It is established that psychosocial stress precedes the onset of depression (Reference Brown and HarrisBrown & Harris, 1989; Reference Bifulco, Brown and MoranBifulco et al, 1998) but the intervening biological mechanisms are not understood. Increased cortisol secretion has been reported widely in depression and is believed to originate from psychosocial stress that induces changes in the brain underlying depression (Reference DinanDinan, 1994; Reference NemeroffNemeroff, 1996). In humans, two small studies reported increased cortisol in subjects with current depression whose illness was preceded by social stress (Reference Dolan, Calloway and FonagyDolan et al, 1985; Reference Deakin, Pennell and UpadhyayaDeakin et al, 1990), but there is scant evidence that hypercortisolaemia occurs in depression in community settings. The present study therefore was carried out in the community under naturalistic conditions. Studies have reported blunting of responses following administration of various serotonin (5-hydroxytryptamine, 5-HT) agonist drugs (Reference Deakin, Pennell and UpadhyayaDeakin et al, 1990; Reference Mitchell and SmytheMitchell & Smythe, 1990; Reference O'Keane and DinanO'Keane & Dinan, 1991). Some studies have reported that impaired 5-HT function is associated with increased baseline cortisol concentrations (Reference Deakin, Pennell and UpadhyayaDeakin et al, 1990; Reference Mitchell and SmytheMitchell & Smythe, 1990). This has contributed to the widespread view that stress-induced increases in cortisol secretion cause depression by undermining 5-HT neurotransmission through glucocorticoid-5-HT interactions in the brain (Reference DinanDinan, 1994). We investigated each step of this hypothetical causal sequence in a large community-based study and we tested the prediction that 5-HT function would be reduced in subjects with current depression but not in subjects who were vulnerable but not yet depressed. In contrast, increased salivary cortisol would occur in both groups.
METHOD
Screening and recruitment
We aimed to recruit three groups of women: 100 women currently depressed by ICD-10 (World Health Organization, 1993) criteria; 300 women vulnerable to depression but not currently depressed; and 100 women not vulnerable to depression and not depressed. Group sizes to detect a cross-sectional group comparison on salivary cortisol were determined by power calculations using data from the large study by Stokes et al (Reference Stokes, Stoll and Koslow1984). Women with children under the age of 16 years living in a south Manchester housing estate were identified from family doctor lists and sent screening questionnaires to assess current mood state (Personal Health Questionnaire, PHQ; Reference SimpsonSimpson, 1984) and vulnerability to depression (Health and Relationships Questionnaire, HRQ, adapted from the Vulnerability to Depression Questionnaire; Reference Brown and HarrisBrown & Harris, 1989).
Interviews and assessments
The women were interviewed twice in their homes. A psychiatrist (P. L. S.) conducted the first interview. The subjects were given a complete description of the study, written informed consent was obtained, demographic details were collected and a Schedules for Clinical Assessment in Neuropsychiatry (SCAN) diagnostic interview (World Health Organization, 1992) was conducted. Four tubes were left for saliva collection over two days at 09.00 and 23.00 h during the follicular phase of the menstrual cycle (day 0-12) or on pill-free days for women taking the oral contraceptive pill.
At the second interview, within 2 weeks of the first, the Life Events and Difficulties Schedule (LEDS; Reference Brown and HarrisBrown & Harris, 1989) for the previous 6 months and the Self Evaluation and Social Support Scales (SESS; Reference Brown, Andrews and BifulcoBrownet al, 1990) were administered by social researchers (C. P., J. D.). These interviews were used to determine vulnerability to depression and current depression.
Serotonin neurotransmission
Serotonin function was assessed by measuring prolactin responses to dexfenfluramine challenge in subgroups (20 depressed, 63 vulnerable and 61 non-vulnerable) during the follicular phase of the menstrual cycle (days 0-12 of cycle, similar test-timing for all groups) or during the contraceptive-pill-free days. The subjects were drug-free and fasted from 23.00 h. Dexfenfluramine (30 mg, oral) and placebo tests were separated by at least 2 days and the order was randomised and double blind. Baseline blood samples were taken an hour before (09.30 h) and at the time of drug/placebo administration (10.30 h). Hourly samples were taken thereafter. Baseline assays were l-tryptophan, branched-chain amino acids, cortisol and prolactin. Prolactin, fenfluramine and norfenfluramine were assayed in subsequent samples.
