Elevated plasma LDL has been implicated in the pathogenesis of atherosclerosis(Reference Gotto1). Oxidised LDL (ox-LDL) stimulates the proliferation of aortic smooth muscle cells and the up regulation of extracellular signal-regulated kinases (ERK)(Reference Yang, Chien, Hsiao, Pan, Wang, Chiu and Lin2). Therefore, the ox-LDL-induced proliferation of vascular smooth muscle cells (VSMC) in the intima of the arterial wall is believed to be a critical event in the development of atherosclerotic plaque.
Several studies have indicated that naturally occurring polyphenols can inhibit migration and proliferation of vascular cells(Reference Iijima, Yoshizumi and Hashimoto3, Reference Paper4). Polyphenols found in green tea have been shown to inhibit LDL-induced human VSMC proliferation and to arrest endothelial cells in the G1 phase of the cell cycle(Reference Locher, Emmanuele, Suter, Vetter and Barton5). Red wine polyphenols have also been found to decrease the proliferation and migration of VSMC by down regulating cyclin A expression and inactivating of the ERK pathway(Reference Iijima, Yoshizumi and Hashimoto3, Reference Liu and Liu6).
The protein proliferating cell nuclear antigen (PCNA) has been implicated in a number of cellular processes, including DNA replication, DNA repair, and cell-cycle regulation(Reference Krishna, Kong, Gary, Burgers and Kuriyan7–Reference Waga, Hannon, Beach and Stillman9). It has also been reported that PCNA synthesis is strictly regulated during the cell cycle, and that there is a clear increase in PCNA protein during the G1/S transition. It is evident that PCNA represents a key protein necessary for the transition of cells from quiescence to the S phase(Reference Morris and Mathews10).
Ellagic acid is a phenolic compound present in berries and nuts(Reference Sellappan, Akoh and Krewer11–Reference de Ancos, Gonzalez and Cano13). It has been found to have antioxidative properties and to inhibit LDL oxidation(Reference Priyadarsini, Khopde, Kumar and Mohan14). Our recent study also indicated that ellagic acid lowers oxidative stress and reduces atherosclerosis in hyperlipidaemic rabbits(Reference Yu, Chang, Wu and Chiang15).
We investigated the effect of ellagic acid on ox-LDL-induced proliferation of rat aortic smooth muscle cells (RASMC). We hypothesised that this effect is due to the activation of ERK 1/2 and the expression of cell-cycle regulatory protein PCNA.
Materials and methods
Isolation and preparation of oxidised low-density lipoprotein
LDL was isolated from normal human plasma by ultracentrifugation in the presence of EDTA (1 mmol/l) in a KBr gradient at 200 000 g for 14 h at 4°C. The LDL fraction was decanted and dialysed against three changes of 1500 ml of NaCl (150 mmol/l) and EDTA (0·25 mmol/l) at pH 7·4. LDL was sterilised using 0·22 μm Millipore filters and stored in the dark at 4°C. Concentrations of LDL are given as μg LDL protein. LDL (1–2 mg/ml) was mildly oxidised at 37°C in the presence of CuSO4 at a final concentration of 5 μmol/l for 24 h. The oxidation was terminated by the addition of EDTA (1·0 mmol/l). Ox-LDL fractions were dialysed against PBS with EDTA (0·1 mmol/l). The purity and the extent of the oxidation of LDL were determined by the formation of thiobarbituric acid reactive products according to the method of Ohkawa et al. with minor modifications(Reference Ohkawa, Ohishi and Yagi16).
