The Mediterranean diet is a collection of eating patterns with different components, traditionally followed by populations from countries bordering the Mediterranean Sea, and is generally characterised by frequent consumption of fruit, vegetables, complex carbohydrates, pulses and fish; low consumption of meat and cheese; low-to-moderate amount of wine intake during meals; and use of olive oil for seasoning (as a major common characteristic). This dietary pattern has been suggested as a possible explanation for the longevity of populations from countries in the Mediterranean regions, and for the relatively low occurrence of CHD in such areas(Reference Trichopoulou, Kouris-Blazos and Wahlqvist1, Reference Trichopoulou, Costacou and Bamia2).
The Mediterranean diet as a whole and some of its aspects have been inversely related to the risk of cancer(Reference Praud, Bertuccio and Bosetti3–Reference Giacosa, Barale and Bavaresco8). In particular, high intakes of fruit and vegetables have been related to a reduced risk of cancer at several sites, with associations stronger for cancers of digestive and respiratory tracts and somewhat weaker for hormone-related cancers. However, most of the epidemiological evidence supporting a favourable role of vegetables and fruit is derived from case–control studies, whereas evidence from cohort studies is less convincing(9).
In the present review, we discuss and summarise the main findings on the relationship between vegetable and fruit consumption and cancer risk from an integrated series of multi-centric case–control studies conducted in Italy and Switzerland.
Methods
Since the early 1990s, a series of case–control studies of various neoplasms were conducted in Italy and Switzerland. These included 768 cases of oral and pharyngeal cancer(Reference Bravi, Bosetti and Filomeno10), 198 cases of nasopharyngeal cancer(Reference Polesel, Serraino and Negri11), 304 cases of oesophageal cancer(Reference Bosetti, La Vecchia and Talamini12), 230 cases of stomach cancer(Reference Lucenteforte, Scita and Bosetti13), 1953 cases of colorectal cancer(Reference Franceschi, Favero and La Vecchia14), 185 cases of liver cancer(Reference Talamini, Polesel and Montella15), 326 cases of pancreatic cancer(Reference Polesel, Talamini and Negri16), 527 cases of laryngeal cancer(Reference Bosetti, La Vecchia and Talamini17), 2569 cases of breast cancer(Reference Franceschi, Favero and La Vecchia18), 454 cases of endometrial cancer(Reference Bravi, Scotti and Bosetti19), 1031 cases of ovarian cancer(Reference Bosetti, Negri and Franceschi20), 1294 cases of prostate cancer(Reference Bosetti, Micelotta and Dal Maso21) and 767 cases of kidney cancer(Reference Bravi, Bosetti and Scotti22), 190 cases of non-Hodgkin's lymphomas(Reference Talamini, Polesel and Montella23) (Table 1); and a total of over 17 000 controls. Cases were individuals admitted to major teaching and general hospitals with incident, histologically confirmed cancers. Controls were patients with no history of cancer, admitted to the same hospitals for acute non-neoplastic condition, unrelated to known risk factors for the corresponding cancer site or dietary modification. The study protocols were revised and approved by the ethical committees of the hospitals involved, according to the regulations at the time of study conduction. All participants gave informed consent.
Table 1 Data on selected cancers for the highest v. the lowest levels of consumption of cruciferous and allium vegetables in Italy and Switzerland, 1991–2009 (Odds ratios, and 95 % confidence intervals)

* Qualitative variable scored as 1 for ‘non-use or low use’, 2 for ‘intermediate use’, and 3 for ‘high use’.
† For endometrial and stomach cancer only, the OR is for consuming ≥ 2 v. 0 portions/week of onion.
Trained personnel interviewed cases and controls during their hospital stay using a structured questionnaire, including information on sociodemographic characteristics, anthropometric measures, lifestyle habits (e.g. tobacco smoking and alcohol drinking), dietary habits, personal medical history, family history of cancer, and, for women, menstrual and reproductive factors, use of oral contraceptives and hormone replacement therapy. The usual diet of the subjects 2 years before diagnosis (or hospital admission, for controls) was investigated using a reproducible and valid FFQ(Reference Decarli, Franceschi and Ferraroni24, Reference Franceschi, Negri and Salvini25), including seventy-eight food items and beverages. Subjects were asked to indicate the average weekly frequency of consumption for each dietary item, and the seasonal consumption for a few vegetables and fruit, with the corresponding duration.