Assays
Salivary cortisol was measured by competitive radioimmunoassay (Reference KaneKane, 1979). Inter-assay precision was <13% at all levels. Intra-assay precision was <6% across the whole assay range. Plasma-free tryptophan, branched-chain amino acids, fenfluramine and norfenfluramine concentrations were determined by a fluorimetric method (Reference Franklin, Cowen and CravenFranklin et al, 1995).
Social adversity
Life events were categorised into severe and non-severe and into recent (within the past month) and non-recent (>1 month, <6 months) using LEDS criteria.
Vulnerability and depression
Vulnerability was defined according to Brown et al (Reference Brown, Andrews and Bifulco1990) as the presence of two or more vulnerability factors, at least one being environmental. Environmental factors are negative evaluation of core relationship (partner or child), social isolation and chronic difficulty. Psychological factors are low self-esteem, subclinical depression and parental separation for more than 1 year before the age of 16 years. Women assessed as being vulnerable to depression have a 25-30% risk of having a new onset of depression in the following year (Reference Brown, Andrews and BifulcoBrown et al, 1990). Depression was defined using ICD-10 criteria (World Health Organization, 1993): depressive episode F32.0-32.2 or recurrent depressive disorder F33.0-33.2. Subsidiary analyses used DSM-IV criteria (American Psychiatric Association, 1994): major depression without psychotic symptoms 296.2-296.3.
Statistical analysis
Average morning and evening salivary cortisol and diurnal change were analysed. Cortisol concentrations at 23.00 h were not normally distributed and vulnerability and life event groups were compared using χ2 and Mann—Whitney U tests. Wilcoxon paired rank tests were used for within-subject comparisons.
Dexfenfluramine challenge was assessed using analysis of covariance of all prolactin data, with a repeated measures factor for sample time and factors for drug/placebo and vulnerability group, and using area under the prolactin curve (AUC) on the fenfluramine day minus AUC on the placebo day (placebo-controlled AUC, PC-AUC). The PC-AUC was normally distributed and therefore analysed by analysis of covariance. The following potential covariates were measured: AUC of fenfluramine and norfenfluramine concentrations; serum cortisol, tryptophan and branched-chain amino acid concentrations; the ratio of tryptophan to branched-chain amino acids; and weight change over the past month (categories were: none; <2 kg; a loss of > 2 kg; a gain of >2 kg). The Statistical Package for the Social Sciences, version 7.5 (Reference CorporationSPSS, 1997) was used.
RESULTS
Sample characteristics
A total of 5558 women were sent questionnaires. Using one reminder, 2112 (38%) responded. Those likely to fall into the depressed, vulnerable or non-vulnerable groups were contacted until each cell was filled. A total of 755 women agreed to participate further in the study. Completed data were collected on 453 women (Table 1). It was not possible to recruit sufficient vulnerable women because often they were currently depressed, hence this cell was below target size and the non-vulnerable target was exceeded. A total of 94 women met the ICD-10 criteria for depression: 48 had mild depression, 37 had moderate depression and 9 had severe depression (as defined by ICD-10); 14.4% of the depressed group were taking antidepressants and 7.2% were taking benzodiazepines. Eleven women were excluded due to current steroid treatment, physical illness or psychosis. All data from women recruited into the study have been included in the analysis.
Table 1 Numbers of subjects with social and biological data by diagnosis; an additional 15 subjects received active fenfluramine but not placebo

| Salivary cortisol | LEDS/SESS | Complete fenfluramine challenge | ||||
|---|---|---|---|---|---|---|
| ICD-10 | DSM-IV | ICD-10 | DSM-IV | ICD-10 | DSM-IV | |
| Non-vulnerable | 177 | 178 | 190 | 192 | 61 | 59 |
| Vulnerable | 166 | 174 | 163 | 170 | 63 | 60 |
| Depression | 94 | 48 | 95 | 49 | 20 | 8 |
| Other diagnoses | 15 | 53 | 15 | 52 | 1 | 18 |
| Total | 452 | 453 | 463 | 463 | 145 | 145 |
Cortisol concentrations
Salivary cortisol concentrations showed diurnal variation (Table 2 and Fig. 1a). Although separated by several weeks, morning cortisol concentrations from the salivary and fenfluramine studies were correlated significantly (r=0.31,n=160, P<0.001).