Cell culture and measurement of cell viability
Rat thoracic aortic smooth muscle cells (RASMC) were obtained from the National Health Research Institute, and cultured in Dulbecco's modified Eagle medium (Gibco, Carlsbad, CA, USA) at 37°C in a humidified incubator with an atmosphere of 95 % air and 5 % CO2. Cells were subcultured into a ninety-six well plate with 1 × 104 cells per well in 100 μl RPMI-1640 medium (Sigma, St Louis, MO, USA) and then treated with ox-LDL (100 μg/ml), with or without ellagic acid (50 μmol/l) for 24 h. All subcultures were done in triplicate. At the end of the incubation period, the cells were harvested and washed with PBS. Then, 10 μl of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-terazolium (MTT) (Sigma) were added to the cells and incubated for 3 h at 37°C. The cells were then incubated overnight with 100 μl solution buffer (10 % SDS; HCl (0·01 mol/l)). The absorbance was measured on an ELISA reader (Multiskan EX; Labsystems, Helsinki, Finland) at a test wavelength of 570 nm, and a reference wavelength of 650 nm(Reference Lee and Surh17).
Cell-cycle analysis
Cellular DNA contents were measured by flow cytometry as described previously(Reference Chen, Hsieh, Chang and Chung18) in order to estimate the proportion of RASMC in different phases of the cell cycle affected by ellagic acid. Approximately 5 × 105 cells/well of subcultured cells, which had been treated with or without ellagic acid, were harvested by centrifugation. They were fixed with 70 % ethanol on ice before being stored at 4°C overnight. The next day, the cells were finally re-suspended in PBS containing propidium iodide (40 μg/ml) andRNase (0·1 mg/ml; Sigma, St Louis, MO, USA), and 5 % triton X-100 (Amresco, Inc., Solon, OH, USA) for 30 min at 37°C. Cells were then analysed in a flow cytometer (Becton-Dickinson, San Jose, CA, USA) equipped with an Ar ion laser at a wavelength of 488 nm in order to determine the cell cycle.
Western blot analysis of extracellular signal-regulated kinase 1/2 and proliferating cell nuclear antigen expression
The expression of ERK 1/2 and PCNA was measured by Western blot analysis with a commercially available kit using rabbit polyclonal anti-p-ERK 1/2 mitogen-activated protein kinases and anti-PCNA primary antibodies (Abcam, Cambridge, Cambs, UK)(Reference Locher, Emmanuele, Suter, Vetter and Barton5, Reference Murakami-Nakai, Maeda, Yonezawa, Kuriyama, Kamisuki, Takahashi, Sugawara, Yoshida, Sakaguchi and Mizushina19). RASMC were stimulated with ox-LDL (100 μg/ml) for 10 min after pretreatment with ellagic acid (50 μmol/l) for 24 h. The experiments were terminated by rinsing the cells with PBS. After lysis of the cells, β-actin, phosphorylated ERK 1/2 and PCNA were extracted according to the manufacturer's instructions. The blots were subsequently incubated with the desired primary antibody. A Zero-D scanTM (Scanalytics, Inc. (BD Biosciences Bioimaging), Rockville, MD, USA) was used for densitometric quantification. The β-actin was used for normalisation of protein expression.
Statistical analysis
All data are expressed as mean values with their standard errors. Comparisons between the four groups were made by one-way ANOVA. Tukey's post hoc test was used to analyse significant effects. A P value of 0·05 was taken as the threshold for statistical significance.
Results
The effect of ellagic acid on the viability of rat aortic smooth muscle cells
Cell viability was assayed by the MTT test. After 24 h incubation with 0·1, 10, 50, and 100 μm-ellagic acid, cell viability was, respectively, 106 (sem 2·2), 106·4 (sem 2·8), 93·8 (sem 2·8) and 39·4 (sem 2·9) % of control levels. Clearly, 100 μm-ellagic acid causes a significant reduction in cell viability.
Oxidised low-density lipoprotein-induced rat aortic smooth muscle cell proliferation
The effect of ox-LDL on the proliferation of RASMC was detected by MTT assay. As shown in Fig. 1, treatment with ox-LDL (50 to 200 μg/ml) for 24 h resulted in increased cell viability; however, doses that exceeded 200 μg/ml had a cytotoxic effect.