OR, and the corresponding 95 % CI, for quintiles (computed among controls) of vegetable or fruit intake were estimated by multiple logistic regression models. For nasopharyngeal and liver cancers and non-Hodgkin's lymphomas, subjects were grouped according to quartiles of intake. Although models were derived independently for each cancer site, according to their potential confounding factors, all of them included terms for age, sex (when appropriate), study centre or area of residence, education and energy intake. Models for liver and breast cancers were also adjusted for alcohol drinking; models for stomach cancer were adjusted for tobacco smoking; and models for cancers of oral cavity and pharynx, nasopharynx, oesophagus, pancreas, larynx and kidney were adjusted for both alcohol drinking and tobacco smoking. Allowance for BMI or physical activity was performed for cancers of oral cavity and pharynx, pancreas, kidney and colorectum. A term for hepatitis was included in the models for liver cancer (B and/or C viruses) and non-Hodgkin's lymphomas (C virus). Concerning female hormone-related cancers, OR for breast cancer were also adjusted for parity, those for ovarian cancer were adjusted for parity and oral contraceptive use, and those for endometrial cancer were adjusted for parity, oral contraceptive use, hormone replacement therapy, age at menarche, menopausal status and history of diabetes.
In the present review, we also provided results on selected vegetables or fruit of specific interest, i.e. those that are cheap and easy to store and transport, and thus the most frequently consumed.
Vegetables
The present data supported a protective role of high intake of vegetables in the risk of several common epithelial cancers (Fig. 1). In particular, the OR for the highest compared with the lowest levels of vegetable intake were 0·19 (95 % CI 0·13, 0·29) for oral and pharyngeal cancer, 0·51 (95 % CI 0·29, 0·90) for nasopharyngeal cancer, 0·32 (95 % CI 0·19, 0·55) for oesophageal cancer, 0·47 (95 % CI 0·27, 0·81) for stomach cancer, 0·57 (95 % CI 0·47, 0·69) for colorectal cancer, 0·72 (95 % CI 0·31, 1·64) for liver cancer, 0·66 (95 % CI 0·39, 1·12) for pancreatic cancer and 0·17 (95 % CI 0·11, 0·27) for laryngeal cancer. Among hormone-related cancers, breast, endometrial and ovarian cancers were inversely related to vegetable consumption, with OR of 0·73 (95 % CI 0·60, 0·88), 0·59 (95 % CI 0·37, 0·94) and 0·47 (95 % CI 0·34, 0·64), respectively, whereas the estimate for prostate cancer was below unity (i.e. 0·87), but not significant. A favourable role of vegetable intake was also observed for kidney cancer (OR 0·65, 95 % CI 0·47, 0·90), as well as for non-Hodgkin's lymphomas (OR 0·49, 95 % CI 0·28, 0·87).

Fig. 1 OR and 95 % CI of selected cancers for the highest quintile v. the lowest quintile of vegetable consumption for all cancer sites, except for nasopharyngeal and liver cancers, and non-Hodgkin's lymphomas (quartiles). Italy and Switzerland, 1991–2009. *Raw vegetables.
