Introduction
Usnea is one of the most speciose genera in the family Parmeliaceae, with estimates ranging from c. 350 taxa (Thell et al. Reference Thell, Crespo, Divakar, Kärnefelt, Leavitt, Lumbsch and Seaward2012; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017) to over 400 (Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020). This hyperdiverse genus has historically been difficult to delineate into species due to a high degree of variability and the lack of characters to draw from within the genus (Clerc Reference Clerc1998; Ohmura Reference Ohmura2001). The integration of molecular techniques to delineate species has been a beneficial resource but on its own is not adequate to distinguish species (Lücking et al. Reference Lücking, Leavitt and Hawksworth2021). For the purposes of this article, the fruticose thallus type with usnic acid in the cortex and the presence of a central cartilaginous axis are accepted as delineating a single genus Usnea, with internal groups Eumitria, Dolichousnea and Usnea s. str. accepted at the infrageneric rank.
Geographically there is an under-representation of Usnea research in Africa and an over-representation in Europe and North America, as is the case with lichenology in general. Dodge (Reference Dodge1956, Reference Dodge1957) reported Usnea species primarily from southern Africa based entirely on morphological data, while Swinscow & Krog (Reference Swinscow and Krog1974, Reference Swinscow and Krog1975, Reference Swinscow and Krog1976a, Reference Swinscow and Krogb, Reference Swinscow and Krog1978, Reference Swinscow and Krog1979, Reference Swinscow and Krog1986, Reference Swinscow and Krog1988) and Krog (Reference Krog, Seyani and Chikuni1994) provided detailed studies of Eastern African species based on both morphological and chemical analyses.
This work provides descriptions of the Usnea species found at a geographical hotspot in West Africa, the republic of São Tomé and Príncipe, an island pair in the Gulf of Guinea region located c. 250–300 km west of the country of Gabon, Africa, very near 0° latitude and 0° longitude (Fig. 1). The islands are oceanic in origin, arising from a volcanic hotspot (the Cameroon volcanic mountain line). Due to this origin type and the relatively old geological age (São Tomé at 15.7 my, Príncipe at 30.4 my) the islands show very high levels of endemism. The islands were not populated by humans until the early 16th century and, although there have been many non-native species introduced at lower elevations of the islands, higher elevations (≥ 800 m São Tomé, ≥ 400 m Príncipe) have remained as largely untouched primary forest.
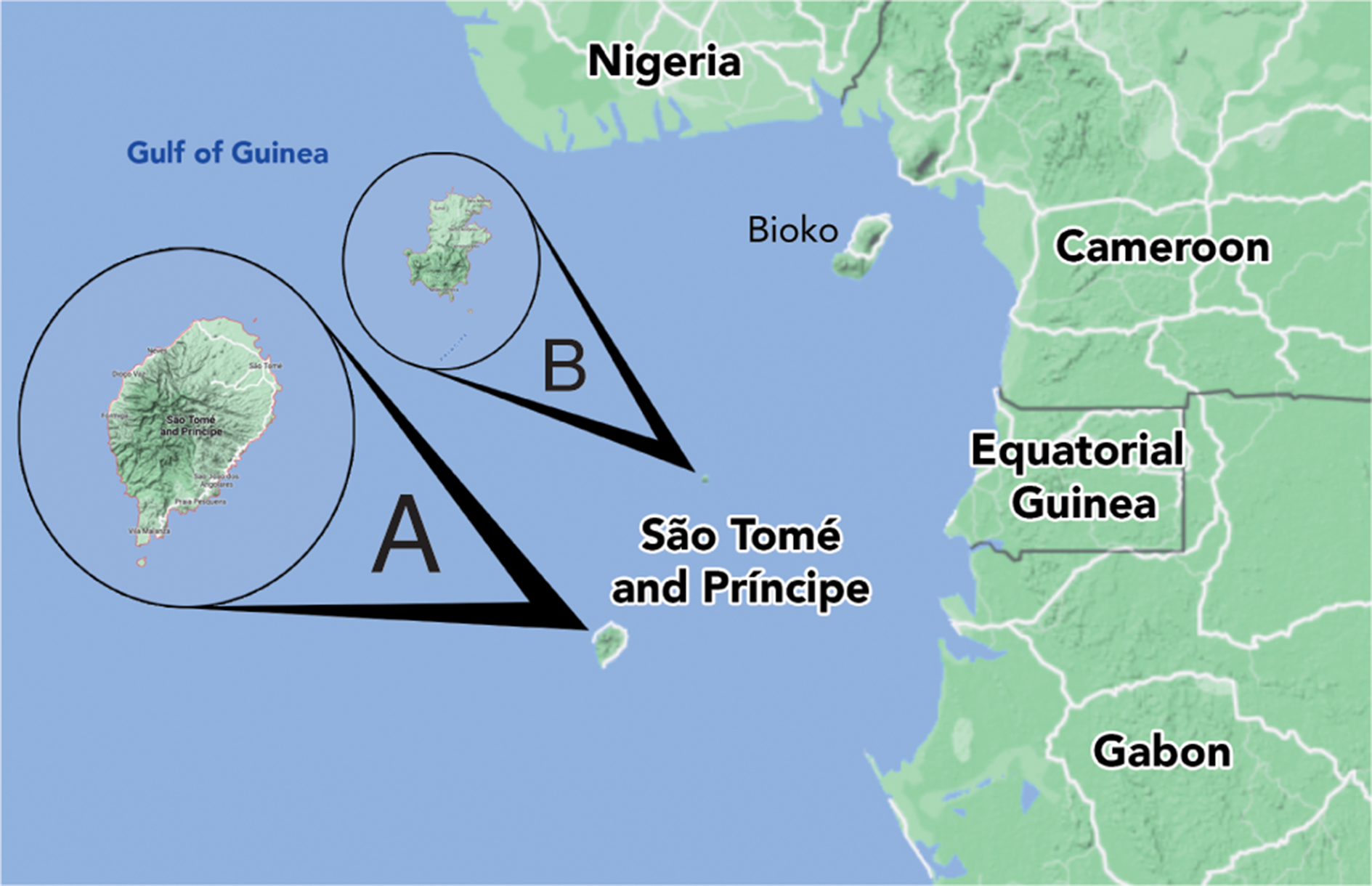
Fig. 1. Map of the Republic of São Tomé and Príncipe showing the islands relative location to continental west Africa and Bioko. A, São Tomé. B, Príncipe. In colour online.
Historically, this island group has been mostly ignored from a lichenological point of view, except for collections made in the late 19th century that were primarily sent to William Nylander for determination, and the expeditions by the first author in 2012 and 2013 (Nadel Reference Nadel2016). Only five species of Usnea were reported by Nylander (Reference Nylander1889), viz. U. articulata (L.) Hoffm., U. ceratina Ach., U. florida (L.) F. H. Wigg., U. longissima Ach. and U. trichodea Ach. All were collected from the larger island of São Tomé and determinations were made without modern tools of thin-layer chromatography and genetic sequencing. A sixth species, U. speciosa Motyka, was added to the list of Usnea from the islands when Motyka (Reference Motyka1936–1938) described it as a new species based on a specimen collected on São Tomé by Adolfo Möller and determined by Nylander (Reference Nylander1889) as U. articulata. The neighbouring island of Bioko (historically Fernando Po) is a part of the same volcanic chain but is of a continental island landform. Usnea collections from Bioko have been used to describe two eumitrioid species, U. baileyi Stirt. and U. firmula (Stirt.) Motyka.
Specimens collected in 2012–2013 show 15 distinct taxonomic units from within Usnea, all within the subgenera of Eumitria (Stirt.) Zahlbr. or Usnea s. str. Morphological, chemical and molecular characteristics were used to determine the different species or species groups. Only U. articulata was re-observed within the modern collection. While U. florida, U. longissima and U. trichodea were not observed, it should be noted that, according to our modern concept of these species, they are found only in the Northern Hemisphere. The similar appearance of the species that do occur in São Tomé surely led to these incorrect determinations. Originally, the specimens collected in 2012–2013 were determined to represent 11 species (Nadel Reference Nadel2016) and were later published in Lücking et al. (Reference Lücking, Nadel, Araujo and Gerlach2020) based solely on molecular data, using the original names or slight variations of them, from the unpublished thesis of the first author. This study, for the first time, combines molecular, morphological and chemical data to publish species determinations from the São Tomé and Príncipe collection. There are, therefore, 14 species or species aggregates that are reported as new to São Tomé and Príncipe, at least one new to Africa, and two species new to science presented here. This paper presents the 15 species of Usnea found during the 2012 and 2013 collecting expeditions.
Materials and Methods
Molecular sequencing and phylogenetic analyses
DNA extractions were obtained from all specimens included in the phylogeny and Sanger sequencing was performed between 2014 and 2015 (Nadel Reference Nadel2016). Approximately 10–15 mg of tissue was cut from the terminal branches and placed in a 1.5 ml microcentrifuge tube with 1000 μl of purified water and centrifuged for 60 s at 1500 rpm. After centrifugation, the samples were transferred to a new microcentrifuge tube where two 1 mm glass beads were added, and mechanical disruption of the sample was performed using a Retsch TissueLyser II Ball Mill Homogenizer for two cycles of 30 s each. Extractions were performed using the Omega E.Z.N.A. HP Plant DNA Mini Kit according to the manufacturer's specifications. To obtain sequences of the internal transcribed spacer (ITS) gene region, polymerase chain reaction (PCR) was performed with the primers USITS4-R (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a) and one newly designed for this study using Geneious v. 7.1.9 (USITS3-F INT: 5ʹ-TGC GGA AGG ATC ATT ACC GAG-3ʹ). AccuPower® PyroHotStart Taq PCR PreMix (Bioneer Corporation) tubes were used with 2 μl of DNA template. Thermal cycling settings followed those of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). PCR products were visualized using a 1.8% agarose gel stained with ethidium bromide and UV transillumination. Successfully amplified product was purified using 2 μl EXOsap-IT (Applied Biosystems) diluted at 1:5 mixed with 3 μl of template. The same primers used for PCR were used for the cycle sequencing reaction. Following precipitation and re-suspension in Hi-Di Formamide (Applied Biosystems), the samples were loaded into an ABI Prism® 3100 Genetic Analyzer (Applied Biosystems) for sequencing.
Resulting sequences were edited and assembled into contigs in Geneious v. 7.1.9 (Biomatters Ltd). Sequences were then manually inspected to determine correct assembly and direction. From the original 87 sequences (Nadel Reference Nadel2016; Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020), 56 were selected for this study by choosing only specimens examined and removing sequences that were deemed of low quality based on ambiguous characters. These 56 sequences were combined with 82 sequences gathered from the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov) (see Supplementary Material Table S1, available online). These NCBI sequences were evaluated on the length, and the quality of sequences as determined by the number of ambiguous nucleotides, and the requirement for publication in a peer-reviewed journal. Final sequences were aligned using MUSCLE v. 3 with default settings and the resultant alignment was inspected and corrected manually where necessary. The resulting alignment consisted of 673 nucleotides for 138 taxa but was trimmed to 500 nucleotides after excluding end regions where many taxa had missing character data due to varying individual sequence lengths. We used jModelTest v. 2.1.10 to determine GTR + I + G as the best fit model of nucleotide substitution. Maximum likelihood analyses (ML) were run in RAxML v. 8.2.12 (Stamatakis Reference Stamatakis2014) and consisted of 1000 replicates using the model GTRGAMMAIX with a starting seed of 12345. Bayesian MCMCMC analyses were performed as implemented in MrBayes v. 3.2.7a (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) using the CIPRES Science Gateway v. 3.3 (http://Phylo.org) (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). The Bayesian analyses consisted of two chains run for 16 million generations with default parameters, uninformative priors and a burn-in of 0.25. BEAGLE v. 3.1.2 (Ayres et al. Reference Ayres, Darling, Zwickl, Beerli, Holder, Lewis, Huelsenbeck, Ronquist, Swofford and Cummings2012) was used to produce a tree based on the Bayesian analysis and the resultant trees were visualized using FigTree v. 1.4.4.