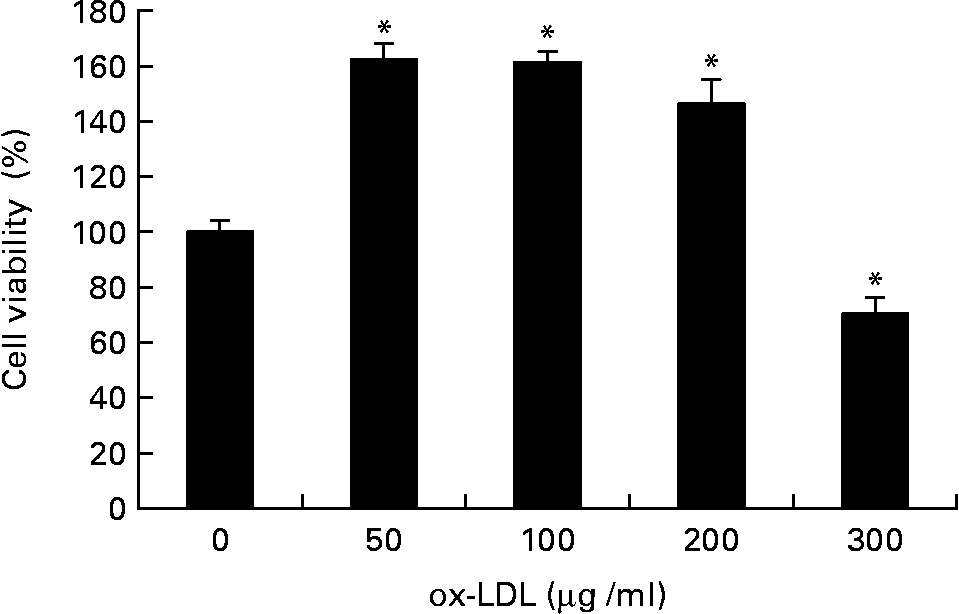
Fig. 1 Effects of different concentrations of oxidised LDL (ox-LDL) on rat aortic smooth muscle cells (RASMC) viability assayed by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-terazolium test. RASMC were treated with increasing concentrations (50–300 μg/ml) of ox-LDL for 24 h in 1 % fetal bovine serum– Dulbecco's modified Eagle medium. Results were from three experiments each and each was performed in triplicate. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different to that of the control group (P < 0·05).
Effect of ellagic acid on oxidised low-density lipoprotein-induced proliferation of rat aortic smooth muscle cells
As shown in Fig. 2, stimulation of RASMC with ox-LDL (100 μg/ml) for 10 min resulted in increased cell viability. Pre-incubation of ox-LDL with ellagic acid (50 μmol/l), however, significantly inhibited ox-LDL-induced cell viability.
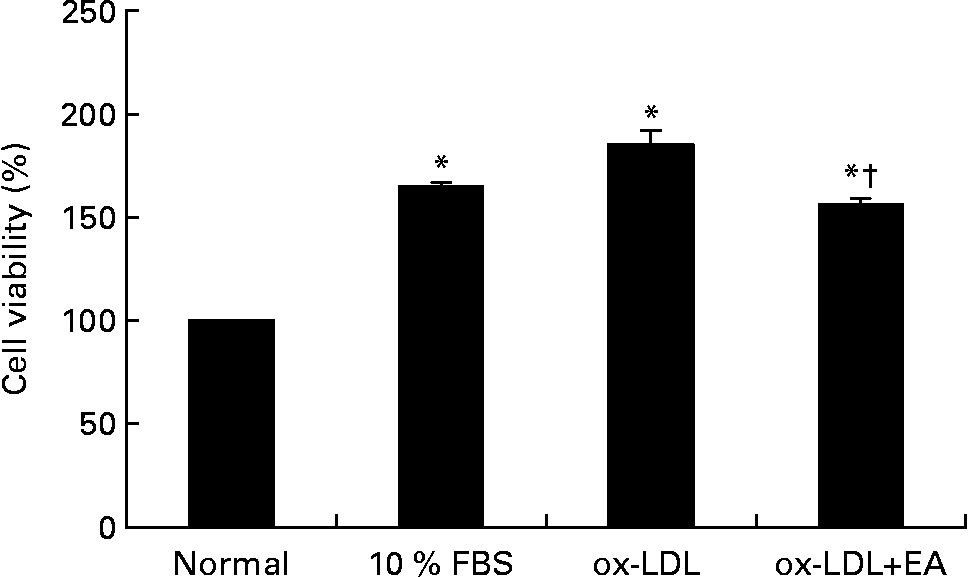
Fig. 2 Effect of ellagic acid (EA) on oxidised LDL (ox-LDL)-induced proliferation of rat aortic smooth muscle cells (RASMC). RASMC were incubated with ox-LDL (100 μg/ml) alone or with 50 μm-ellagic acid for 24 h in 0·1 % fetal bovine serum (FBS)–Dulbecco's modified Eagle medium. Values are means (n 3), with their standard errors represented by vertical bars. * Mean value was significantly different to that of the normal group (P < 0·05). † Mean value was significantly different to that of the ox-LDL group (P < 0·05).