Cooking food may destroy selected nutrients and enzymes, alter the structure and thus digestibility of the food, and create by-products. However, for some foods and food components (e.g. lycopene), cooking not only kills potentially harmful organisms, but also actually improves the bioavailability of certain nutrients and digestibility. Findings from our case–control studies supported inverse relationships between consumption of both raw and cooked vegetables and the risk of cancers, with the exception of breast cancer, for which an inverse association was found essentially for raw vegetables. In general, these relationships appeared somewhat stronger for raw than cooked vegetables for upper digestive tract cancers. OR for the highest v. the lowest intakes (generally quintiles) of raw and cooked vegetables were respectively, 0·25 and 0·50 for oral and pharyngeal cancer(Reference Bravi, Bosetti and Filomeno10), 0·54 and 0·67 for nasopharyngeal cancer(Reference Polesel, Serraino and Negri11), 0·32 and 0·79 for oesophageal cancer(Reference Bosetti, La Vecchia and Talamini12), 0·59 and 0·57 for colorectal cancer(Reference Franceschi, Favero and La Vecchia14), 0·77 and 0·57 for pancreatic cancer(Reference Polesel, Talamini and Negri16), 0·22 and 0·32 for laryngeal cancer(Reference Bosetti, La Vecchia and Talamini17), 0·73 and 0·96 for breast cancer(Reference Franceschi, Favero and La Vecchia18), 0·47 and 0·65 for ovarian cancer(Reference Bosetti, Negri and Franceschi20), 0·87 and 0·74 for prostate cancer(Reference Bosetti, Micelotta and Dal Maso21), and 0·74 and 0·68 for kidney cancer(Reference Bravi, Bosetti and Scotti22).
Cruciferous vegetables (cabbages, cauliflowers, broccoli, brussels sprouts and turnip greens) are important sources of sulphur-containing compounds (glucosinolates), whose major breakdown products (isothiocyanates and indoles) have anti-carcinogenic properties in in vitro and animal studies(Reference Bosetti, Filomeno and Riso26). Our data showed significantly reduced OR for regular ( ≥ 1 portion/week) compared with no/occasional intake for cancers of the oral cavity and pharynx, oesophagus, colorectum, breast and kidney (Table 1)(Reference Bosetti, Filomeno and Riso26).
Frequent consumption of allium vegetables, particularly onion, represents a peculiar feature of the Mediterranean diet. Garlic and onion are a source of several organosulphur compounds in addition to amino acids, vitamins and micronutrients, and have anti-inflammatory, anti-thrombotic, cholesterol-lowering and antioxidant effects(Reference Galeone, Pelucchi and Levi27, Reference Galeone, Pelucchi and Dal Maso28). Data from the present series of case–control studies support a favourable role of allium vegetable intake in cancer risk in Italy and Switzerland (Table 1). In particular, a significantly decreased risk for high intakes of onion and garlic was found for cancers of oral cavity and pharynx, oesophagus, colorectum, larynx, endometrium, ovary and kidney (only garlic)(Reference Galeone, Pelucchi and Levi27–Reference Turati, Pelucchi and Guercio29).
Fruit
Fruit was a favourable correlate of the risk of several cancers, particularly of the digestive tract; however, associations were generally weaker than for vegetables. The OR for the highest compared with the lowest levels of intake were 0·39 (95 % CI 0·26, 0·59) for oral and pharyngeal cancer, 0·52 (95 % CI 0·31, 0·87) for oesophageal cancer, 0·53 (95 % CI 0·30, 0·93) for stomach cancer, 0·72 (95 % CI 0·60, 0·87) for colorectal cancer, 0·60 (95 % CI 0·39, 0·98) for pancreatic cancer and 0·52 (95 % CI 0·35, 0·77) for laryngeal cancer (Fig. 2). A strong association with fruit was also found for non-Hodgkin's lymphomas (OR 0·51, 95 % CI 0·30, 0·85). The OR for liver cancer was around 0·5; however, it did not reach statistical significance. No appreciable association was observed for hormone-related and kidney cancers, with OR ranging from 0·83 (endometrial cancer) to 1·02 (kidney cancer).

Fig. 2 OR and 95 % CI of selected cancers for the highest quintile v. the lowest quintile of fruit consumption for all cancer sites, except for nasopharyngeal and liver cancers, and non-Hodgkin's lymphomas (quartiles). Italy and Switzerland, 1991–2009. *Non-citrus fruits.