Field collection
Specimen collection took place over two field collecting trips during April–May 2012 and April–May 2013. Over the course of the two years, approximately four weeks were spent on the main island of São Tomé and five weeks on the smaller island of Príncipe. On São Tomé, Usnea were collected primarily from elevations between 800 m and the highest peak at 2009 m. The collections from Príncipe were primarily collected at elevations between 400 and 715 m. Specimens were collected along with relevant ecology and substratum data, when possible, as well as precise geolocation and altitude measurements using a Garmin Oregon 450 GPS. Attempts to separate species were made in the field with placement into separate ‘Rite in the Rain’ packets, with further separation of mixed collections occurring later. Packets containing Usnea were dried without heat by taking advantage of the dehumidifier effect of the hotel air conditioning. A subset of 69 specimens was included in this study.
Morphological, anatomical and chemical studies
The following account is based on field studies and on herbarium specimens deposited in BM, CAS, G, LBL, M, S, TUR-V and W. Type material of most of the species discussed in this paper was studied.
The morphology of specimens was examined using a Leica MS5 stereomicroscope, with measurements taken using a Leica DM2000 microscope. The species concept and morphological terms used in this study follow Clerc (Reference Clerc1998, Reference Clerc2011), Herrera-Campos et al. (Reference Herrera-Campos, Clerc and Nash1998) and Ohmura (Reference Ohmura2001). Anatomical measurements of cortex, medulla and central axis were carried out in longitudinal sections of branches at ×40 magnification. The percentage thickness of cortex/medulla/axis of the total branch diameter (CMA) and the ratios of axis/medulla (A/M), axis/cortex (A/C) and medulla/cortex (M/C) of all the cited specimens were calculated according to Clerc (Reference Clerc1984, Reference Clerc1987), Gerlach et al. (Reference Gerlach, Zeynep, Naciri, Araujo Caviro, Borges da Silveira and Clerc2019, Reference Gerlach, Borges da Silveira, Rojas and Clerc2020) and Clerc & Naciri (Reference Clerc and Naciri2021). Measurements for CMA values follow the categories described by Clerc (Reference Clerc2011). These values are presented with extreme values in parentheses, standard deviations in plain text and the mean value in italics. Analyses of the anatomical structure of the cortex were made following the methods of Ohmura (Reference Ohmura2001), on thin hand-cut sections and observed at ×1000 magnification with a Leica DM2000 microscope.
Chemical analyses were performed on all cited specimens using thin-layer chromatography (TLC) following Culberson & Ammann (Reference Culberson and Ammann1979), with solvent B modified according to Culberson & Johnson (Reference Culberson and Johnson1982). K, C and Pd spot tests, according to Hale (Reference Hale1979), were directly applied to the medulla in longitudinal sections of the branches.
Results
ITS sequence data for the study were generated by the first author in 2014 and 2015 for his thesis (Nadel Reference Nadel2016) and later included as part of a larger phylogenetic study (Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020). Fifty-six sequences from that initial work are included in this study. Of these, 33 represent species that had not been sequenced prior to 2015 according to a search of GenBank. This includes U. beckeri (7), U. exasperata (3), U. firmula (5), U. krogiana (1), U. longiciliata (6), U. nodulosa (6), U. sorediosula (1), U. submollis (2), as well as Usnea sp. 1 (MN0523) and Usnea sp. 2 (MN0526).
The best ML tree (LNL −5257.88) inferred from the analysis of the ITS rDNA region was compared with the Bayesian probability percent and evaluated for strength of tree branches. At the subgenus level, both Dolichousnea (Y. Ohmura) Articus and Eumitria are highly supported by the Bayesian analysis (posterior probability (PP) = 1) and supported, although moderately in the case of Dolichousnea (ML = 79), by ML. Usnea s. str. is not supported by ML but has strong support using Bayesian inference with a PP of 0.99 (Fig. 2). There are 31 moderately to highly supported nodes with ML bootstrap values ≥ 70 and Bayesian PP ≥ 0.90 (Figs 2–5). Usnea subgenus Usnea s. str. encompasses most of the clades. This includes the species where single collections were made: U. erinacea aggregate, U. krogiana and U. sorediosula, as well as the U. articulata aggregate (Fig. 3) and the U. beckeri and U. longiciliata clades (Fig. 4). There is strong support for the monophyly of both new species, U. beckeri and U. longiciliata (ML = 97–100, PP = 1). Most of the highly supported regions fall within the clades containing the Eumitria subgenus (Fig. 5) and the two new species presented here, Usnea beckeri and U. longiciliata (Fig. 4). The Eumitria tree (Fig. 5) shows that monophyly of U. firmula is supported on a node with a 100/1 ML/PP value. These sequences are the first to have been isolated from this species. The U. baileyi and U. pectinata aggregates are also moderately to highly supported clades within Eumitria alongside U. firmula.

Fig. 2. Molecular phylogeny of Usnea including the three subgenera, Dolichousnea, Eumitria and Usnea s. str., based on the ITS rDNA sequences. Bold branches represent support from both maximum likelihood (ML) and Bayesian posterior probability (PP) inference (ML ≥ 70 and PP ≥ 0.90). See Supplementary Material Table S1 (available online) for voucher, locality and chemistry information.

Fig. 3. Molecular phylogeny of the Usnea articulata aggregate based on the ITS rDNA sequences. Bold branches represent maximum likelihood (ML) ≥ 70 and Bayesian posterior probability (PP) ≥ 0.90. The phylogeny shows the polyphyletic nature of the group. Usnea submollis and U. exasperata are included along with U. ghattensis, but the branch lacks support at the base.

Fig. 4. Molecular phylogeny of the lineage within Usnea s. str. which includes the two novel species, Usnea longiciliata and U. beckeri and the associated holotypes. The phylogeny is based on ITS rDNA sequence data and analyzed using maximum likelihood (ML) and Bayesian posterior probability (PP) inference. Bold branches are supported with both ML (≥ 70) and PP (≥ 0.90).
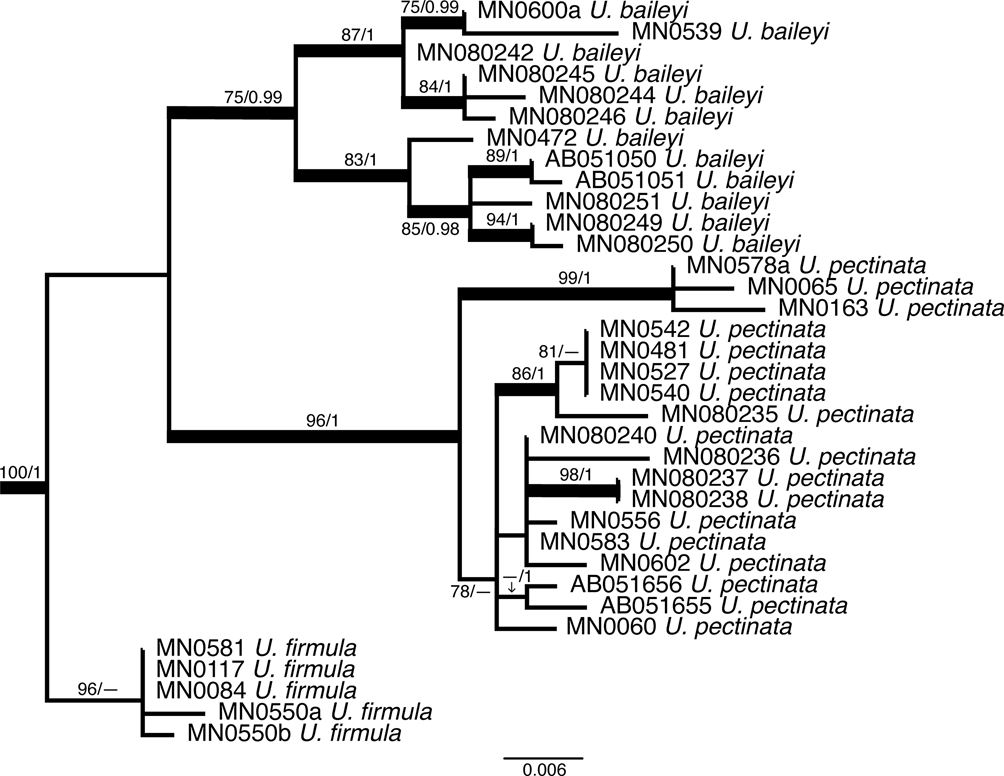
Fig. 5. Molecular phylogeny of the subgenus Eumitria with bold branches showing support (maximum likelihood (ML) ≥ 70 and Bayesian posterior probability (PP) ≥ 0.90) for the monophyly of Usnea firmula while U. baileyi and U. pectinata show varying levels of support and require further study.
Taxonomy
Usnea articulata aggr.
Incl.: Usnea articulata (L.) Hoffm., Deutschl. Fl. (Erlangen) 2, 133 (1796) [1795].—Lichen articulatus L., Sp. Pl. 2, 1156 (1753); type: sine loco, (lectotype—Dillenius, Hist. Musc., 1741, Tab. 11, fig. 4, fide Jørgensen, James & Jarvis, Bot. J. Linn. Soc. 115, 372 (1994)); Burnley [England], s. n. et d., Sherard s. n. (epitype—OXF, fide Jørgensen, James & Jarvis, Bot. J. Linn. Soc. 115, 372 (1994)). Chemistry (epitype): usnic, protocetraric and fumarprotocetraric acids (trace) (TLC: P. W. James).
Usnea pseudocyphellata Motyka, Lich. Gen. Usnea Monogr., 125 (1936–1938); type: Kamerun, Geb. Bula, vorzüglich an alten Bäumen, häufig, 02.1910, Deister (holotype—LBL!). Chemistry: usnic and protocetraric acids. %CMA: 2/41.5/13, A/M: 0.3, A/C: 6, M/C: 19.5.
Usnea speciosa Motyka, Lich. Gen. Usnea Monogr., 112, 124 (1936–1938); type: [São Tomé] Insula Stis Thomae (in Sinu Guineensi), 1300–2100 m, 1885, Moller (holotype—W!). Chemistry: diffractaic and barbatic (tr.) acids. %CMA = 2.5/42/11; A/M = 0.3; A/C = 4.4; M/C = 17.
Complete descriptions and further synonyms can be found in Swinscow & Krog (Reference Swinscow and Krog1976a, Reference Swinscow and Krog1978, Reference Swinscow and Krog1988).
CMA
(Specimens collected in São Tomé only, n = 6). %C = (1.5–)2.0–2.5–3.0; %M = (38–)38.7–40.9–43.1(–43.5); %A = 10–13.2–17.1(–19); A/M = (0.2)0.3–0.4–0.5; A/C = (4.0–)4.3–5.3–6.3(–6.6); M/C = 12.6–17–21.9(–26.4).
Chemistry
(Specimens collected in São Tomé only, n = 6). 1) Medulla K−, Pd+ red orange; protocetraric and fumarprotocetraric acids, unknown 4–5/6/6 greyish spot (n = 2). 2) Medulla K−, Pd−; diffractaic and barbatic (trace) acids (n = 4).