Effect of ellagic acid on the rat aortic smooth muscle cell cycle and proliferating cell nuclear antigen expression
To clarify the effect of ellagic acid on cell-cycle regulation, we analysed the phases of cell cycle using flow cytometry. As shown in Fig. 3, the percentages of G2/M and S phase increased after stimulation of RASMC with ox-LDL and decreased after pre-treatment of ox-LDL with ellagic acid. Using Western blotting, we examined whether the effect of ellagic acid on the cell cycle was associated with the expression of PCNA (Fig. 4). The gene expression patterns were consistent with the cell-cycle analysis data. Cells that had been treated with ox-LDL only increased in number in the S phase; this increase was accompanied by increasing the expression of PCNA. However, cells pre-treatment with ox-LDL and ellagic acid decreased in number with the S phase.

Fig. 3 Effect of ellagic acid (EA) on rat aortic smooth muscle cells (RASMC) cell cycle induced by oxidised LDL (ox-LDL). RASMC were incubated with ox-LDL (100 μg/ml) alone or with 50 μm-EA for 24 h in 1 % fetal bovine serum (FBS)–Dulbecco's modified Eagle medium. (A) Normal cells – G0/G1, 86·86 %; G2/M, 6·38 %; S, 7·76 %. (B) 10 % FBS-treated cells – G0/G1, 75·91 %; G2/M, 12·32 %; S, 11·7 %. (C) ox-LDL-treated cells – G0/G1, 71·01 %; G2/M, 13·57 %; S, 15·42 %. (D) Cells treated with ox-LDL and EA – G0/G1, 83·2 %; G2/M, 7·32 %; S, 9·4 %.

Fig. 4 Effects of ellagic acid (EA) on oxidised LDL (ox-LDL)-induced protein-proliferating cell nuclear antigen (PCNA) expression. (A) Western blot of PCNA. (B) PCNA expression as a ratio to the control. Rat aortic smooth muscle cells were incubated with ox-LDL (100 μg/ml) with or without 50 μm-EA for 24 h in 1 % fetal bovine serum (FBS)–Dulbecco's modified Eagle medium. Proteins were extracted from the cells and analysed by Western blot analysis. The immunoblots shown are representative of three independent experiments. Values are means (n 3), with their standard errors represented by vertical bars. * Mean value was significantly different to that of the normal group (P < 0·05). † Mean value was significantly different to that of the ox-LDL group (P < 0·05).
Effect of ellagic acid on extracellular signal-regulated kinase 1/2 phosphorylation
Activation of ERK 1/2 was accompanied by phosphorylation of the Tyr204 residue. RASMC were stimulated with ox-LDL (100 μg/ml) for different periods of time; enhanced phosphorylation of ERK 1/2 occurred after cells had been exposed to ox-LDL for 5 min, but then began to decline (Figs. 5 (A) and (B)). Compared with the control group (1 % fetal bovine serum), exposure of RASMC to ox-LDL caused a marked increase in the phosphorylation of ERK 1/2. The ox-LDL-stimulated phosphorylation of ERK 1/2 was inhibited by ellagic acid (Fig. 6).