A reduced risk of cancers of the digestive tract and larynx was observed in our data for increasing intakes of citrus fruit, rich in vitamin C, flavanones and other compounds with antioxidant, anti-mutagenic and anti-proliferative properties(Reference Foschi, Pelucchi and Dal Maso30). Compared with subjects consuming less than 1 portion of citrus fruit per week, the OR for those consuming 4 or more portions per week were 0·47 (95 % CI 0·36, 0·61) for oral cavity and pharyngeal cancer, 0·42 (95 % CI 0·25, 0·70) for oesophageal cancer, 0·69 (95 % CI 0·52, 0·92) for stomach cancer, 0·82 (95 % CI 0·72, 0·93) for colorectal cancer and 0·55 (95 % CI 0·37, 0·83) for laryngeal cancer. For other neoplasms, including cancers of breast, ovary, endometrium, prostate and kidney, no evidence of a beneficial effect of citrus fruit was found(Reference Foschi, Pelucchi and Dal Maso30).
Apples contain several phytochemicals, including flavonoids and phenolic acids, and, when compared with other fruits, they had the second highest level of antioxidant activity (after cranberries) and phenolic compounds, and the highest level of free phenolic compounds(Reference Gallus, Talamini and Giacosa31). Subjects eating at least one apple a day had a reduced risk of cancers of oral cavity and pharynx (OR 0·79, 95 % CI 0·62, 1·00), colorectum (OR 0·80, 95 % CI 0·71, 0·90), larynx (OR 0·58, 95 % CI 0·44, 0·76), breast (OR 0·82, 95 % CI 0·73, 0·92) and ovary (OR 0·85, 95 % CI 0·72, 1·00). OR for oesophageal and prostate cancers were 0·75 (95 % CI 0·54, 1·03) and 0·91 (95 % CI 0·77, 1·07), respectively (Fig. 3)(Reference Gallus, Talamini and Giacosa31).
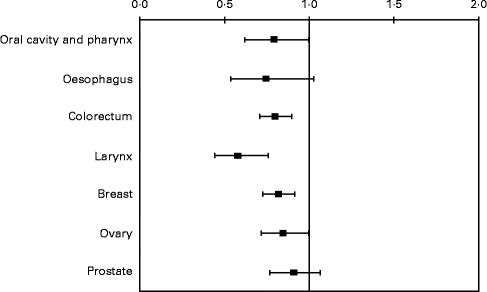
Fig. 3 OR and 95 % CI of selected cancers for consumption of ≥ 1 v. < 1 apple/d. Italy, 1991–2002.
Tomatoes are a typical aspect of the Italian diet and, in general, of the Mediterranean diet, and are the main source of lycopene, a carotenoid with strong antioxidant properties that is not converted to vitamin A(Reference La Vecchia32). Compared with the lowest quintile of tomato intake, the OR of colorectal cancer for the highest quintile was 0·8 (95 % CI 0·6, 0·9)(Reference La Vecchia32). We estimated an OR of 0·65 (95 % CI 0·4, 1·0) for cancer of oral cavity, pharynx and oesophagus, and one of 0·43 (95 % CI 0·3, 0·6) for cancer of the stomach, for the highest compared with the lowest quartile of intake(Reference La Vecchia33).
Fibres, flavonoids and proanthocyanidins
With regard to specific components of vegetables and fruit, dietary fibre intake was inversely associated with most cancers considered, particularly with those of the digestive tract (Fig. 4). The OR for an increment of intake equal to the difference between the 80th and 20th percentile were 0·51 (95 % CI 0·40, 0·66) for oral and pharyngeal cancer, 0·70 (95 % CI 0·51, 0·96) for oesophageal cancer and 0·68 (95 % CI 0·60, 0·78) for colorectal cancer. The OR for the highest v. the lowest categories of intake were 0·58 (95 % CI 0·34, 0·96) for nasopharyngeal cancer, 0·47 (95 % CI 0·28, 0·79) for stomach cancer, 0·4 (95 % CI 0·2, 0·7) for pancreatic cancer, 0·3 (95 % CI 0·2, 0·4) for laryngeal cancer and 0·68 (95 % CI 0·53, 0·88) for ovarian cancer. Conversely, a lack of association was observed for cancers of breast, endometrium, prostate and kidney(Reference Negri, Franceschi and Parpinel34–Reference Pelucchi, La Vecchia and Chatenoud44).