Diagnostic characters
Usnea articulata is morphologically and anatomically well characterized by a pendulous thallus with strongly swollen segments with pseudocyphellae (Fig. 6C) of various shapes, the absence of papillae, a very thin cortex (1–3%), a large and lax medulla (38–45%), a thin central axis (10–20%) and thus a tenuicorticata-type of CMA (Gerlach et al. Reference Gerlach, Borges da Silveira, Rojas and Clerc2020) (Fig. 6A) with a fairly high M/C.

Fig. 6. Usnea articulata MN0586a. A, transversal section of a main branch. C, thin and elongated pseudocyphellae. E, main, irregular branch with constricted lateral branches and transversal furrows. Usnea exasperata MN0269. B, transversal section of a main branch. D, main, almost cylindrical branch with numerous irregular annulations. F, main branch with non-constricted lateral branches. Scales: A & B = 0.5 mm; C–F = 1 mm. In colour online.
Variability
Whereas European specimens seem to correspond exclusively to the protocetraric acid chemotype, the African specimens show quite a variable chemistry with the protocetraric acid, salazinic acid, psoromic acid and diffractaic acid chemotypes (Swinscow & Krog Reference Swinscow and Krog1988). No correlations between morphology and these chemotypes could be found (Swinscow & Krog Reference Swinscow and Krog1976b). The degree to which segments are swollen is also variable. Some specimens have branches that are not swollen at all (Fig. 6E). In these cases, the presence of pseudocyphellae, the absence of papillae, the anatomy and the chemistry are diagnostic. Pseudocyphellae might be of various shapes from rounded to elongated, thin to large, or even almost absent in some specimens. Rarely, pseudocyphellae enlarge and produce soredia; such morphotypes correspond exactly to the type specimen of U. pseudocyphellata Motyka.
Distribution and ecology
Usnea articulata occurs in south-western Europe, the British Isles, Macaronesia, Africa, South America and Australia (Truong et al. Reference Truong, Rodriguez and Clerc2013b). It is a frequent and abundant species in tropical Africa (Dodge Reference Dodge1956; Swinscow & Krog Reference Swinscow and Krog1976b). In São Tomé and Príncipe, it occurs in primary, Rubiaceae-dominated forest growing mainly on twigs and branches of tropical hardwood and fallen into leaf litter, between elevations of 1162 and 1863 m on the island of São Tomé only. It is found associated with U. bicolorata, U. exasperata, U. firmula, U. pectinata Taylor and U. submollis.
Discussion
In Africa, Usnea articulata seems to be a highly variable species. Motyka (Reference Motyka1936–1938) considered this species to be restricted to Europe and North Africa and he described many ‘articulata-morphotypes’ occurring in Central Africa as new species or new varieties (Motyka Reference Motyka1936–1938, Reference Motyka1956). Today, most of these taxa are considered synonyms of Usnea articulata (Swinscow & Krog Reference Swinscow and Krog1976b, Reference Swinscow and Krog1978). Usnea speciosa is a robust morphotype with elongated pseudocyphellae and diffractaic acid whose description was based on a specimen collected in São Tomé. Swinscow & Krog (Reference Swinscow and Krog1976b) considered this taxon to be a different species characterized by the effigurate-linear pseudocyphellae and the diffractaic acid chemotype. However, firstly the specimen MN586a collected in São Tomé has numerous effigurate-linear pseudocyphellae (Fig. 6C) as well as protocetraric acid in the medulla and, secondly, there are specimens with both protocetraric and diffractaic acids, such as the type of U. flavescens Motyka (Swinscow & Krog Reference Swinscow and Krog1976b). One specimen (MN0068c) has the typical enlarged and ±circular pseudocyphellae that become sorediate and are diagnostic for U. pseudocyphellata. In our phylogenetic tree, U. articulata belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). Figure 3 shows U. articulata being paraphyletic with two highly supported clades. However, there are no correlations with the chemistry or with any morphological characters. Furthermore, the lack of support in the deeper nodes does not allow any definitive conclusion to be drawn. For this reason, we here consider our specimens to be part of an aggregate. Studies of this cosmopolitan species on a worldwide scale with the tools of integrative taxonomy are needed to resolve this group (Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020).
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, on road from Bom Successo to ‘Macambara’ Radio Station, 1162–1319 m, 2012, M. Nadel & J. Shevock MN0068c (CAS); trail between Pico Cálvario and mesa below Pico de São Tomé, 1647 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0216 (CAS); trail between Pico Cálvario and mesa below Pico de São Tomé, 1863 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0216 (CAS); primary trail from Bom Successo to Lagoa Amelia, 1298 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0551 (CAS); primary trail from Bom Successo to Lagoa Amelia, 1440 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0575b (CAS); overlook above Lagoa Amelia, 1470 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0571 (CAS); secondary trail from Bom Successo to Lagoa Amelia, 1191 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0578a (CAS); ibid., 1196 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0586a (CAS).
Usnea baileyi (Stirt.) Zahlbr.
Denkschr. Kaiserl. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 83, 182 (1909).—Eumitria baileyi Stirt., Scott. Natural. 6, 100 (1881) [1881–1882]; type: [Australia], Queensland, F. M. Bailey 16 (lectotype—BM!) (fide Rogers & Stevens Reference Rogers and Stevens1988).
Complete descriptions, images and synonyms can be found in Swinscow & Krog (Reference Swinscow and Krog1974, Reference Swinscow and Krog1988), Stevens (Reference Stevens1999), Ohmura (Reference Ohmura2001), Clerc (Reference Clerc, Nash, Gries and Bungartz2007), Truong & Clerc (Reference Truong and Clerc2013) and Temu et al. (Reference Temu, Clerc, Tibell, Tibuhwa and Tibell2019).
CMA
(Specimens collected in São Tomé only, n = 5). %C = (3.0–)3.5–5.1–7.7(–9.5); %M = 2.5–4.3–6.2(–7.0); %A = (67–)72.9–81–87; A/M = (9.5–)12.1–21.5–30.9(–32.5); A/C = (6.8–)11.1–18.8–26.5; M/C = 0.7–0.9–1.4(–1.8); %TBA = 35–45.6–59(–63).
Chemistry
(Specimens collected in São Tomé only, n = 2). Medulla K+ yellow turning orange, Pd+ yellow turning red-orange, hyphae of the tubular axis K+ yellow, Pd−. Norstictic and salazinic (trace) acids, unknowns 4.5–5/5–5/5–6 pale brownish spots, fatty acid?/3/4 (n = 2).
Diagnostic characters
Usnea baileyi is characterized by a stiff, shrubby to subpendant thallus (Fig. 8D), a pale basal part concolorous with main branches (Fig. 8G), tapering branches with cylindrical segments, long and slender fibrils (Fig. 8C), the number of which is inversely proportional to the number of tubercules (juvenile fibrils) or fibercles (scars of broken fibrils) or soralia (enlarged fibercles producing soredia and isidiomorphs), a thin, reddish pigmented medulla and a broad tubular central axis (Fig. 8H).
Variability
The morphology of Usnea baileyi is highly variable depending on the growth of fibrils and whether they are shed or not. Its chemistry is also very variable with numerous chemotypes (see Rogers & Stevens Reference Rogers and Stevens1988).
Distribution and ecology
Usnea baileyi is known to occur on every continent except Antarctica and Europe (Swinscow & Krog Reference Swinscow and Krog1974; Stevens Reference Stevens1999; Ohmura Reference Ohmura2001; Truong & Clerc Reference Truong and Clerc2013; Esslinger Reference Esslinger2019). It is primarily a corticolous species growing on a wide variety of trees and shrubs, occasionally also lignicolous (fencepost) or saxicolous. It is a species with a rather wide ecological range, from humid to arid places in subtropical and tropical areas of the world (Rogers & Stevens Reference Rogers and Stevens1988). It occurs on both São Tomé and Príncipe in various forest types, from 200–1400 m elevation on São Tomé and 500–600 m on Príncipe. The species was found both in undisturbed forest and more disturbed areas alongside roads and trails. The growth form was always corticolous, sometimes fallen from the canopy onto the forest floor and boulders. It was found associated with U. pectinata. Usnea baileyi is newly reported for São Tomé and Príncipe.
Discussion
Usnea baileyi with its tubular central axis and phylogenetic position belongs to the subgenus Eumitria. (Ohmura Reference Ohmura2001, Reference Ohmura2002; Temu et al. Reference Temu, Clerc, Tibell, Tibuhwa and Tibell2019). In the phylogenetic tree (Figs 2 & 5), our specimens cluster in the Eumitria subgenus clade and are nested in the U. baileyi clade, sister of the U. pectinata clade. As Temu et al. (Reference Temu, Clerc, Tibell, Tibuhwa and Tibell2019) and Lücking et al. (Reference Lücking, Nadel, Araujo and Gerlach2020) have already shown, U. baileyi seems to be quite heterogenous and more detailed studies are necessary to understand the heterogeneity of this pantropical species (Clerc Reference Clerc, Nash, Gries and Bungartz2007). For differences from U. firmula, see under that species.
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: trail past Ponta Furada at the end of the road on west coast, 215 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0472 (CAS); Parque Natural Obô de São Tomé, on the primary trail from Bom Successo to Lagoa Amelia, 1246 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0549a (CAS); unimproved road between Bemposta and Chamico, 862 m, 2013, M. Nadel, J. Shevock, T. Daniel & Q. Quade MN0600a (CAS). Island of Príncipe: trail from Terreiro Velho to Morro de Leste, 581 m, 2013, M. Nadel & O. Rocha MN0535 (CAS); trail from Terreiro Velho to Morro de Leste, 596 m, 2013, M. Nadel & O. Rocha MN0539 (CAS).
Usnea beckeri P. Clerc & Nadel sp. nov.
MycoBank No.: MB 843679
Thallus pendulous, stiff and brittle, up to 25 cm long, dark green-coloured, with apothecia and without soralia. Main branches cylindrical to slightly irregular, smooth. Lateral branches not constricted. Axis moderately thin to moderately thick (30–50%). Medulla dense, containing the stictic acid gr., US1 and US2 sensu Ohmura (Reference Ohmura2001). Apothecia mainly lateral, with non-eroded cortical rim and long (2–15 mm) cilia. Ascospores 8–9 × 5–6 μm.
Type: Republic of São Tomé and Príncipe, Gulf of Guinea, Island of Príncipe, trail up to the summit of Pico Papagaio from Santa Trindade, 536 m elevation, 15 April 2013, M. Nadel, J. R. Shevock, T. Daniel & O. Rocha 489 (holotype—CAL-1318542).
(Fig. 7)

Fig. 7. Usnea beckeri holotype. A, thallus; note the small, detached branch fragments illustrating how brittle the branches are. B, apothecia, serial and subterminal. C, anatomy of a main branch. D, smooth branches. Scales: B = 2 mm; C = 0.5 mm; D = 1 mm. In colour online.
Thallus pendulous and brittle, up to 25 cm long, dark green-coloured, with filamentous ramifications (Fig. 7A); basal part short, pale to concolorous with branches; main branches 0.6–0.9 mm thick, usually smooth (Fig. 7D), cylindrical to slightly irregular, tapering only at the extremities, often partly blackened especially close to the basal part; segments cylindrical or slightly to distinctly swollen in cross-section, sometimes very short, especially on main branches, with distinct annulations, sometimes with regenerated cortex between segments giving the impression of a double annulation; lateral branches not constricted at attachment point; terminal branches thin, smooth, capillaceous, with few ramifications; foveolae and transverse furrows absent; maculae and pseudocyphellae absent; papillae absent; tubercles (young fibrils?) small (50 μm), few, scattered on main branches only; fibrils absent; fibercles small (50 μm), few, scattered on main branches only; soralia absent.