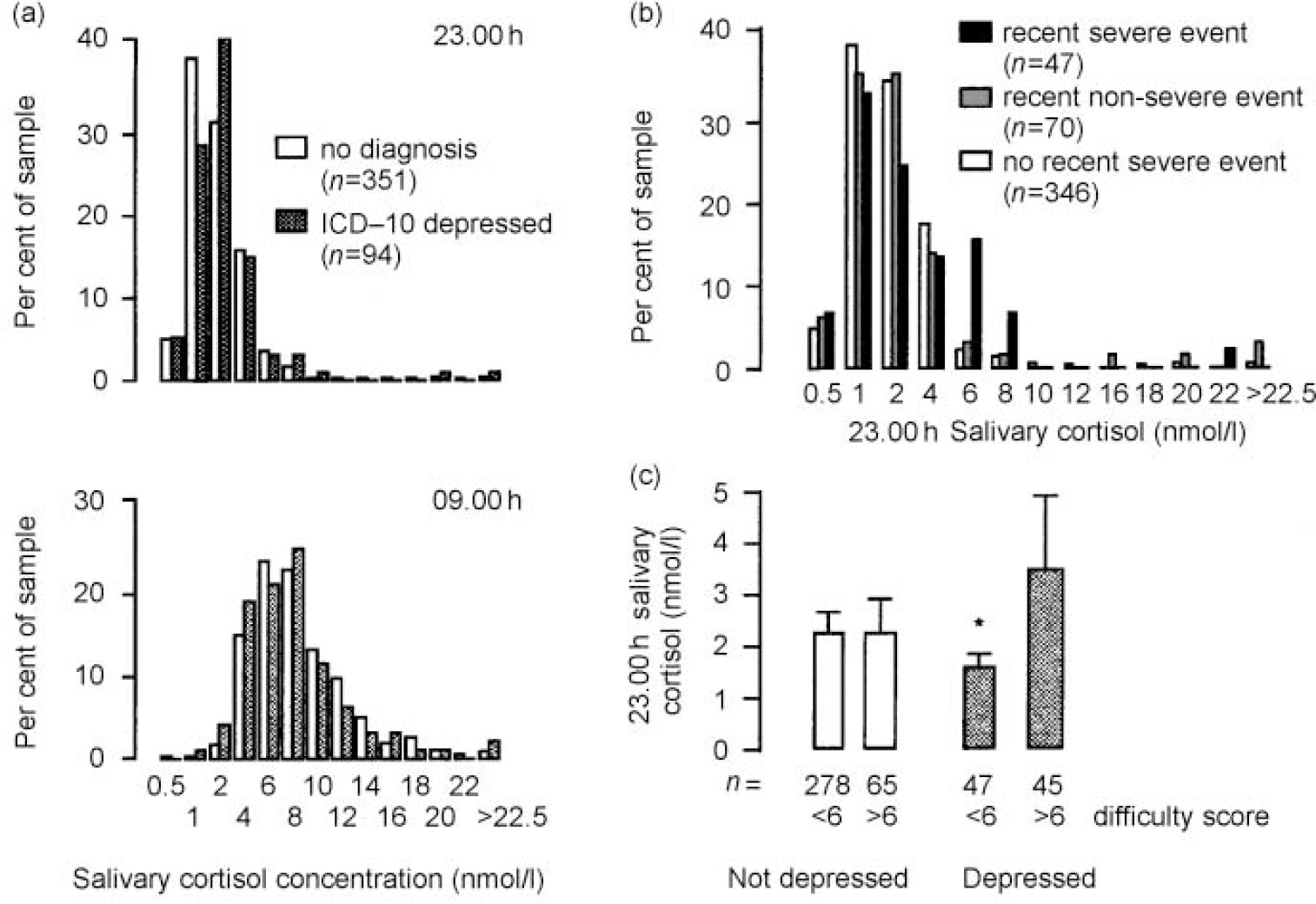
Fig. 1 Influence on salivary cortisol: (a) no increase in 9 a.m. or 11 p.m. salivary cortisol concentration in depressives; (b) severe recent life events are associated with increased 11 p.m. cortisol concentration (P<0.002, χ2 test); (c) chronic difficulties increase evening cortisol only in depressed subjects (* P=0.01 for high v. low difficulty score in the depressed; two-tailed Mann—Whitney U test).