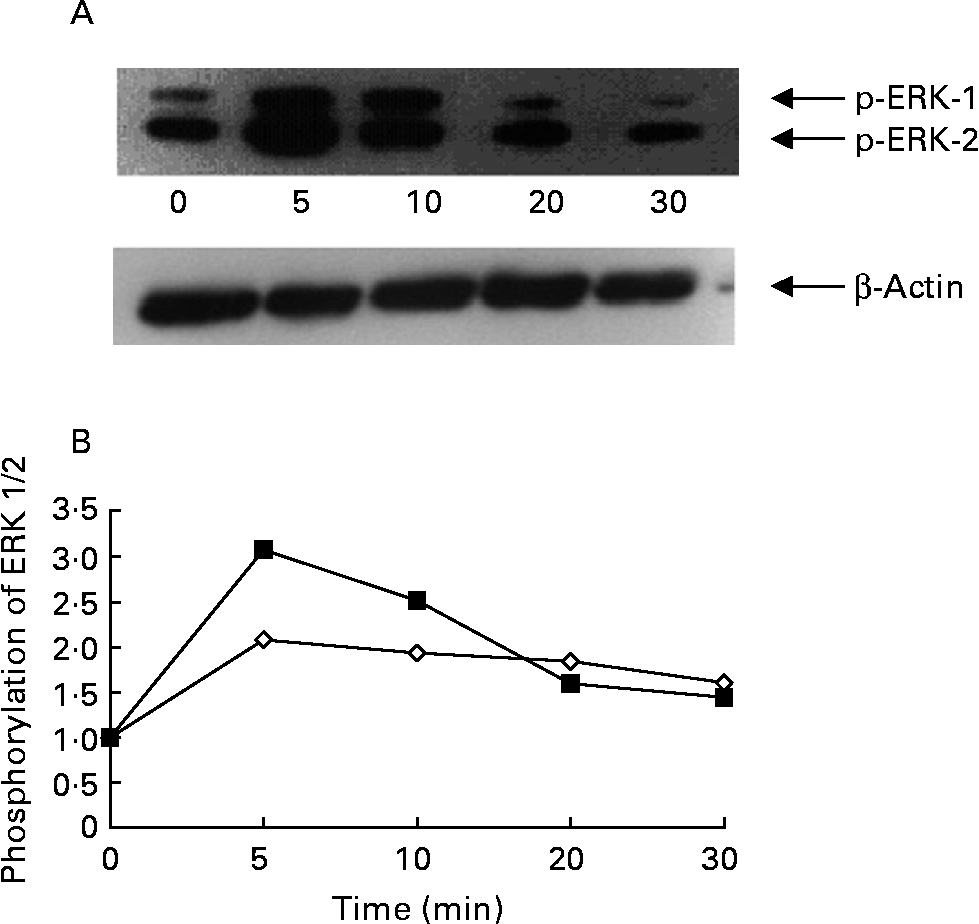
Fig. 5 Time course of oxidised LDL (ox-LDL)-induced phosphorylation of extracellular signal-regulated kinases (ERK) 1 and 2 in rat aortic smooth muscle cells (RASMC). (A) Western blot of phosphorylated ERK (p-ERK) 1 and 2. (B) Phosphorylation of ERK 1 (⋄) and ERK 2 (■) expressed as a ratio to the control. Cells were cultured in twelve-well plates until confluence and then transferred to medium with serum-free medium for 24 h. After 24 h cultivation, the RASMC were stimulated with ox-LDL (100 μg/ml) for the indicated time periods (0 min to 30 min).