Apothecia never numerous, sometimes absent, 1–5 mm wide, mainly lateral and subterminal, flat to slightly cupular, with pruinose discs surrounded by (sometimes few) long cilia (2–15 mm long) and a non-eroded cortical rim (Fig. 7B); ascospores ellipsoid, 8–11 × 5–7 μm.
Cortex slightly shiny in section, of the merrillii-type plectenchyma, often with incomplete circular cracks, thin to moderately thin, %C = (4.5–)4.8–5.7–6.6(–7.0) (n = 9); medulla dense, moderately thin to moderately thick, %M = (18.5–)19.9–23.7–27.5(–29); axis moderately thin to moderately thick, %A = (29–)32.8–41.3–49.8(–53); A/M = (1.0–)1.2–1.9–2.6(–2.8); A/C = (4.5–)5.1–7.6–10.1(–12.1); M/C = 3.3–4.2–5.1(–6.3) (Fig. 7C).
Chemistry
(n = 4). Medulla K+ yellow turning slowly orange, Pd+ orange. Stictic, constictic, cryptostictic, menegazziaic and norstictic (tr.) acids, and the unknown substances US1 and US2 sensu Ohmura (Reference Ohmura2001).
Etymology
The first author of this taxon names it in honour of his good friend Uwe Becker (Cologne, Germany), who did his Ph.D. on the lichens of the inselbergs in Zimbabwe, promoting the knowledge of African lichens; in shared memory of the good times we spent together, collecting lichens on these extraordinary inselbergs. Uwe is now a distinguished and successful children's author.
Diagnostic characters
The main diagnostic characters of Usnea beckeri are the dark green and brittle pendulous-filamentous thallus, with apothecia, without soralia, the overall smooth, cylindrical to slightly irregular branches with distinct annulations and circular cracks, the non-constricted lateral branches, the moderately thick and shiny cortex, the dense medulla, the moderately thick axis and the presence of the stictic acid gr. with the unknown US1 and US2 in the medulla.
Variability
Some specimens consist of a network of thick main branches of approximately the same diameter, without smaller branches ending in thin capillaceous apices. In this case, branches are distinctly irregular. Apothecia can be totally absent. Some thalli are almost shrubby or subpendulous.
Distribution and ecology
Known only from the island of Príncipe, observed in primary, Rubiaceae-dominated hardwood tropical forests between 501 and 715 m elevation. It is associated with the other novel species, U. longiciliata, and has a corticolous growth habit, although many specimens were collected from the ground, including on rocky areas and leaf litter where it had fallen from the canopy.
Discussion
A search among the type specimens and protologues of all the pendulous non-sorediate and apotheciate species occurring in Africa and South America to find an already published name was unsuccessful. Usnea beckeri strongly resembles the other new species U. longiciliata. Both are fertile, non-sorediate taxa that share the peculiar dark green coloration of the thallus, the long cilia on the apothecia, the dense medulla and the presence of the unknowns US1 and US2 in the medulla. They differ essentially in their life form, pendulous in U. beckeri (Fig. 7A) and shrubby in U. longiciliata (Fig. 8A), in the cortex type, of the merrillii-type in U. beckeri and of the ceratina-type in U. longiciliata, in the presence of numerous fibercles, the larger ascospores and in the absence of the stictic acid gr. in U. longiciliata. Usnea himalayana C. Bab. looks superficially like U. beckeri. However, the former taxon has pseudocyphellae, a much thinner central axis and a lax medulla producing salazinic acid. For differences with U. nodulosa and U. submollis, see under these taxa. In the phylogenetic tree (Fig. 2), U. beckeri belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). It is monophyletic and belongs to a highly supported but unresolved clade containing U. nodulosa, Usnea sp. 1 and Usnea sp. 2 (Fig. 4).

Fig. 8. Usnea firmula MN0084. A, thallus with irregular main branches. B, lateral apothecia. E, jet black-pigmented basal part. F, anatomy of a main branch with wide tubular axis. Usnea baileyi MN0539. C, main ‘fish bone’ like branches with long and slender fibrils. D, thallus with tapering branches. G, concolorous basal part. H, anatomy of a main branch with tubular axis. Scales: A & D = 1 cm; B, C, F & H = 0.5 mm; E & G = 1 mm. In colour online.
Paratypes
Sāo Tomé and Príncipe: Island of Príncipe: trail from Santa Trindade to Pico Papagaio, 501 m, near rope ladder, 2012, M. Nadel & J. Shevock MN0412 (CAS); trail from Santa Trindade to Pico Papagaio, 536 m, 2013, M. Nadel, J. Shevock, T. Daniel & O. Rocha MN0489 (CAS); trail from Santa Trindade to Pico Papagaio, 674 m, 2013, M. Nadel, J. Shevock, T. Daniel & O. Rocha MN0496 (CAS); ridge leading to Pico Príncipe, 715 m, 2013, M. Nadel & O. Rocha MN0515 (CAS); trail from Terreiro Velho to Morro de Leste, 565 m, 2013, M. Nadel & O. Rocha MN0532, MN0533 (CAS); trail from Terreiro Velho to Morro de Leste, 606 m, 2013, J. Shevock, T. Daniel, O. Rocha & M. Nadel MN0543, MN0544 (CAS).
Usnea bicolorata Motyka
Lich. Gen. Usnea Monogr., 336 (1936–1938); type: Africa centralis, in montibus Kivu, de Witte s. n. (holotype—LBL-920!). Chemistry: usnic, protocetraric and barbatic acids. %CMA = 4.5/35/21; A/M = 0.6; A/C = 4.4; M/C = 7.4.
Usnea griseola Motyka, Annls Univ. Mariae Curie-Skłodowska, Sect. C, Biol. 11, 120 (1959) [1956]; type: Nkanda, im Hang gegen dem Satel, an Hypericum und Ericaceen, 2700 m, 26.xi.1954, Stauffer 3398/984 (holotype—G60597!). Chemistry: usnic, protocetraric (main), salazinic and alectorialic acids. %CMA = 6/38/12; A/M = 0.3; A/C = 2; M/C = 6.4.
Usnea bicolorata var. pseudorubescens Motyka, Annls Univ. Mariae Curie-Skłodowska, Sect. C, Biol. 11, 122 (1959) [1956]; type: Muhavura, auf schräg aufsteigendem Stamm eines Rhodblattbaumes 862, Oberseite, 3300 m, 19.xi.1954, Stauffer 3370/872 (holotype—G66569!). Chemistry: usnic, protocetraric, alectorialic and barbatic acids. %CMA = 4.5/33.5/24; A/M = 0.7; A/C = 5; M/C = 7.2.
Detailed description and images of U. bicolorata are given in Swinscow & Krog (Reference Swinscow and Krog1979, Reference Swinscow and Krog1988) and Ohmura et al. (Reference Ohmura, Lin and Wang2010). However, none of these authors were successful in tracing the type specimen. In the type specimen of U. bicolorata, as in the types of U. griseola and U. bicolorata var. pseudorubescens, branches are not tapering but irregular (i.e. the largest part of the main branches is not close to the basal part). Furthermore, lateral branches are slightly but distinctly constricted and the CMA-type is close to the tenuicorticata-type (M/C > 7).
CMA
(Specimens collected in São Tomé only, n = 3). %C = (4.5–)4.7–6.2–7.5; %M = 32–34.5–37.6(–38); %A = 15–21.3–27.8(–28); A/M = 0.4–0.6–0.7; A/C = 3.0–3.1–3.2; M/C = 4.4–5.9–7.8(–8.1).
Chemistry
(Specimens collected in São Tomé only, n = 1). Medulla K−, Pd+ red-orange; protocetraric acid. The two other specimens collected are K−, Pd+ red-orange (no TLC performed).
Diagnostic characters
The main diagnostic characters of U. bicolorata are the shrubby sorediate thallus, the jet black-pigmented basal part, the irregular branches, the constricted lateral branches, the orange-red subcortical pigment, the large (> 1/2 branch diameter) and irregular soralia that can encircle the branches, the cornuta-tenuicorticata type of CMA and the presence of protocetraric acid in the medulla.
Variability
The development of soralia, from punctiform and minute to large, ±convex and encircling the branches, varies greatly depending on the maturity of the thallus as well as on environmental conditions. The chemistry is variable with different chemotypes (Ohmura et al. Reference Ohmura, Lin and Wang2010).
Distribution and ecology
Usnea bicolorata is known to occur in tropical Africa (Motyka Reference Motyka1936–1938, Reference Stevens1956; Dodge Reference Dodge1957; Swinscow & Krog Reference Swinscow and Krog1975, Reference Swinscow and Krog1988) and in Taiwan (Ohmura et al. Reference Ohmura, Lin and Wang2010). In Taiwan, U. bicolorata was found on the bark of Pinus at an elevation of c. 2600 m. It is a common species on shrubs in the montane forests and ericaceous zone at 1900–3500 m elev. in East Africa (Swinscow & Krog Reference Swinscow and Krog1988). In São Tomé and Príncipe, it was found in primary Rubiaceae-dominated forests on the island of São Tomé between 1230 and 1298 m, growing on hardwood branches with U. articulata, U. firmula and U. submollis. Usnea bicolorata is newly reported for São Tomé and Príncipe.
Discussion
Both specimens collected in São Tomé are juvenile and small with punctiform and minute underdeveloped soralia. However, all other characters fit well with the definition of U. bicolorata. Several other sorediate Usnea species are known to have an orange-reddish subcortical pigmentation, such as U. crocata Truong & P. Clerc, U. dorogawensis Asahina, U. grandisora Truong & P. Clerc, U. poliothrix Kremp., U. subcornuta Stirt. and U. subdasaea Truong & P. Clerc. However, U. bicolorata is the only species among them with a distinct jet black-pigmented basal part. For differences with U. sorediosula Motyka, the other African species with an orange-reddish subcortical pigmentation, see under that species. The var. australiensis G. N. Stevens with galbinic acid, ‘pale or black’ trunk, ‘isidia sparse or quite dense in pseudocyphellae’ [soralia] should be further studied since it might not correspond to U. bicolorata. Usnea bicolorata could not be sequenced since it was separated out from mixed collections after the sequencing had already been completed in 2014–2015.
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé on the primary trail from Bom Successo to Lagoa Amelia, 1298 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0552c (CAS); secondary trail from Bom Successo to Lagoa Amelia, 1230 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0579c (CAS).
Usnea erinacea aggr.
Incl.: Usnea erinacea Vain. Dansk Bot. Ark. 4 (no. 11), 3 (1926); type: Mexico, Chimantla, 1846, Liebmann (holotype—TUR!). Chemistry: salazinic and norstictic acids. %CMA = 7.5/17.5/50; A/M = 2.9; A/C = 6.7; M/C = 2.3.
Usnea sanguinea Swinscow & Krog, Lichenologist 11, 243 (1979); type: Tanzania, Northern Province, Arusha National Park, Juniper Hill, Swinscow & Krog T3/16 (holotype—BM!). Chemistry: salazinic and norstictic acids. %CMA: 10.5/15/49, A/M: 3.3, A/C: 4.7, M/C: 1.4
A detailed description is provided by Clerc (Reference Clerc2004, Reference Clerc, Nash, Gries and Bungartz2007).
CMA
(Specimens collected in São Tomé only, n = 1). %C = 8; %M = 28; %A = 29; A/M = 1; A/C = 3.6; M/C = 3.5.
Chemistry
(Specimen collected in São Tomé only, n = 1). Medulla K−, Pd+ red-orange; protocetraric acid, unknown 3/6/5 greyish green.
Diagnostic characters
The main diagnostic characters are the shrubby apotheciate, non-sorediate thallus with a cortical red pigmentation, the tapering branches and the non-constricted lateral branches.
