnetii onto the farm or the route of transmission. Nonetheless, it is assumed that goats showed more clinical signs than sheep and that goats appeared more susceptible to C. burnetii infection.
The PCR results from blood samples showed a slightly higher infection rate in goats. This difference may not have been as a result of susceptibility to the infection, but might be related to the date of onset of the infection in the goat herd. The PCR results of the other shedding routes did not show any significant distinctions.
In human serum samples, Fournier & Raoult [Reference Fournier and Raoult26] as well as Schneeberger et al. [Reference Schneeberger27] showed that DNA of C. burnetii can be detected up to 17 days after onset of the disease. Similarly, Rolain & Raoult [Reference Rolain and Raoult28] found DNA of C. burnetii in blood samples of patients with active Q fever. Marmion et al. [Reference Marmion29] detected C. burnetii DNA in peripheral blood mononuclear cells (PBMC) and bone marrow until up to 12 years after an acute Q fever infection in humans, but only in cases of chronic fatigue syndrome. As C. burnetii infects monocytes/macrophages [Reference Angelakis and Raoult2] bacterial DNA may remain detectable until these cells are renewed. Therefore it can be expected that PCR positivity of EDTA blood samples may be detected for longer as EDTA contains cellular components. The detection of C. burnetii DNA in serum or EDTA blood samples in humans suggests either a recent infection or chronic infection (e.g. endocarditis). The 3% of sheep and 8% of goats that were PCR positive in blood is consistent with a recent infection in this situation. A follow-up study should be performed to elucidate whether the animals have an acute infection or chronic Q fever. To the best of our knowledge, this is the first report on the detection of C. burnetii in blood samples of infected animals.
Unfortunately, due to organizational problems and legal reasons, the blood samples of the residents, the stockmen, and the plant breeders were taken 14 months after the first sampling of the sheep and goats. Only the stockmen and five of the farm residents had visited their family doctor prior to this, when Q fever was diagnosed. Two adult residents with clinical symptoms received antibiotic treatment (adult 1: penicillin and doxycycline each for 10 days; adult 2: doxycycline for 10 days). Remarkably, three children (aged 4, 11, 15 years) showed an increased antibody activity against C. burnetii in our investigations in July 2010. Nonetheless, only in the case of the 4-year-old child was the family doctor consulted during the acute infection, the child received antibiotic treatment against Q fever (erythromycin for the first 10 days, after no improvement ciprofloxacin for a further 10 days). The other two children did not notice any clinical signs or associate them with Q fever, which indicates seroconversion only. These individuals only became aware of their serological status because of our investigations in July 2010. The children reported that they had been in the stables frequently to stroke the lambs and watch the births during the lambing period. Even if the samples of the animals and the humans were not taken at the same time, the temporal relationship between the infections of the different host species suggests transmission between species.
The immediate vicinity of the dwellings, the office buildings and the stables (Fig. 1) explains why people living or working on the farm or its immediate surroundings were exposed to a high risk of infection. It is likely that most of the people with a positive antibody titre contracted the disease during the lambing period in spring 2009 whether they were symptomatic or not.
The titres (Table 2) were also consistent with the assumption that the infections took place some time previously and therefore probably at the time of lambing. IgM antibodies appear within 7 days on average and the IgG antibodies about 14 days after the onset of the acute infection [Reference Guigno30]. IgM antibodies reach their maximum levels after 4–8 weeks and then decrease gradually over the following 10–12 weeks [Reference Dupuis31], and in some cases up to 17 weeks [Reference Hunt, Field and Murphy32]. At least 4–6 months after the acute infection, as in this study, the antibodies will normally have disappeared or be present only at low levels [Reference Wagner-Wiening, Brockmann and Kimmig33, Reference Kimmig, Wagner-Wiening and Neumeister34]. IgG antibodies against phase I and phase II antigen generally decrease gradually after 4–6 months, but phase II IgG antibodies can persist at a low titre (about 1:64 to 1:1024) over several years [Reference Kimmig, Wagner-Wiening and Neumeister34]. The titres in this study are consistent with this pattern in that the IgM antibodies were at a low level or negative and the phase II IgG antibodies were elevated at a level between 1:64 and 1:1024 in all cases except one (50-year-old male resident) (Table 2). We expect that the IgG antibodies against phase I antigen will decrease. The male shepherd (45 years, with phase I IgG antibodies of 1:1024) remains under serological follow-up because of the risk of chronic infection with IgG antibodies against a phase I IgG antigen of 1:1024 and persistently high phase II IgG antibodies [Reference Wagner-Wiening, Brockmann and Kimmig33]. It is not known whether he showed any clinical symptoms of chronic infection at the time of sampling. While we expect that the titres in these individuals represent recent infection it is possible that some of the positive results reflect past exposure particularly as the occupations of the individuals tested would put them at high risk of previous infection.