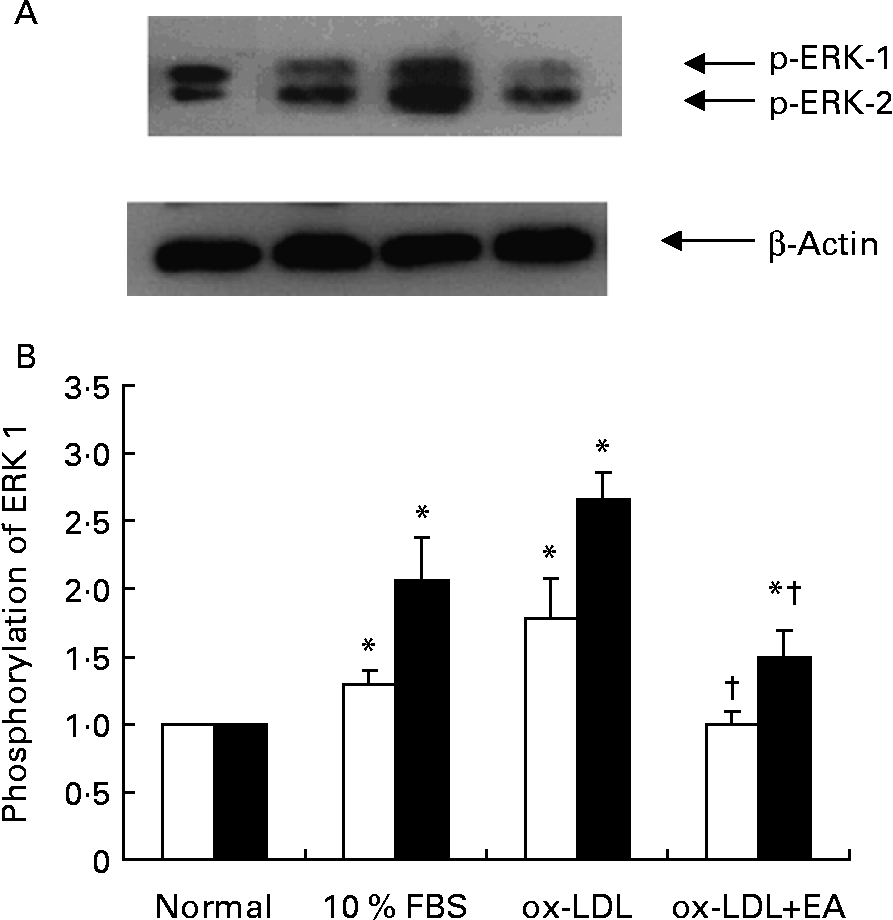
Fig. 6 Effects of ellagic acid (EA) on oxidised LDL (ox-LDL)-induced extracellular signal-regulated kinases (ERK) 1 and 2 phosphorylation. (A) Western blot of phosphorylated ERK (p-ERK) 1 and 2. (B) Phosphorylation of ERK 1 (□) and ERK 2 (■) expressed as a ratio to the control. Rat aortic smooth muscle cells were stimulated with ox-LDL (100 μg/ml) for 10 min after pretreatment with 50 μm-EA for 24 h in 1 % fetal bovine serum (FBS)–Dulbecco's modified Eagle medium. Proteins were extracted from the cells and analysed by Western blot analysis. The immunoblots shown are representative of three independent experiments. Values are means (n 3), with their standard errors represented by vertical bars. * Mean value was significantly different to that of the normal group (P < 0·05). † Mean value was significantly different to that of the ox-LDL group (P < 0·05).
Discussion
The present study demonstrates that ellagic acid significantly inhibited ox-LDL-induced proliferation of RASMC perhaps by inactivating the ERK pathways and blocking cell-cycle progression. Ellagic acid is a phenolic compound found in fruits including grape juice (10·2 mg/100 g), grape wine (5·6 mg/100 g), blueberries (0·9 mg/100 g), blackberries (42·4 mg/100 g), raspberries (17·9 mg/100 g) and strawberries (19·8 mg/100 g)(Reference Mertens-Talcott, Talcott and Percival20). The typical dietary intake of ellagic acid in humans is approximately 40–80 mg/d if 200 g strawberries or blackberries are eaten(Reference Mertens-Talcott, Talcott and Percival20). In the present study, we found that 50 μm-ellagic acid treatment effectively reduced ox-LDL-induced RASMC proliferation. Previous studies also indicated that other polyphenols, such as vitamin E (50 μmol/l) and resveratrol (30–50 μmol/l) also inhibited proliferation of VSMC(Reference Liu and Liu6, Reference Chai, Binion and Chisolm21). This dose (50 μmol/l) of ellagic acid is equivalent to the dietary intake of approximate 200 g of berries subject to absorption(Reference Mertens-Talcott, Talcott and Percival20–Reference Whitley, Stoner, Darby and Walle23).