Fig. 4 OR and 95 % CI of selected cancers for fibre consumption. Italy and Switzerland, 1991–2009. Comparisons: difference between 80th and 20th percentile of the control distribution for oral and pharyngeal, oesophageal and colorectal cancer; 4th v. 1st quartile of consumption for nasopharyngeal cancer; 5th v. 1st quintile of consumption for other cancer sites.
Results on flavonoids from the present series of Italian and Swiss case–control studies showed a significant protective role of flavanones on upper aero-digestive tract cancers (OR for the highest v. the lowest quintiles of intake 0·51, 95 % CI 0·37, 0·71 for oral cavity and pharyngeal cancer; 0·38, 95 % CI 0·23, 0·66 for oesophageal cancer; 0·60, 95 % CI 0·41, 0·89 for laryngeal cancer), flavonols (OR 0·64, 95 % CI 0·54, 0·77) and anthocyanidins (OR 0·67, 95 % CI 0·54, 0·82) on colorectal cancer, flavonols (OR 0·80, 95 % CI 0·66, 0·98) and flavones (OR 0·81, 95 % CI 0·66, 0·98) on breast cancer, and flavonols (OR 0·63, 95 % CI 0·47, 0·84) and isoflavones (OR 0·51, 95 % CI 0·37, 0·69) on ovarian cancer(Reference Rossi, Negri, Bosetti and Fraga45, Reference Rossi, Bosetti and Negri46). Specific results on proanthocyanidins are shown in Fig. 5. Significant inverse associations were found for cancers of the stomach, colorectum, pancreas, endometrium and prostate, with OR for an increment of intake equal to the difference between the 20th and the 80th percentile of 0·58 (95 % CI 0·44, 0·77) for stomach cancer(Reference Rossi, Rosato and Bosetti47), 0·88 (95 % CI 0·81, 0·95) for colorectal cancer(Reference Rossi, Negri and Parpinel48), 0·62 (95 % CI 0·49, 0·79) for pancreatic cancer(Reference Rossi, Lugo and Lagiou49), 0·81 for endometrial cancer (95 % CI 0·66, 0·98)(Reference Rossi, Edefonti and Parpinel50) and 0·87 (95 % CI 0·76, 0·99) for prostate cancer (unpublished results).

Fig. 5 OR for an increment of proanthocyanidins with ≥ 3 mers intake equal to the difference between the 80th and 20th percentile. Italy and Switzerland, 1991–2009.*Monomers and dimers.
Discussion
Data from this integrated network of studies support a favourable role of vegetable intake on the risk of several common cancers, particularly of the digestive tract. Fruit intake was inversely related to the risk of digestive tract cancers as well, with associations generally less stronger than those for vegetable intake.
Potential limitations include selection and information bias. However, participation of cases and controls was high and similar, and attention was paid in excluding from the control group subjects who were admitted for any condition that might have affected dietary habits. Furthermore, cases and controls came from comparable catchment areas and were interviewed by the same trained personnel in a hospital setting(Reference D'Avanzo, La Vecchia and Katsouyanni51). Among the strengths are the large sample size, the use of a reproducible and valid FFQ(Reference Decarli, Franceschi and Ferraroni24, Reference Franceschi, Negri and Salvini25), and the ability to adjust for a number of potential confounding factors for the cancers investigated, as well as energy intake.
In terms of population-attributable risks, 20–60 % of digestive tract cancers in Italy could be attributable to low consumption of vegetables and fruit. In particular, the risks attributable to low consumption of vegetables and fruit in men and women were 25 and 17 % for oral and pharyngeal cancer, 40 and 29 % for oesophageal cancer, and 18 and 15 % for laryngeal cancer, respectively(Reference La Vecchia, Altieri and Tavani52). In both sexes combined, the attributable risks were 60 % for stomach cancer and 43 % for colorectal cancer. For breast cancer, the attributable risk was 21 %(Reference La Vecchia, Altieri and Tavani52).