Table 2 Salivary cortisol (nmol/l; 23.00 h, 09.00 h and diurnal) in each study group

| Non-vulnerable | Vulnerable | ICD-10 depressed | DSM-IV depressed | |
|---|---|---|---|---|
| (n=177) | (n=166) | (n=94) | (n=48) | |
| 23.00 h | ||||
| Mean | 2.37 | 2.14 | 2.47 | 2.54 |
| Median | 1.5 | 1.5 | 1.5 | 1.5 |
| Interquartile range | 1.5 | 1.0 | 1.13 | 1.5 |
| 09.00 h | ||||
| Mean1 | 8.12 | 7.68 | 7.382 | 7.113 |
| Median | 7.5 | 6.75 | 6.50 | 6.25 |
| Interquartile range | 5.0 | 5.0 | 4.63 | 4.25 |
| Diurnal | ||||
| Mean | 5.73 | 4.92 | 4.924 | 4.574 |
| Median | 5.0 | 5.0 | 4.5 | 4.0 |
| Interquartile range | 5.0 | 4.5 | 5.0 | 4.25 |
Subjects meeting the criteria for ICD-10 depression did not have increased salivary cortisol concentrations either in the evening or morning (Fig. 1a and Table 2). Indeed, there were reductions in the 09.00 h salivary cortisol and diurnal change in the depressed subjects of borderline statistical significance by two-tailed tests (Table 2). Similarly, average morning serum cortisol (from those who had dexfenfluramine tests) was reduced in depressed subjects (mean=319.9 and s.e.=125.7 nmol/l, n=24) compared with non-depressed subjects (mean=403.2 and s.e.=190.4 nmol/l,n=135, P=0.04, one-way analysis of variance).
Severe recent life events were associated with increased 23.00 h salivary cortisol concentrations (Fig. 1b; P<0.002, χ2 test), whereas recent non-severe events and severe events occurring more than a month previously were not. This association between life events and high evening salivary cortisol concentration remained unchanged when depressed patients were excluded. The association with life events was no more (or less) apparent in the depressed group.
The vulnerable, non-depressed group showed no evidence of increased cortisol concentrations. Almost all the currently depressed women fulfilled LEDS/SESS vulnerability criteria, largely owing to psychosocial difficulties and poor relationships. In a subsidiary analysis we investigated whether the hypothalamic-pituitary-adrenal (HPA) axis becomes responsive to these vulnerability factors in depression. Although those with current depression as a group did not have greater cortisol concentration than controls, dividing the depressed group into high— and low-difficulty groups by median difficulty score revealed that, in depression, chronic stress is associated with increased cortisol secretion (Fig. 1c). This was not seen in the non-depressed.
Dexfenfluramine challenge tests
Prolactin responses to dexfenfluramine were greater in depressed subjects than in vulnerable or non-vulnerable subjects by repeated-measures analysis (Fig. 2a; group × drug × sample: F=2.7; 12,846; P=0.002) and by PC-AUC, with dexfenfluramine AUC and average l-tryptophan concentrations as covariates. Baseline cortisol and branched-chain amino acid concentrations were not significant covariates. Exploration of the effects of life events on 5-HT function was hampered by small subgroups, but fenfluramine responses were greater in the depressed with recent life events; in the absence of life events, prolactin responses tended to be reduced (Fig. 2b; life event × group interaction: F=5.7; 2,137; P=0.004).