Distribution and ecology
The Usnea erinacea aggregate occurs on a wide variety of trees, on rocks, and rarely on dead trunks or fences and scrubs in the subtropical/tropical areas over a wide altitudinal range (200–3000 m), mainly in South America (Truong et al. Reference Truong, Bungartz and Clerc2011; Gerlach et al. Reference Gerlach, Clerc and Borges da Silveira2017), Africa (Swinscow & Krog Reference Swinscow and Krog1988) and Australia (Stevens Reference Stevens1999). In São Tomé and Príncipe, it was found on the island of São Tomé only, at 2009 m on the summit of the highest peak on the island. The specimen was blown by wind onto the concrete monument on the peak but had a clearly corticolous growth habit. The surrounding vegetation consisted of small trees and shrubs covered in large amounts of lichen and liverworts including U. exasperata. The Usnea erinacea aggregate is newly reported for São Tomé and Príncipe.
Discussion
Usnea erinacea as currently understood is polyphyletic (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a; Gerlach et al. Reference Gerlach, Clerc and Borges da Silveira2017; Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020) and requires a thorough study with the tools of integral taxonomy. It contains several species differing mainly in the type of reddish pigmentation, in the pigmentation of the basal part, in the anatomical characters (cortex structure and thickness) and in the chemistry. The small specimen (2 cm long) without apothecia collected on São Tomé Island (MN0264a) has a variegated reddish cortical pigmentation, a jet black-pigmented basal part, a matt cortex and protocetraric acid in the medulla. All these characters do not fit with the concept of either U. erinacea s. str. or U. sanguinea. In our phylogenetic tree (Fig. 2), MN0264a belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). It appears as the sister species of U. crocata, another pigmented taxon, but this relationship is not supported. It is most probably an undescribed species. More specimens from Africa and South America are needed to formally describe this species.
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, on the summit of Pico de São Tomé, 2009 m, 2012, M. Nadel & O. Rocha MN0264a (CAS).
Usnea exasperata (Müll. Arg.) Motyka
In Zahlbruckner & Hauman, Nat. Hist. Danish Lich. 5, 24 (1936).—Usnea dasypogoides var. exasperata Müll. Arg., Flora (Regensburg) 73, 336 (1890); type: Kilimandscharo [Kilimanjaro], b. 3000 m, x.1889, Hans Meyer (holotype—G00066257!)
A detailed description, synonyms and images of U. exasperata can be found in Swinscow & Krog (Reference Swinscow and Krog1978, Reference Swinscow and Krog1988).
CMA
(Specimens collected in São Tomé only, n = 3). %C = 9.0–10.5–12; %M = 24.5–25.7–27–3(–27.5); %A = 27–28.3–29.8(–30); A/M = 1.0–1.1–1.2; A/C = (2.2–)2.3–2.7–3.0; M/C = 2.1–2.5–3.0.
Chemistry
(Specimens collected in São Tomé only, n = 3). Medulla K+ slowly yellowish, Pd+ citrine yellow; psoromic and 2ʹ-O-demethylpsoromic acids.
Diagnostic characters
(Specimens collected in São Tomé only, n = 3). The main diagnostic characters of this form of U. exasperata are the pendulous thallus, the irregular branches, the cylindrical to slightly acute-angled segments in cross-section (Fig. 6D), the lateral branches widened at ramification points (Fig. 6F), the smooth cortex with linear cracks developing into pseudocyphellae, ±circular and ±stipitate soralia that might aggregate but not fuse together, the matt and relatively thick cortex, the dense to compact medulla with, on average, the same thickness as the central axis in cross-section (Fig. 6B), and the presence of psoromic acid in the medulla.
Variability
Swinscow & Krog (Reference Swinscow and Krog1978) discussed in detail the variability of U. exasperata. They accepted a broad concept of the species, with the inclusion of a total of 17 taxa described by Motyka (Reference Motyka1956) into synonymy with U. exasperata. The morphotype encountered on São Tomé corresponds to the ‘smooth form’ described by Swinscow & Krog (Reference Swinscow and Krog1978: figs 11 & 12) in East Africa.
Distribution and ecology
Usnea exasperata was so far known to occur only in East Africa (Motyka Reference Motyka1936–1938; Swinscow & Krog Reference Swinscow and Krog1978). The localities in São Tomé extend the distribution of this species to West Africa; it was found on the island of São Tomé at high elevation (1800–2009 m) in the primary, Rubiaceae-dominated forest, corticolous on the shrubs and hardwood trees and fallen on leaf litter. It was associated with U. articulata, U. erinacea aggr. and U. pectinata aggr. Usnea exasperata is newly reported for São Tomé and Príncipe.
Discussion
Among the material collected in São Tomé, U. exasperata could only be superficially confused with U. articulata. However, the latter species has a much thinner cortex, a larger and laxer medulla and thinner central axis, and protocetraric and/or diffractaic acids in the medulla. Only the chemotype of U. exasperata with psoromic acid was found in São Tomé. The type specimen of U. exasperata has protocetraric and fumarprotocetraric acids in the medulla. Among the 17 taxa put into synonymy by Swinscow & Krog (Reference Swinscow and Krog1978), only U. elegantissima Motyka shares this chemotype with U. exasperata and would be the available name for this ‘smooth morphotype’ in case future molecular studies would split this taxon into distinct species corresponding to the chemotypes. In the phylogenetic tree (Fig. 2), U. exasperata belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). It appears monophyletic, although not strongly supported, and is nested into a weakly supported clade containing U. articulata, U. submollis and U. ghattensis G. Awasthi (Fig. 3). This would confirm the close relationship with U. articulata as already mentioned by Swinscow & Krog (Reference Swinscow and Krog1976b).
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, trail between Pico Cálvario and Pico Mesa below Pico de São Tomé, 1863 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0243 (CAS); on the summit of Pico de São Tomé, 2009 m, 2012, M. Nadel & O. Rocha MN0260 (CAS); trail between Pico Mesa and the Rio Cantador Valley, 1837 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0269 (CAS).
Usnea firmula (Stirt.) Motyka
Lich. Gen. Usnea Monogr., 51 (1936–1938).—Eumitria firmula Stirt., Scott. Natural. 6(3), 100 (1881) [1881–1882]; type: [Bioko] Fernando Po, lava beds, G. Thomson s. n., undated [19th century] (holotype—BM). Chemistry: protocetraric acid.
Syn. nov.: Usnea baileyi var. pinnatifida Swinscow & Krog, Norw. J. Bot. 21(2), 172 (1974); type: Tanzania, Central Province, Mpwapwa District, Kiboriani Mountains, highest peak N of Mpwapwa, 1800 m, evergreen bush on quartzite rocks of the SSE slope, 11.v.1972, T. Pocs & L. Mezosi (holotype—BM-6566/I!). Chemistry: protocetraric acid.
Complete descriptions, images and synonyms can be found in Swinscow & Krog (Reference Swinscow and Krog1974, Reference Swinscow and Krog1988).
CMA
(Specimens collected in São Tomé only, n = 4). %C = (2.0–)2.5–3.4–4.0; %M = (1.0–)1.2–2.1–3.0; %A = (86–)86.5–89.3–92; A/M = 27.7–48–71.3(–81.3); A/C = 20.9–28.9–39.7(–44.7); M/C = (0.3–)0.4–0.7–1.0; %TBA = (79–)79.3–83–86.7(–87).
Chemistry
(Specimens collected in São Tomé only, n = 2). Medulla K+ yellow turning orange, Pd+ red-orange, hyphae of the tubular axis K+ pale greenish yellow, Pd −. Protocetraric acid, unknowns 4.5–5–5/3–3/5–5.5 pale brownish spots.
Diagnostic characters
Usnea firmula is characterized by a stiff shrubby to subpendant thallus (Fig. 8A), a jet black-pigmented basal part with black pigmentation often spreading on the main branches (Fig. 8E), irregular branches with cylindrical segments (Fig. 8A), long and slender fibrils the number of which is inversely proportional to the number of tubercules (juvenile fibrils), the absence of soralia, the frequent occurrence of apothecia mainly growing laterally on short branches (Fig. 8B), a thin, reddish pigmented medulla and a broad tubular central axis (Fig. 8F).
Variability
The morphology of Usnea firmula is highly variable depending on the growth of fibrils and whether they are shed or not. The presence of apothecia is also variable, from absent to numerous.
Distribution and ecology
Usnea firmula was described on the basis of a specimen collected on the island of Bioko (Fernando Po) situated to the NW of São Tomé, also in the Gulf of Guinea. It is so far known only from Africa (Cameroon, Kenya, Republic of Equatorial Guinea and Tanzania). In São Tomé and Príncipe it occurs on the island of São Tomé in the primary, Rubiaceae-dominated forest between 1162 and 1323 m, growing corticolous on hardwood branches often fallen to the forest floor and in leaf litter. It is found associated with U. articulata, U. bicolorata and U. submollis. This collection of Usnea firmula is noted as new for São Tomé and Príncipe (Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020).
Discussion
Usnea firmula, with its tubular central axis and phylogenetic position (Figs 2 & 5), belongs to the subgenus Eumitria. It differs from U. baileyi mainly by its black-pigmented basal part, its irregular branches, the absence of soralia, and the frequent occurrence of apothecia. There also seems to exist differences in the %CMA and %TBA, but the number of specimens studied here is too small to draw definitive conclusions. The species also differ in their chemistry: Usnea baileyi contains norstictic acid whereas U. firmula produces protocetraric acid in the medulla. However, Swinscow & Krog (Reference Swinscow and Krog1974) described two varieties of U. baileyi (var. pinnatifida Swinscow & Krog and var. planiuscula Swinscow & Krog), both with protocetraric acid in the medulla. Usnea baileyi var. pinnatifida corresponds well to U. firmula with an extended jet black-pigmented basal part, the irregular branches, and the chemistry. It is considered here as a synonym of U. firmula. Usnea baileyi var. planiuscula is a different taxon that significantly differs from both U. firmula and U. baileyi. Further studies are necessary to determine its status. Usnea firmula and its affiliation to the Eumitria subclade is phylogenetically confirmed here, as in Lücking et al. (Reference Lücking, Nadel, Araujo and Gerlach2020). All sequenced specimens are clustered in a partly supported monophyletic clade sister to that formed by U. baileyi and U. pectinata (Figs 2 & 5).
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, on road from Bom Successo to ‘Macambara’ Radio Station, 1162–1319 m, 2012, M. Nadel & J. Shevock MN0084 (CAS); trail from ‘Macambara’ Radio Station leading into the forest, 1323 m, 2012, M. Nadel & J. Shevock MN0117 (CAS); primary trail from Bom Successo to Lagoa Amelia, 1298 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0550a, MN0550b (CAS); secondary trail from Bom Successo to Lagoa Amelia, 1241 m, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0581 (CAS).
Usnea krogiana P. Clerc
Lichenologist 38, 199 (2006); type: Espagne [Spain], Iles Canaries, La Gomera, Alajero, Lomo de la Mulata, sur les pentes SE du Garajonay, surplombant la route menant à Lajero, 1320–1340 m, sur Erica arborea dans des plantations de pins en pente SSE, avec Teline canariensis, 23.ix.1986, P. Clerc (holotype—G00066621). Chemistry: stictic (major), constictic, cryptostictic, menegazziaic and norstictic acids (minor). %CMA = 11/9/60; A/M = 6.6; A/C = 5.5; M/C = 0.8.
A detailed description and images of U. krogiana can be found in Clerc (Reference Clerc2006).
CMA
(Specimens collected in São Tomé only, n = 1). %C = 12; %M = 9; %A = 57; A/M = 4.7; A/C = 4.7; M/C = 0.8.