If we accept that all the seropositive individuals acquired their infection during this outbreak then 2/3 people in the farm village were infected during or just after the lambing period. This high rate of positivity would be consistent with the close contact between humans and animals in this village and with the rather long exposure time generated through shedding during three lambing periods [two lambing periods in sheep and one in goats (Fig. 2)].
The rate of infected humans with clinical symptoms compared to those who were seropositive without any clinical signs within the time of exposure was 1:2·2. The ratio of symptomatic or asymptomatic reported cases to non-reported cases was 1:1·6. This contrasts with Q fever outbreaks described previously where the relationship between reported cases and non-reported cases without or with only mild clinical symptoms was 1:3·5 (S. F. Fischer, unpublished data). A possible reason for this might be the awareness of Q fever by the people living or working at the farm in question and therefore an awareness of Q fever by their family doctors.
We propose that Q fever infection was introduced into the herd by a trainee stockman and his sheepdog, who were at Upper Lindenhof for an apprenticeship from the beginning of 2008 until April 2009 (Fig. 2). We suspect the C. burnetii infection was carried from the parental farm (where Q fever was known to be present in spring 2008) to Upper Lindenhof. Unfortunately as we were unable to obtain any samples from the trainee and his dog this hypothesis could not be confirmed.
The temporal relationship makes us suspect that they were possible vectors for the bacteria carrying it from one farm to the other, e.g. in the dog's coat or in the trainee's working clothes. As one of the stockmen became ill in December 2008 it can be speculated that Q fever had already been on the farm at the end of 2008. At that time the infection in the flock was probably disregarded, because the human patient was not checked for Q fever until May 2009 and there was no lambing period in the sheep and goat flocks. Thus, the typical clinical signs such as abortion, stillbirth and the delivery of weak offspring did not occur in the flocks. Other obvious clinical signs in the non-lambing period were not established, so possible infected animals could not be observed.
Nevertheless, retrospectively it is impossible to be certain through which route the bacteria were transmitted to the farm. Infection through introduction of new stock can be ruled out in this case, because only rams were purchased, but their serological and PCR results (blood, faecal, prepuce swabs) were negative in the tests in summer 2009. Finally four possible options of introduction remained:
-
(1) The theory concerning the trainee stockman, since the stockman's illness in December 2008 was Q fever. We believe that this is the most likely route of transmission.
-
(2) The possibility that shepherds, who attended the sheep-shearing workshop at the end of January 2009, transmitted C. burnetii to the flock via their work clothes, instruments or vehicles.
-
(3) The possibility that other animal species, e.g. pigs, cattle, and poultry kept in the valley section of the research station might already be infected. Some of the employees were in contact with both sections and all animals and machinery. Unfortunately, it was not possible to sample the animals at Lower Lindenhof, so we cannot rule out this infection route.
-
(4) The possibility of transmission by wildlife or rodents cannot be excluded, especially because we were unable to sample any of them.
However, these are only four known routes as to how C. burnetii could have been transmitted to this farm and several other unknown routes could also be possible.
No clinical Q fever infections, either in humans or in other host species, were notified at Lower Lindenhof and in the surrounding villages at the time of the outbreak. The isolated site of Upper Lindenhof and the fact that the farm was enclosed by forest on three sides, thus restricting the windborne spread of C. burnetii [Reference Tissot-Dupont35], probably explains the localized nature of the outbreak.
These investigations were the beginning of a series of experiments which took place at Upper Lindenhof at the Research Station for Animal Husbandry, Animal Breeding and Small Animal Breeding, University of Hohenheim. It is hoped that further investigations will enhance our understanding of the pathogenesis, the epidemiology and a possible treatment of Q fever in small ruminants in order to reduce or even avoid human infections.
ACKNOWLEDGEMENTS
The investigation was funded by the Federal Ministry of Education and Research, Germany, Grant 01KI0734. We thank CVUA Fellbach for providing the results of their investigations in April 2009. Moreover, we are grateful for the investigations performed and the support given by the Research Station for Animal Husbandry, Animal Breeding and Small Animal Breeding, University of Hohenheim and its employees. Furthermore, we thank Frances Sherwood-Brock for reading the English manuscript. The authors also thank Ingola von Keyserlingk, Sabine Baumann, Sandra Schöbel, Marie Hasselmann (LAVES), and Malte Diederichsen (Clinic for Swine and Small Ruminants) for their excellent laboratory technical assistance.
DECLARATION OF INTEREST
None.