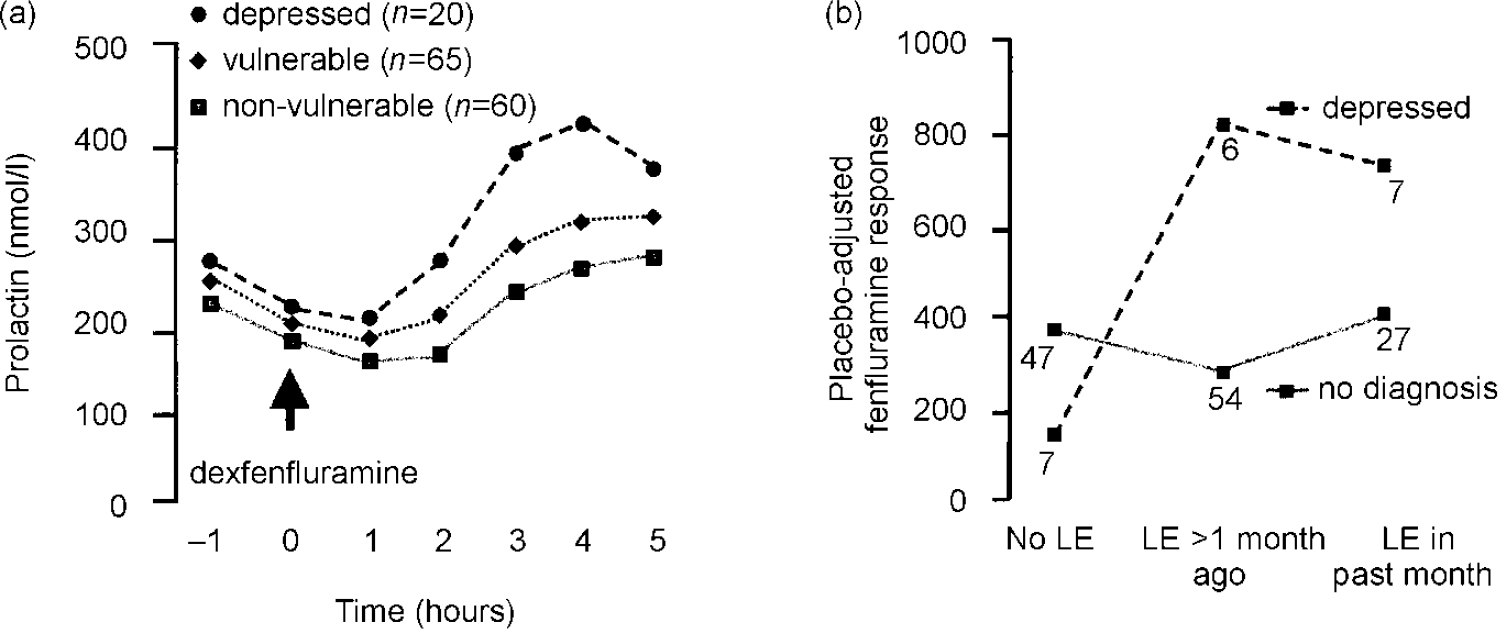
Fig. 2 Serotonin function as assessed by dexfenfluramine challenge test. (a) Prolactin response to dexfenfluramine is enhanced in depressed subjects (sample × drug × diagnostic group, P=0.005). (b) Placebo-adjusted fenfluramine responses by life events (LE) and depression: main effect of depression, P=0.03; main effect of life event,P=0.01; life event × depression interaction,P=0.004.
Tryptophan concentrations were reduced in those with recent severe life events compared with those with no recent severe events (Table 3). No reduction was found in other categories of life event severity or recency. Depression (ICD-10 or DSM-IV) was not associated with low tryptophan concentration. However, in the depressed subjects there were significant increases in branched-chain amino acid concentrations and this resulted in statistically significant reductions in the ratio of tryptophan to branched-chain amino acids (Table 3). None of the measures of tryptophan availability was a significant covariate in the analysis of covariance.
Table 3 Tryptophan, branched-chain amino acids and tryptophan:branched-chain amino acid ratio by vulnerability and by recency of life events
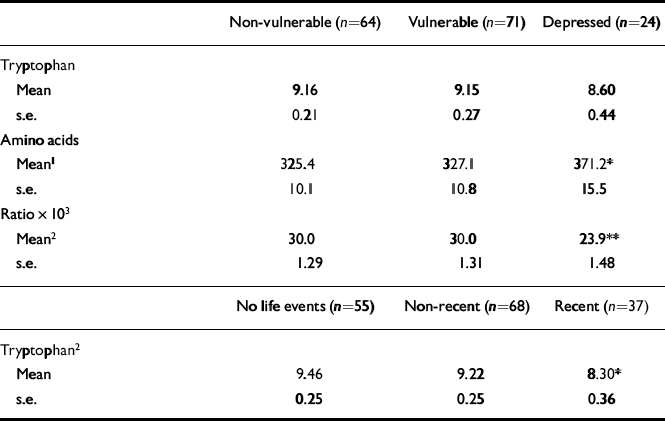
| Non-vulnerable (n=64) | Vulnerable (n=71) | Depressed (n=24) | |
| Tryptophan | |||
| Mean | 9.16 | 9.15 | 8.60 |
| s.e. | 0.21 | 0.27 | 0.44 |
| Amino acids | |||
| Mean1 | 325.4 | 327.1 | 371.2* |
| s.e. | 10.1 | 10.8 | 15.5 |
| Ratio × 103 | |||
| Mean2 | 30.0 | 30.0 | 23.9** |
| s.e. | 1.29 | 1.31 | 1.48 |
| No life events (n=55) | Non-recent (n=68) | Recent (n=37) | |
| Tryptophan2 | |||
| Mean | 9.46 | 9.22 | 8.30* |
| s.e. | 0.25 | 0.25 | 0.36 |
Self-rated weight loss did not influence the concentration of tryptophan or branched-chain amino acids. Fenfluramine responses were greater in the subgroup reporting mild degrees of weight loss (<2 kg) compared with the no-weight-loss group and the other groups; however, those with more severe degrees of weight loss and those with weight gain had entirely normal responses (data not shown).