Chemistry
(Specimens collected in São Tomé only, n = 1). Medulla K+ yellow turning slowly orange-red, Pd+ citrine yellow; soralia K+ yellowish, Pd+ yellow; norstictic and salazinic acids. This chemotype is new for U. krogiana.
Diagnostic characters
Usnea krogiana is an easily recognizable species with the small stiff and shrubby thallus, the basal part that is sharply delimited with a black pigment, the tapering branches with cylindrical segments, the small and minute fibercles/soralia, the matt and relatively thick cortex, the thin and compact medulla and the large central axis (> 50%).
Variability
Usnea krogiana is not a very variable species and can be easily recognized in the field. Chemistry, however, shows a little variation with three chemotypes: 1) stictic acid gr. and norstictic acid; 2) norstictic acid; 3) norstictic and salazinic acids.
Distribution and ecology
Prior to the São Tomé collection (Nadel Reference Nadel2016; Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020), Usnea krogiana was known to occur only in Macaronesia (Azores and Canary Islands) and in the West Indies (Cuba, Dominican Republic, Haiti and Puerto Rico) (Clerc Reference Clerc2006). In São Tomé and Príncipe it was found only on the island of São Tomé, at Lagoa Amelia, a vegetation covered lake in a dormant volcanic crater, growing on a shrubby species of Schefflera.
Discussion
The specimen collected on São Tomé corresponds well morphologically and anatomically to the type. The presence of salazinic acid together with norstictic acid had not been previously recorded in this species. For differences from other shrubby sorediate taxa, see Clerc (Reference Clerc2006). Usnea krogiana cannot be confused with any other taxa discussed in this article. The sequences generated (Nadel Reference Nadel2016) and analyzed here and in Lücking et al. (Reference Lücking, Nadel, Araujo and Gerlach2020) mark the first time that U. krogiana has been sequenced. It belongs to clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). It is interesting to note that in our phylogenetic tree (Fig. 2), this Macaronesian species belongs to an unsupported clade mainly containing species originally described from Macaronesia (U. macaronesica P. Clerc and U. boomiana P. Clerc), together with U. arianae P. Clerc et al.
Specimen examined
Sāo Tomé and Príncipe: Island of São Tomé: Lagoa Amelia, 1418 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0560 (CAS).
Usnea longiciliata P. Clerc & Nadel sp. nov.
MycoBank No.: MB 843680
Thallus shrubby, stiff, dark green-coloured, with apothecia and without soralia. Lateral branches not constricted. Axis thick (> 50%). Medulla dense, not compact, containing the unknown substances US1 and US2 sensu Ohmura (Reference Ohmura2001). Apothecia mainly subterminal, with long (5–15 mm) cilia. Ascospores 9–12 × 6–8.5 μm.
Type: Republic of São Tomé and Príncipe, Gulf of Guinea, Island of Príncipe, trail up to the summit of Pico Papagaio from Santa Trindade, 509 m elev., fallen from tree onto trail, 15 April 2013, M. Nadel 485 (holotype—CAS1318659). Chemistry: US1, US2 (sensu Ohmura Reference Ohmura2001). %CMA = 10/10.5/59; A/M = 5.6; A/C = 5.9; M/C = 1.1. Ascospores: 9–12 × 6–8.5 μm.
(Fig. 9)

Fig. 9. Usnea longiciliata holotype. A, thallus. B, transversal section of a main branch. C, subterminal apothecia with long fibrils (cilia). D, branch with tubercles (young fibrils) and fibercles. Scales: B = 0.2 mm; C = 2 mm; D = 0.5 mm. In colour online.
Thallus shrubby and stiff, up to 6 cm long, dark green-coloured, with ±isotomic-dichotomous ramifications (Fig. 9A); basal part short, brownish-blackish (not jet black) close to the holdfast; main branches 0.6–1 mm thick, tapering or cylindrical, rarely slightly irregular; segments cylindrical, terete in cross-section; lateral branches not constricted at attachment point; terminal branches moderately thin, almost unbranched; foveolae and transverse furrows absent; maculae and pseudocyphellae absent; papillae absent; tubercles (young fibrils) numerous (Fig. 9D); fibrils numerous, 3–5 mm long, slender on young thalli, almost absent on mature thalli; fibercles few on young thalli, numerous on mature thalli; soralia absent.
Apothecia numerous on mature thalli, 1–6 mm wide, mainly terminal, sometimes pseudoterminal, flat to slightly cupular, with pruinose discs surrounded by long cilia (5–15 mm long), with an eroded cortical rim (Fig. 9C); ascospores ellipsoid, 9–12 × 6–8.5 μm.
Cortex slightly shiny in section, of the ceratina-type plectenchyma, moderately thick, %C = (7.5–)8.3–9.8–11.3(–11.5) (n = 5); medulla dense, thin, %M = 10.5–13–16.2(–16.5); axis thick, %A = (46–)48.8–54.2–59; A/M = (2.8–)2.9–4.4–5.6; A/C = (4.3–)4.5–5.6–6.7(–6.9); M/C = 0.9–1.4–1.9(–2.2) (Fig. 9B).
Chemistry
(n = 5). Medulla K+ pale yellow, Pd−; unknowns US1 and US2 (sensu Ohmura Reference Ohmura2001).
Etymology
The name longiciliata refers to the long cilia (fibrils) surrounding the apothecial discs.
Diagnostic characters
The shrubby, stiff and apotheciate thallus, the long fibrils (cilia) on the edge of the apothecia, the numerous fibercles, the non-constricted lateral branches, the cylindrical to tapering main branches, the thick central axis and thin but dense medulla and the distinctive chemistry are the main diagnostic characters of this species.
Variability
Young thalli might lack apothecia (Nadel 405). They usually have numerous fibrils and few fibercles. Mature thalli lose fibrils and therefore have numerous fibercles (type specimen).
Distribution and ecology
Usnea longiciliata is currently endemic to the island of Príncipe. It is a corticolous species occurring in the primary Rubiaceae forest between 465 and 580 m in the central region of the island, often fallen to the forest floor on small twigs and branches. It is found associated with the other new species, U. beckeri.
Discussion
A search among the type specimens and protologues of all the shrubby, non-sorediate and apotheciate species occurring in Africa and South America to find a previously published name was unsuccessful. Usnea hakonensis Asahina and U. beckeri are the only other species for which the unknown substances US1 and US2 are diagnostic (Ohmura Reference Ohmura2001). Morphologically and anatomically it does not resemble any other shrubby and apotheciate African or South American species. For differences from U. beckeri, U. nodulosa and U. submollis, see under these taxa. In our phylogenetic tree (Fig. 2), U. longiciliata belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a). It is monophyletic and sister clade to that formed by U. bismolliuscula, U. nodulosa and U. beckeri (Fig. 4).
Paratypes
Sāo Tomé and Príncipe: Island of Príncipe: trail from Santa Trindade to Pico Papagaio, 465 m, between roça and rope ladder, 2012, M. Nadel & J. Shevock MN0405 (CAS); ibid., 488 m, between roça and rope ladder, 2012, M. Nadel & J. Shevock MN0407 (CAS); trail from Santa Trindade to summit Pico Papagaio, 543 m, 2013, M. Nadel, J. Shevock, T. Daniel & O. Rocha MN0492 (CAS); slope between Pico Príncipe and Ribeira Banzu, 480 m, 2013, M. Nadel & O. Rocha MN0525 (CAS); trail from Terreiro Velho to Morro de Leste, 585 m, 2013, J. Shevock, T. Daniel, O. Rocha & M. Nadel MN0537 (CAS).
Usnea nodulosa Swinscow & Krog
Lichenologist 11, 232 (1979); type: Uganda, Masaka District, Bukoto County, Jubiya forest, elev. 1100 m, on twigs of exposed shrubs, 1971, Swinscow 3U 32/5 (holotype—BM!). Chemistry: usnic, constictic and barbatic acids. %CMA = 3/39/16; A/M = 0.4; A/C = 5.7; M/C = 13.9. Ascospores: 7.5–10 × 5–6 μm.
A detailed description is provided in Swinscow & Krog (Reference Swinscow and Krog1979, Reference Swinscow and Krog1988).
CMA
(Specimens collected in São Tomé only, n = 7). %C = (3.0 –)3.2–3.9–4.6(–5.0); %M = (33–)34.4–36.4–38.4(–39.5); %A = (14–)16.1–19.1–22; A/M = 0.4–0.5–0.6(–0.7); A/C = 4.0–5.0–6.0(–7.0); M/C = (6.8–)7.6–9.5–11.4(–11.8).
Ascospores
(Specimens collected in São Tomé only, n = 2). 7–9 × 5.5–7.0 μm.
Chemistry
(n = 8). Medulla, central axis, apothecial discs: K+ dark yellow, Pd+ red-orange. 1) Constictic and barbatic acids (type specimens, n = 3); 2) stictic, constictic, cryptostictic, menegazziaic and barbatic acids (São Tomé specimens, n = 5), new chemotype.
Diagnostic characters
The main diagnostic characters of U. nodulosa are the erect, bushy thallus with numerous terminal apothecia containing small ascospores, without soralia, the constricted lateral branches, the numerous fibrils and fibercles, the tenuicorticata-type of CMA with glossy cortex and lax medulla, and the presence of barbatic acid with either the stictic acid group (including constictic acid) or with constictic acid alone in the medulla.
Variability
The position and dimension of the apothecia are variable. Some thalli have a majority of terminal apothecia while in others, apothecia are mainly lateral. The density of fibrils/fibercles is also variable, as is the size of the thallus.
Distribution and ecology
Usnea nodulosa was so far known only from Uganda, Kenya and Tanzania (Swinscow & Krog Reference Swinscow and Krog1978, Reference Swinscow and Krog1988). In Eastern Africa, U. nodulosa is a corticolous species occurring mainly on twigs of exposed shrubs in hot, dry bushland at 1000 to 1500 m elev. The occurrence in São Tomé and Príncipe is an important extension of its distribution area to the western part of Africa. It was found in the primary Rubiaceae-dominated forest on both São Tomé and Príncipe islands. On São Tomé it was found at mid elevations between 862 and 1229 m and on Príncipe one collection was made at 598 m. It is found associated with U. pectinata. Usnea nodulosa is new for São Tomé and Príncipe.
Discussion
The tubercles (or nodules) of U. nodulosa with pseudocyphellae at their apices described by Swinscow & Krog (Reference Swinscow and Krog1978) are reinterpreted here as fibercles (scars left after fibrils are shed). In comparison, U. submollis has no or few fibercles, but numerous small verrucose true papillae, larger ascospores and a different chemistry (norstictic and salazinic acids). Usnea beckeri has a smooth cortex without papillae or tubercles and a different chemistry (stictic acid group, US1 and US2). Usnea longiciliata has a different chemistry (US1 and US2), non-constricted lateral branches and a thicker axis. Usnea albomaculata Motyka is a similar species with fewer fibercles, conspicuous medullary extrusions at the level of the axils and fibercles, and a different chemistry (psoromic acid). Usnea picta (J. Steiner) Motyka has lateral branches that are not constricted, another CMA-type (isotype (G): %CMA = 7/24.5/37; A/M = 1.5; A/C = 5.3; M/C = 3.5), a dense to compact medulla and a different chemistry (salazinic acid). Usnea nodulosa was sequenced here for the first time. In our phylogenetic tree (Fig. 2), it belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a) and is the sister species of U. beckeri (Fig. 4).