Increased proliferation of intimal VSMC is an important component in the development of atherosclerosis, and ox-LDL is a mitogen for VSMC(Reference Yang, Chien, Hsiao, Pan, Wang, Chiu and Lin2). In the present study, we found that ox-LDL induced RASMC proliferation. Ox-LDL at low concentrations (0–100 μg/ml) increased cell viability in a dose-dependent manner; however, ox-LDL at doses of 200 μg/ml began to elicit a cytotoxic effect. This finding of ox-LDL exhibiting a biphasic effect on VSMC is similar to that reported by other researchers; low concentrations (20–120 μg/ml) had a proliferative effect while high doses (> 200 μg/ml) had a cytotoxic effect(Reference Liu and Liu6, Reference Auge, Pieraggi, Thiers and Negre-Salvayre24, Reference Chatterjee25). The increased mitogenic effect of ox-LDL is attributed to the chemical changes brought about by the process of oxidation of LDL components such as the generation of H2O2, and the conversion of phosphatidylcholine to lysophosphatidylcholine and 4-hydroxy-2-nonenal(Reference Stiko, Regnstrom, Shah, Cercek and Nilsson26, Reference Reuf, Rao, Li, Bode, Patterson, Bhatnagar and Runge27). In addition, certain oxidation products of unsaturated NEFA have been shown to stimulate mitogenic signalling pathways in VSMC (Reference Rao, Alexander and Runge28).
Previous studies have indicated that ox-LDL stimulates VSMC proliferation via activation of the ERK pathway(Reference Yang, Chien, Hsiao, Pan, Wang, Chiu and Lin2). It is known that the ERK pathway is activated by oxidative stress. Liu et al. postulated that ox-LDL might induce a response to oxidative stress by stimulating the generation of intracellular reactive oxygen species(Reference Liu and Liu6). Previous study also indicated that reactive oxygen species are able to induce tyrosine phosphorylation of mitogen-activated protein kinases and cell growth(Reference Baas and Berk29). The present results indicated that treatment with ox-LDL significantly increased in cell proliferation and activation of ERK 1/2.
Our previous study found that ellagic acid exhibits free-radical scavenging activities and reduces oxidative stress in cell and animal models(Reference Yu, Chang, Wu and Chiang15, Reference Yu, Wang, Liu and Chen30). We proposed that the inhibitory effect of ellagic acid on ox-LDL-induced cell proliferation might be due to its antioxidative ability. It might decrease reactive oxygen species production induced by ox-LDL and therefore inhibit ERK 1/2 expression.
PCNA synthesis is strictly regulated during the cell cycle and its protein is necessary for the transition of cells from quiescence to the S phase(Reference Morris and Mathews10). In the present study, we found that the amount of PCNA in the G2/M and S phases increased after treatment of ox-LDL but decreased after prior addition of ellagic acid to the ox-LDL (Fig. 3). The amount of PCNA protein was consistent with the cell-cycle analysis data (Fig. 4). It is therefore likely that ellagic acid reduced ox-LDL-induced RASMC proliferation by suppressing PCNA transcription.
In conclusion, cell proliferation stimulated by ox-LDL was inhibited by ellagic acid according to the MTT test and G0/G1 cell-cycle arrest in smooth muscle cells. The expressions of ERK 1/2 and PCNA were also suppressed by ellagic acid. Therefore, ellagic acid inhibits ox-LDL-induced aortic smooth muscle cell proliferation by inactivating the ERK 1/2 pathway and by suppressing the expression of PCNA. These results may advance the understanding of the role of antioxidants in the prevention of atherosclerosis.
Acknowledgements
The research was supported by grants from the National Science Council, Taiwan (NSC93-2320-B-039-017).