DISCUSSION
The major findings of this study run counter to prevailing views: depression in the community is not associated with increased cortisol concentration and is associated with increased rather than decreased 5-HT responsivity.
Hypothalamic—pituitary—adrenal axis function and depression
Increased secretion of cortisol in depression is widely held to be central to the pathogenesis of symptoms and to be caused by psychosocial stress (Reference Stokes, Stoll and KoslowStokes et al, 1984; Reference DinanDinan, 1994; Reference NemeroffNemeroff, 1996). This hypothesis has been difficult to reconcile with the low prevalence of hypercortisolaemia in milder depressive illnesses in which social stress plays an important aetiological role. In our study, cortisol was responsive to recent psychosocial stress but was not raised in depression. The majority of the cases of depression were of mild severity, but even in more severe cases and those fulfilling DSM-IV criteria (n=48; Table 1) there was no trend for increased cortisol concentrations. Indeed, we found evidence that morning cortisol in serum was reduced in depressed subjects and the same trend was seen in salivary cortisol. Reduced 09.00 h salivary cortisol has been reported in chronic fatigue syndrome (Reference Strickland, Morriss and WeardenStricklandet al, 1998), in some studies of post-traumatic stress disorder and after traumatic events (Reference Yehuda, Giller, Levengood, Friedman, Charney and DeutchYehuda et al, 1995). These disorders are associated with anxiety and in the present study the currently depressed subjects with comorbid anxiety diagnoses had lower morning serum cortisol concentrations (data not shown).
Hypothalamic—pituitary—adrenal axis function and psychosocial stress
In the present study high ratings of psychosocial difficulty were associated with increased evening cortisol concentration, but only in the depressed group (Fig. 1c). This raises the possibility that some cases of community depression may involve a primary dysregulation of the HPA axis, which results in exaggerated cortisol responses to persistent adversity. Further analysis may reveal whether the dysregulation arises from early life experiences or from constitutional factors, and whether there is some symptomatic or personality correlate. A primary dysregulation of the HPA axis may account for the many reports of increased cortisol in in-patients with current depression, with the stress of admission interacting with a sensitised HPA axis (Reference Maes, Calabrese and MeltzerMaes et al, 1994). Indeed, a number of studies suggest that admission is an important influence on the prevalence of HPA axis dysregulation in depression. Our results contrast with a community-based study that found increased evening salivary cortisol in children and adolescents during depression (Reference Goodyer, Herbert and AlthamGoodyer et al, 1996). The explanation may lie in differences in age, gender, social class and, possibly, severity of the two samples. Our finding that life events are associated with greater salivary cortisol concentrations was not reported in two community-based high-risk studies, but both studies specifically excluded currently depressed individuals, who would have had high rates of life events, and they were smaller studies (Goodyear et al, 2000; Reference Harris, Borsanyi and MessariHarris et al, 2000).