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, on road from Bom Successo to ‘Macambara’ Radio Station, 1162–1319 m, 2012, M. Nadel & J. Shevock MN0066 (CAS); secondary trail from Bom Successo to Lagoa Amelia, 1229 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0580 (CAS); unimproved road between Bemposta and Chamico near São Luís, 885 m, 2013, M. Nadel, J. Shevock, T. Daniel & Q. Quade MN0595 (CAS); unimproved road between São Luís and Chamico, 898 m, 2013, M. Nadel, J. Shevock, T. Daniel & Q. Quade MN0599 (CAS); unimproved road between Bemposta and Chamico, near Bemposta, 862 m, 2013, M. Nadel, J. Shevock, T. Daniel & Q. Quade MN0600b, MN0603 (CAS). Island of Príncipe: slope between Pico Príncipe and Ribeira Banzu, 598 m, 2013, M. Nadel & O. Rocha MN0521b (CAS).
Usnea pectinata aggr.
Incl.: Usnea pectinata Taylor, London J. Bot. 6, 191 (1847); type: Himalaya, Sylhet [Bangladesh], s. d., Wallich s. n. (isotype—M!). Chemistry: stictic, constictic, cryptostictic, menegazziaic and norstictic acids. %CMA = 4/7.5/77; A/M = 10.1; A/C = 20.3; M/C = 2.
Usnea gigas Motyka, Lich. Gen. Usnea Monogr., 388, 400 (1936–1938); type: Congo Belge, Banana, Pengle Irumu, forêt vierge de l'Ituri, épiphyte sur les branches dans la couronne des arbres, 24.xi.1914, Becquaert (isotype—S!). Chemistry: protocetraric acid (TLC by H. Krog in 1977). %CMA = 5.5/10/68; A/M = 6.7; A/C = 12; M/C = 1.8.
Usnea himantodes Stirt., Scott. Natural., N. S. 1(7), 75 (1883) [1883–1884]; type: Australia, New South Wales, Illawarra, 1882, Kirton s. n. (holotype—BM!). Chemistry: stictic, constictic, cryptostictic, menegazziaic and norstictic acids (tr.). %CMA = 8.5/13.5/56; A/M = 4.1; A/C = 6.6; M/C = 1.6.
Usnea mexicana Vain., Dansk Bot. Ark. 4, 3 (1926); type: Mexico, Paso de Doňa Juana, in arboribus, ii.1841, Liebman 7703 (lectotype—TUR-V!). Chemistry: diffractaic and constictic acids. %CMA = 5.5/6.5/76; A/M = 11.7; A/C = 13.8; M/C = 1.2.
A detailed description and images of the different morphologies and chemistry of the U. pectinata aggregate are given in Ohmura (Reference Ohmura2001), Swinscow & Krog (Reference Swinscow and Krog1978, Reference Swinscow and Krog1988; as U. gigas), Herrera-Campos et al. (Reference Herrera-Campos, Clerc and Nash1998; as U. mexicana), Stevens (Reference Stevens1999; as U. himantodes), Clerc (Reference Clerc, Nash, Gries and Bungartz2007; as U. mexicana) and Truong et al. (Reference Truong, Rodriguez and Clerc2013b; as U. mexicana).
CMA
(Specimens collected in São Tomé only, n = 10). %C = (3.5–)3.9–5.6–7.3(–8.0); %M = (8.0)10.1–14.5–18.9(–21.5); %A = (43–)49.2–59.8–70.4(–75); A/M = (2.0–)2.2–4.8–7.4(–9.5); A/C = (6.6–)7.1–11.8–16.5(–18.3); M/C = 1.6–2.8–4.0(–5.1).
Chemistry
(Specimens collected in São Tomé only, n = 11). 1) Medulla K−, Pd+ red-orange; protocetraric acid (n = 3). 2) Medulla K+ yellow turning dark red, Pd+ yellow; diffractaic, barbatic (tr.) and ±protocetraric (tr.) acids (n = 1). 3) Medulla K+ yellow turning dark red, Pd+ yellow; salazinic and constictic acids (n = 1). 4) Medulla K+ yellow, Pd+ orange; constictic acid (n = 1). 5) Medulla K+ dirty yellow, Pd+ orange; constictic, diffractaic and barbatic acids (tr.). (n = 1). 6) Medulla K+ dirty yellow, Pd+ orange; salazinic, protocetraric and fatty acids 5/3/4 (n = 1). 7) Medulla K+ dirty yellow, Pd+ orange; salazinic, diffractaic and ±protocetraric (tr.) acids (n = 3).
Diagnostic characters
The main diagnostic characters of the U. pectinata aggregate are the pendulous, stiff and brittle thallus with long fibrils in an almost fishbone-like arrangement (Fig. 10A), the irregular branches that are circular to almost alate in cross-section (Fig. 10C-H) (see Swinscow & Krog Reference Swinscow and Krog1978: fig. 7), the thick (< 50%) and often yellowish brown to dark brown pigmented axis (Fig. 10B) that is often somewhat fistulose (only in the thickest branches), and the frequent thick and whitish zones of cortex regeneration between the branch segments.

Fig. 10. Usnea pectinata. A, typical fish bone-like arrangement of fibrils and branches (MN0241). B, transversal section of a main branch, large and pigmented central axis (MN0583). C, main branches almost smooth, slightly ridged (MN0556). D, main branches with thin annular cracks (MN0527). E, main branches with thick annular cracks (MN0527). F, main branches partly decorticated (MN0163). G, main branches almost fully decorticated (MN0163). H, main branches ridged or acute-angled (MN0583). Scales: A = 1 cm; B = 0.5 cm; C–H = 1 mm. In colour online.
Variability
This aggregate displays a huge morphological and chemical variation (see the discussion in Swinscow & Krog (Reference Swinscow and Krog1978)).
Distribution and ecology
The Usnea pectinata aggr. is found on all continents with a tropical/subtropical climate. It grows on tree bark or commonly on rocks as, for example, in Japan (Ohmura Reference Ohmura2001). In Eastern Africa, it is a widespread species locally common on trunks and large branches of trees in open woodland and by waysides at 1000–2000 m elev. (Swinscow & Krog Reference Swinscow and Krog1988). It was found on both São Tomé (825–1863 m elev.) and Príncipe (443–596 m) in open primary forests of rubiaceous tropical hardwoods, as well as roadside in disturbed secondary forests. It was often found fallen from the canopy into leaf litter but also found growing on shrubs, tree branches and tree trunks. It was associated with U. articulata, U. baileyi, U. exasperata and U. nodulosa. This collection of the Usnea pectinata aggregate is noted as newly reported for São Tomé and Príncipe (Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020).
Discussion
As a consequence of the huge morphological and chemical variation, more than 20 species have been described in this aggregate. Figure 10C–H illustrates part of the important main branch morphological variation in this aggregate. In our phylogenetic tree (Fig. 2), Usnea pectinata s. str., corresponding to the stictic acid chemotype with an unpigmented central axis (AB051655 & AB051656), is nested inside a group of specimens with a pigmented central axis and various chemotypes (Fig. 5) corresponding to some of the other species described in the aggregate. A thorough and worldwide study using the tools of integrative taxonomy seems to be necessary to clarify whether there is one very variable species or many small well-defined taxa (Temu et al. Reference Temu, Clerc, Tibell, Tibuhwa and Tibell2019; Lücking et al. Reference Lücking, Nadel, Araujo and Gerlach2020). Usnea gigas is the sexually reproducing taxon of this aggregate.
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: road to São Nicolau, 825 m, 2012, M. Nadel & J. Shevock MN0060 (CAS); Parque Natural Obô de São Tomé, on road from Bom Successo to ‘Macambara’ Radio Station, 1162–1319 m, 2012, M. Nadel & J. Shevock MN0065, MN0068a (CAS); trail from Bom Successo to Pico Cálvario, 1192 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0163 (CAS); trail between Pico Cálvario and Pico Mesa, 1863 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0241 (CAS); primary trail from Bom Successo to Lagoa Amelia, 1381 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0556 (CAS); secondary trail from Bom Successo to Lagoa Amelia, 1191 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0578a (CAS); ibid., 1216 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0583 (CAS); road between Bemposta and Chamico, near Bemposta, 862 m, 2013, M. Nadel, J. Shevock, T. Daniel & Q. Quade MN0602 (CAS). Island of Príncipe: trail up to summit of Pico Papagaio from Santa Trinidade, 443 m, 2013, M. Nadel, J. Shevock, T. Daniel & O. Rocha MN0481 (CAS); trail from Terreiro Velho to Morro de Leste, 522 m, 2013, M. Nadel & O. Rocha MN0527 (CAS); ibid., 596 m, 2013, M. Nadel & O. Rocha MN0540 (CAS); ibid., 585 m, 2013, J. Shevock, T. Daniel, O. Rocha & M. Nadel MN0542 (CAS).
Usnea sorediosula Motyka
Lich. Gen. Usnea Monogr., 330 (1936–1938); type: Usambara [Tanzania], Waldsteppe am Kumba Sumpf, 1894, Holst 3526 (holotype—G0066557!). Chemistry: protocetraric acid, unknown substance (TLC by H. Krog in 1973). %CMA = 13/14/46; A/M = 3.3; A/C = 3.5; M/C = 1.0.
Nomenclatural note: Usnea barbata var. sorediosula (Müll. Arg.) Müll. Arg., Bot. Jb. 20, 245 (1894) is based on U. dasypogoides var. sorediosula Müll. Arg. Flora (Regensburg) 68, 499–500 (1885); type: Prope Andrangolovaka in Madagascaria centrali, Hidlebrandt (holotype—G66446!). Motyka wrote ‘Usnea sorediosula (Müll. Arg.) Mot., nova species. U. barbata v. sorediosula Müll. Arg. pro parte in herb. Typus in Mus. Botan. Univ. Geneva. Locus classicus: Usambara, silva stepposa ad paludem Kumba, 1883 C. Holst.’. Clearly he was basing his taxon U. sorediosula on a specimen in G identified as U. barbata var. sorediosula, but not the type species of this taxon, so he was describing a new species and not making a new combination. The type specimen of U. dasopogoides var. sorediosula Müll. Arg. does not have a red-orange subcortical pigment.
A detailed description and images of U. sorediosula are provided in Swinscow & Krog (Reference Swinscow and Krog1975, Reference Swinscow and Krog1988). However, what Swinscow & Krog (Reference Swinscow and Krog1975, Reference Swinscow and Krog1988) called pseudocyphellae are interpreted here as soralia.
CMA
(Specimen collected in São Tomé). %C = 9; %M = 19; %A = 43; A/M = 2.3; A/C = 4.7; M/C = 2.1.
Chemistry
(Specimen collected in São Tomé). Medulla K−, Pd+ orange-red; protocetraric acid.
Diagnostic characters
The main diagnostic characters for U. sorediosula are the shrubby sorediate thallus with a red-orange subcortical pigment, the concolorous basal part, the tapering to cylindrical branches, the lateral branches that are not constricted, the punctiform, strongly irregular soralia aggregating into consoralia, with numerous isidiomorphs, the moderately thick cortex (8–10%) and central axis (40–50%), and the presence of protocetraric acid in the medulla.
Variability
See under ‘Discussion’ below.
Distribution and ecology
Usnea sorediosula is known from tropical Africa (Motyka Reference Motyka1936–1938; Swinscow & Krog Reference Swinscow and Krog1975, Reference Swinscow and Krog1988). The report from the Philippines (Galinato et al. Reference Galinato, Baguinon and Santiago2018) is highly dubious and should be checked. In Eastern Africa, U. sorediosula is said to be widespread but uncommon on twigs of shrubs in open woodland and bush scrub at 1000–2000 m elevation. In São Tomé and Príncipe, it was found only on the island of São Tomé, fallen from the canopy into the leaf litter of primary Rubiaceae-dominated hardwood forests at 1230 m elevation.