The favourable effect of vegetables and fruit against most forms of cancers has been related to several of their constituents, in the absence, however, of a clear understanding on which of these may be the key relevant ones. Indeed, vegetables and fruit are sources of a wide variety of micronutrients and other bioactive compounds, including carotenoids, folate, vitamins C, D and E as well as vitamin B6, flavonoids, dietary fibre and selenium. These may act against cancer through their antioxidant activities, free radical-trapping capacity, modulation of detoxification enzymes, anti-mutagenic and anti-proliferative properties, stimulation of the immune system, as well as modulation of hormone concentration and hormone metabolism(Reference Steinmetz and Potter53). Ascorbic acid, carotenoids and other antioxidant vitamins were inversely related to upper digestive and respiratory tract neoplasms(9, Reference Pelucchi, Bosetti and Rossi54). Likewise, lycopene was inversely related to several digestive tract neoplasms, and carotenoids, vitamin E and calcium showed an inverse relationship with breast cancer(9, Reference Pelucchi, Bosetti and Rossi54). However, despite the promising results of several dietary vitamins and food components on cancer risk from epidemiological studies, trials usually failed to show a protective effect of vitamin supplementation on cancer risk(Reference Willett55).
Interestingly, a varied diet including several different types of fruit and vegetables was protective against several cancers in our data(Reference Lucenteforte, Garavello and Bosetti56–Reference La Vecchia, Munoz and Braga60). Different fruits and vegetables contain many different bioactive compounds, and these may exert synergistic interaction. It is, therefore, possible that a combination of plant-based bioactive components has a favourable role in cancer risk.
Given the high correlation between several micronutrients, it is difficult to disentangle their role and detect which specific component or group of components is responsible for a protective role against cancer. Since several antioxidants may influence cancer risk and act to prevent carcinogenesis, examining overall antioxidant exposure rather than individual antioxidants has recently been proposed. Inverse relationships of the Non-Enzymatic Antioxidant Capacity (NEAC) from diet, measured in terms of ferric reducing–antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC) and total radical-trapping antioxidant parameter (TRAP), were reported in our studies for colorectal and stomach cancer(Reference La Vecchia, Decarli and Serafini61). The OR of colorectal cancer for the highest v. the lowest quintiles were 0·68 (95 % CI 0·57, 0·82) for FRAP, 0·69 (95 % CI 0·57, 0·83) for TEAC and 0·69 (95 % CI 0·57, 0·83) for TRAP(Reference La Vecchia, Decarli and Serafini61). The corresponding OR for stomach cancer were 0·54 (95 % CI 0·33, 0·88) for FRAP, 0·67 (95 % CI 0·42, 1·07) for TEAC and 0·57 (95 % CI 0·39, 0·90) for TRAP, respectively (submitted).
The beneficial effect of high intakes of vegetables and fruit in these studies may be also due, at least in part, to the fact that their high consumption often accompanies lower intakes of foods that may be positively related to the risk of cancer. They may, therefore, represent a general indicator of healthy dietary and lifestyle habits. Indeed, high intakes of fruit and vegetables are key features of the Mediterranean diet(Reference Trichopoulou, Bamia and Trichopoulos62), which has been inversely related to overall mortality as well as to specific health outcomes including CVD and cerebrovascular diseases, as well as cancer(Reference Trichopoulou, Kouris-Blazos and Wahlqvist1, Reference Trichopoulou, Costacou and Bamia2, Reference Couto, Boffetta and Lagiou6, Reference Giacosa, Barale and Bavaresco8).