Serotonin function in depression: dexfenfluramine challenge tests
Unexpectedly, we found that prolactin responses to dexfenfluramine were increased in the currently depressed individuals, especially those with recent life events, although the latter observation is based on small numbers. This result contrasts with three previous studies of psychiatric inpatients that have reported attenuated prolactin responses to d/l— ord-fenfluramine (Reference O'Keane and DinanO'Keane & Dinan, 1991; Reference Lichtenberg, Shapira and GillonLichtenberget al, 1992; Reference Siever, Murphy and SlaterSieveret al, 1984). A further study reported decreased responses in an endogenous subgroup but the difference was not statistically significant when low baseline prolactin levels were covaried (Reference Mitchell and SmytheMitchell & Smythe, 1990). Individuals with endogenous depression had smaller responses than patients with milder depression in one study (Reference Lopez-Ibor, Saiz Ruis and IglesiasLopez-Ibor et al, 1988) but in another the reverse was observed (Reference Maes, Jacobs and SuyMaes et al, 1989) and neither had healthy control groups. In a large study of primary care attenders with depression no change was found (Reference Park, Williamson and CowenPark et al, 1996). In these studies control groups have not been defined beyond matching on basic variables and by exclusion criteria. Our finding of increased prolactin responses is based on two exceptionally large control groups drawn from the same locality and socio-economic grouping as the depressed group, one of which also controlled for psychosocial vulnerability. Furthermore, the finding survives correction for all known influences on prolactin responses to fenfluramine: placebo challenge, baseline cortisol and prolactin, menstrual phase, drug and metabolite levels, tryptophan availability and weight loss.
One possible explanation for increased prolactin responses to dexfenfluramine is that weight loss and/or reduced tryptophan availability in the depressed group caused reduced 5-HT release and a secondary adaptive up-regulation of 5-HT receptor responsivity. This mechanism has been suggested for increased responses following dieting (Reference Walsh, Oldman and FranklinWalsh et al, 1995; Reference Cowen, Clifford and WalshCowen et al, 1996). In depressed patients, the ratio of tryptophan to branched-chain amino acids, which compete with each other for transport into the brain, was indeed reduced by 20% but this was not a significant covariate of responses. Furthermore, subjects with self-rated weight loss of > 2 kg showed no trend to increased dexfenfluramine responsiveness and there was no relationship between weight loss and measures of tryptophan availability. These considerations suggest that 5-HT receptor upregulation may not adequately explain the findings.
Serotonin receptor subtypes: role of 5-HT2 receptors in depression
There is evidence that prolactin responses to dexfenfluramine are mediated through 5-HT2c receptors (Reference Goodall, Cowen and FranklinGoodall et al, 1993). Thus, a more straightforward explanation for increased prolactin responses is that they are due to increased neurotransmission through 5-HT synapses with 5-HT2c receptors in untreated mild-moderate depression in the community. In more advanced or serious depressive illness, perhaps in combination with the effects of hospital admission, responses may become attenuated, as suggested by some fenfluramine studies discussed above. Our finding is in keeping with the theory that 5-HT2 systems are activated by adversity and mediate the anxiety component of depression (Reference DeakinDeakin, 1988). Together with our previous evidence of attenuated 5-HT1A-mediated tryptophan responses, the results are compatible with the theory that depression involves an imbalance between excessive 5-HT2C and reduced 5-HT1A functioning (Reference DeakinDeakin, 1988; Reference Deakin and GraeffDeakin & Graeff, 1991).
Activation of 5-HT systems in response to stress
We have shown that psychosocial stresses activate the HPA axis but that this is not the mechanism by which psychosocial stress causes depression. Depression occurs in the absence of sustained hypercortisolaemia. ubjects with depression had reduced morning cortisol, which may be related to coexistent anxiety. Central 5-HT2 neurotransmission is enhanced in depression and is responsive to life events. This is compatible with the idea that this 5-HT system is concerned with central responses to adversity. Life events and depression influence peripheral amino acid metabolism. More research is needed to determine whether this affects central 5-HT function and vulnerability to depression.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Mild—moderate depressive illnesses are common in community settings and are mostly undetected and untreated.
-
• Recent life events evoke physiological stress responses, as measured by salivary cortisol concentrations.
-
• Common depression found in community settings is not associated with sustained increases in cortisol concentrations, but does show abnormal serotonin (5-HT) function.
LIMITATIONS
-
• Half of the cases of depression were of mild severity.
-
• Prolactin responses to dexfenfluramine are a probe of 5-HT2C but not 5-HT1A function — the receptor type implicated in cortisol interactions.
-
• More frequent cortisol sampling would have enabled greater detailed study of cortisol secretion throughout the day and, for example, examination of secretory peaks in the pathogenesis of depression.
Acknowledgements
This research was funded by Wellcome Trust Grant 036979/Z/92. We thank Elizabeth Barrow, Cheryl Beresford and Jane Douglas for nursing assistance with neuroendocrine testing.








eLetters
No eLetters have been published for this article.