Discussion
Usnea sorediosula differs from U. bicolorata, the other species in São Tomé with red-orange subcortical pigmentation and protocetraric acid in the medulla, by the concolorous basal part, the tapering or cylindrical main branches, the non-constricted lateral branches, the thicker cortex and central axis, and by the much lower M/C. Usnea sorediosula was sequenced here for the first time. In our phylogenetic tree (Fig. 2), it belongs to the clades 3 & 4 of Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a).
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, secondary trail from Bom Successo to Lagoa Amelia, 1230 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0579 (CAS).
Usnea submollis J. Steiner
Verh. zool.-bot. Ges. Wien 53, 229 (1903); type: [Kenya], British Ost-Africa, Machakos, 1896, Liechtenstein & Pospichill (holotype—W!). Chemistry: alectorialic acid (trace in apothecium) (TLC by Swinscow & Krog (Reference Swinscow and Krog1978)). %CMA = 3.5/39.5/14; A/M = 0.4; A/C = 4; M/C = 11.3. Ascospores: 11–14.5 × 5–9 μm.
A detailed description and images of U. submollis are given in Swinscow & Krog (Reference Swinscow and Krog1978, Reference Swinscow and Krog1988).
CMA
(Specimens collected in São Tomé only, n = 4). %C = 3.5–4.0–4.7(–5.0); %M = (35–)35.4–37.4–39; %A = (14–)14.1–17.3–20; A/M = 0.4–0.5–0.6; A/C = 3.2–4.3–5.5(–6.0); M/C = (7.0–)7.6–9.4–11.
Ascospores
(Specimens collected in São Tomé only, n = 3). 11–14 × 6.5–9 μm.
Chemistry
(Specimens collected in São Tomé only, n = 3). Medulla and apothecial disc K+ yellow turning red, Pd+ yellow turning orange-red; norstictic, salazinic, protocetraric (weak) acids.
Diagnostic characters
The main diagnostic characters of U. submollis are the erect, bushy thallus with numerous terminal or lateral apothecia containing large ascospores, without soralia, the constricted lateral branches, the numerous small, verrucose and true papillae, the tenuicorticata-type of CMA with glossy cortex and lax medulla, and the presence of norstictic and salazinic acids in the medulla.
Variability
The general aspect of the thallus is very variable, with small thalli that are more compact and large, almost subpendulous, thalli with large and distinct primary branches. Apothecia vary from lateral to terminal dispositions.
Distribution and ecology
Usnea submollis is known to occur only in tropical Africa (Motyka Reference Motyka1936–1938, Reference Motyka1956; Dodge Reference Dodge1957; Swinscow & Krog Reference Swinscow and Krog1978, Reference Swinscow and Krog1988). Motyka (Reference Motyka1936–1938) mentions a specimen collected on lava beds on Fernando Po. In Eastern Africa, this taxon is commonly found mainly on branches and twigs in montane forest and the ericaceous zone at elevations of 1500–3500 m. (Swinscow & Krog Reference Swinscow and Krog1978). In São Tomé and Príncipe, it was found only on the island of São Tomé, in the primary tropical Rubiaceae-dominated forest on small branches fallen into the leaf litter at elevations between 1298 m and 1354 m. It is associated with U. articulata, U. bicolorata and U. firmula. Usnea submollis is newly reported for São Tomé and Príncipe.
Discussion
Usnea submollis differs from all other species discussed here by the larger ascospores. Moreover, it differs from U. beckeri and U. longiciliata by its constricted lateral branches and different chemistry. Usnea submollis was sequenced here for the first time. In our phylogenetic tree (Fig. 2), both specimens form a monophyletic clade (although not strongly supported) nested inside the U. articulata-U. exasperata clade (Fig. 3). For differences with U. nodulosa, see under that species.
Specimens examined
Sāo Tomé and Príncipe: Island of São Tomé: Parque Natural Obô de São Tomé, trail from ‘Macambara’ leading into the forest, 1323 m, 2012, M. Nadel & J. Shevock MN0116 (CAS); trail from Bom Successo to Pico Cálvario, 1354 m, 2012, M. Nadel, J. Shevock & A. Stanbridge MN0191 (CAS); primary trail from Bom Successo to Lagoa Amelia, 1298 m, 2013, M. Nadel, J. Shevock, T. Daniel & E. Soares MN0552 (CAS), MN0553 (CAS).
Usnea sp. 1
This specimen (MN0523) is very similar to U. beckeri. However, its position in the phylogenetic tree (Fig. 4) casts some doubts on its identity. This is a very large specimen (thallus 40–50 cm long) that was collected on the ridge leading to Pico Príncipe, the highest ridge on the smaller island. This was the most difficult site to visit on either island due to logistical access issues, rugged terrain and heavy rainfall. It differs from U. beckeri by the much thicker branches, strong and thick annulations on primary branches and fistulose axis. It has a much thinner cortex (2%), a thinner medulla (15.5%), a much thicker axis (65%) and as a consequence higher A/C (29.3) and M/C (6.9). More specimens are needed to recognize this morphotype as a new species or as an extreme morphotype of U. beckeri.
Specimen examined
Sāo Tomé and Príncipe: Island of Príncipe: on slope between Ribeira Banzu and Pico Príncipe, 497 m, M. Nadel & O. Rocha MN0523 (CAS).
Usnea sp. 2
Through its shrubby to subpendant thallus (Fig. 11A), the inflated branches (Fig. 11B), the presence of perforations in the cortex (Fig. 11C), the thin and shiny cortex, and the large and lax medulla with conglutinated hyphae (Fig. 11E), this specimen (MN0526) is similar to U. bismolliuscula (for a description of this species see Ohmura (Reference Ohmura2001)). It differs, however, by the absence of the typical irregular and confluent soralia, the presence of the numerous papillae (young fibrils) and fibercles (Fig. 11D), and the presence of barbatic acid together with the stictic acid group. In the phylogenetic tree (Fig. 4) it appears to be nested inside the U. nodulosa-beckeri clade that is highly supported and also a sister clade of U. bismolliuscula s. str. Usnea bismolliuscula is so far the only known species with perforations in the cortex. However, we are reluctant to describe a new taxon based on only one specimen and will wait for more material to formally describe it.
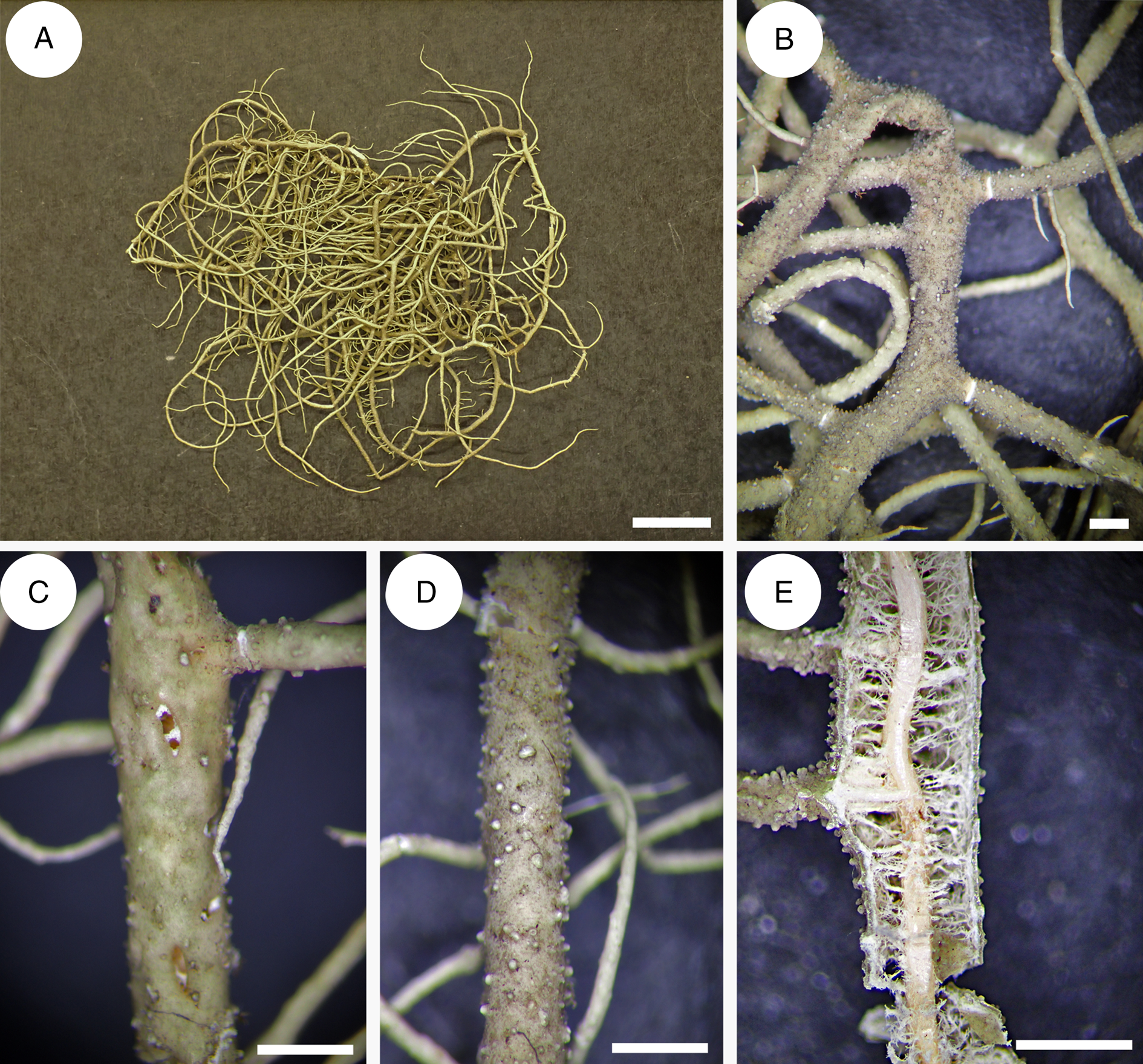
Fig. 11. Usnea sp. 2 (MN0526). A, thallus. B, irregular main branch with constricted lateral branches. C, perforations in the cortex. D, tubercles (young fibrils) and fibercles. E, transversal section of a main branch, medulla with conglutinated hyphae. Scales: A = 1 cm; B–E = 1 mm. In colour online.
Specimen examined
Sāo Tomé and Príncipe: Island of Príncipe: along Ribeira Banzu between Pico Príncipe and São Carlos do Fundão, 262 m, M. Nadel & O. Rocha MN0526 (CAS).
Acknowledgements
We warmly thank the curators of the BM, CAS, LBL, M, S, TUR-V and W herbaria for the loan of several collections of Usnea type specimens. Carlos Boluda and Yamama Naciri (G) are thanked for fruitful discussions, Yoshihito Ohmura (TNS) for confirming the cortex type of the new species, Bob Drewes and the California Academy of Sciences for past funding and the amazing opportunity to collect lichens in São Tomé and Príncipe, and Dennis Desjardin for providing academic guidance. Special thanks to Brian Perry and James Shevock for many hours of mentorship and assistance throughout this project.
Author Contribution
MN conceived the study, performed PCRs, DNA isolation and sequencing and designed Figs 1–5. PC was responsible for the taxonomic section, performed TLC and designed Figs 6–11. Both PC and MN wrote the manuscript.
Author ORCIDs
Miko Nadel, 0000-0001-8367-4139; Philippe Clerc, 0000-0003-1453-0865.
Competing Interests
The authors declare none.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282922000238.