A beneficial role of vegetable and fruit consumption in the risk of cancer has been reported either from a number of case–control studies(Reference Chuang, Jenab and Heck63–Reference Lindblad, Wolk and Bergstrom65) or from some large cohort investigations(Reference Terry, Giovannucci and Michels66–Reference Flood, Velie and Chaterjee72). However, also a number of prospective studies, which generally have a higher level of evidence than case–control studies, have reported null associations, casting some doubts about the possible benefits of high vegetable and fruit consumption(Reference van Gils, Peeters and Bueno-de-Mesquita73–Reference Heinen, Verhage and Goldbohm77). Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) study, across ten countries, have supported significant inverse associations between fruit consumption and upper aero-digestive tract cancers (mouth, pharynx, larynx and oesophagus) and lung cancer (in smokers), as well as between fruit and vegetable consumptions and colorectal cancer. For the other cancer sites considered (i.e. stomach, pancreas, breast, cervix, prostate, bladder and lymphoma), no significant association was reported for both fruit and vegetables consumption(Reference Bradbury, Appleby and Key78). The combined analysis of two major US cohorts, the Nurses' Health Study and the Health Professionals' Follow-up Study (HPFS), indicated no relevant role of fruit and vegetable intake on overall cancer incidence(Reference Hung, Joshipura and Jiang75) as well as on colon and rectal cancer(Reference Michels, Edward and Joshipura76), but suggested possible inverse associations with kidney cancer(Reference Lee, Giovannucci and Smith-Warner71), premenopausal breast cancer(Reference Zhang, Hunter and Forman79) and lung cancer in women but not in men(Reference Feskanich, Ziegler and Michaud80). Prospective data from the Netherlands Cohort Study on Diet and Cancer have shown a favourable role of vegetable and fruit intake in colorectal(Reference Voorrips, Goldbohm and van Poppel81) and lung cancers(Reference Voorrips, Goldbohm and Verhoeven82), and no significant association with pancreatic(Reference Heinen, Verhage and Goldbohm77), ovarian(Reference Mommers, Schouten and Goldbohm83), kidney(Reference van Dijk, Schouten and Kiemeney84) and breast cancers(Reference Verhoeven, Assen and Goldbohm85). Moreover, high intake of fruit, but not vegetables, reduced urothelial cancer risk(Reference Zeegers, Goldbohm and van den Brandt86), and intakes of specific groups of vegetables (e.g. raw vegetables) and fruits (e.g. citrus fruits) were inversely related to subtypes of oesophageal and stomach cancers(Reference Steevens, Schouten and Goldbohm87). The Pooling Project of prospective Studies of Diet and Cancer, an international consortium of prospective cohort studies(Reference Smith-Warner, Spiegelman and Ritz88), found no association between fruit and vegetable consumption and the risk of breast cancer overall (but an inverse association for oestrogen receptor-negative breast tumours; twenty cohorts/24 690 cases)(Reference Jung, Spiegelman and Baglietto89), pancreatic cancer (fourteen cohorts/2212 cases)(Reference Koushik, Spiegelman and Albanes90), colon cancer overall (but an inverse association for distal colon cancer; fourteen cohorts/5838 cases)(Reference Koushik, Hunter and Spiegelman91) and ovarian cancer (twelve cohorts/2130 cases)(Reference Koushik, Hunter and Spiegelman92); a modest inverse association with lung cancer (eight cohorts/3206 cases)(Reference Smith-Warner, Spiegelman and Yaun93); and a significant inverse association with kidney cancer (thirteen cohorts/1474 cases)(Reference Lee, Mannisto and Spiegelman94).
In conclusion, our findings, as well as those from other studies conducted in Mediterranean countries(Reference Benetou, Orfanos and Lagiou95, Reference Masala, Assedi and Bendinelli96), indicate a large favourable effect of vegetable and fruit intake on the risk of several cancer sites, and this may well be due to the relative abundance of vegetables and fruit in the Mediterranean diet.
Acknowledgements
The present review was supported by the Italian Foundation for Cancer Research (FIRC) and by the Italian Ministry of Health, General Directorate of European and International Relations. F. T. was supported by a fellowship from the FIRC.
The authors' contributions are as follows: C. L. V. and F. L. designed and carried out the study; F. T. and M. R. drafted the article; C. P. and C. L. V. revised the paper critically for important intellectual content.
None of the authors has any conflicts of interest to declare.








