Current dietary recommendations encourage Americans to consume three or more 1 ounce-equivalents of whole grains per d(1) (three or more 30 g servings/d). These recommendations are largely based on epidemiological evidence for decreased chronic disease and decreased obesity with increased whole-grain consumption(Reference Slavin, Jacobs and Marquart2, Reference Slavin3). However, the nutritional value of whole grains is dependent on grain type, and other factors influence the nutritional quality of whole grains such as particle size, preparation and bioavailability of nutrients.
Grains are composed of three main parts: the bran layer, the endosperm, and the germ. Most milling processes reduce the amount of fibre in grains by removing the germ and bran. Bran, the outermost layer of grain, is composed of four components: the aleurone layer, seed cuticle, inner pericarp, and outer pericarp(Reference MacDougall, Selvendran, Cho and Dreher4). The aleurone layer in wheat is composed of one layer of cells covering the endosperm and the germ which contributes significantly to the dietary fibre content of wheat bran(Reference MacDougall, Selvendran, Cho and Dreher4). The aleurone layer also contributes significantly to the nutrient content of the grain with over 80 % of naturally occurring niacin, 60 % of the naturally occurring pyridoxine, and 60 % of the total minerals of wheat(Reference Pedersen, Knudsen and Eggum5). The endosperm is the main component of the grain, comprising 81–85 % of the grain by weight(Reference Amrein, Granicher and Arrigoni6). Wheat endosperm contributes the majority of the total dietary fibre in refined flours, but total fibre in refined wheat flour is relatively low (2·7 g fibre per 100 g flour)(Reference MacDougall, Selvendran, Cho and Dreher4, 7).
The aims of the present study were: (1) to identify how the particle size of wheat bran influences SCFA production and (2) to identify how the wheat bran fraction influences SCFA production in vitro. We hypothesised that smaller or finer particles will produce greater SCFA concentrations and that the aleurone layer will produce greater SCFA concentrations than whole-wheat bran or aleurone by-product.
Experimental methods
Two samples of whole-wheat bran (large-particle bran and small-particle bran), aleurone, and two samples of aleurone by-product (coarse by-product and fine by-product) were fermented in a batch in vitro fermentation system. Aleurone by-product is the remaining fraction after the aleurone layer is removed from the bran. All samples were provided by Cargill Inc. (Minneapolis, MN, USA). Table 1 shows the particle size and composition of each sample. Particle size was measured using a laser diffraction particle size analyser (LS12-320; Beckman Coulter Inc., Fullerton, CA, USA) with the Tornado dry powder system. Particle-size measurements were taken when the laser beam detected 6 % obscurity. Nutrient composition data were measured at Medallion Laboratories (Minneapolis, MN, USA). Total dietary fibre was measured by AOAC 991.43, protein by the Kjeldahl method, starch by AOAC 979.10, fat by hydrolysis and then gravimetrically, and moisture gravimetrically.
Table 1 Particle size and composition of wheat bran, aleurone and aleurone by-product
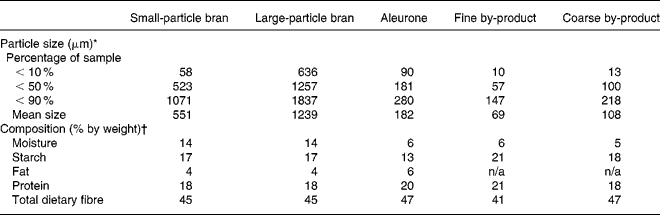
n/a, Not applicable.
* Particle size was determined using a laser diffraction particle size analyser (LS12-320; Beckman Coulter Inc., Fullerton, CA, USA) with the Tornado dry powder system. Particle size was measured at 6 % obscurity. The laser diffraction particle size analyser obtained particle-size measurements when 6 % of the light was attenuated due to light scattering or absorption by the bran particles.
† Composition data provided by Cargill Inc. (Minneapolis, MN, USA). The remaining percentage composition was analysed as ash.
Chemical reagents were obtained from Fisher Scientific (Pittsburgh, PA, USA), Sigma Aldrich (St Louis, MO, USA) and VWR Scientific (West Chester, PA, USA). Serum bottles (100 ml) were prepared for each sample, one for each of the five time points: 0, 4, 8, 12 and 24 h. Serum bottles were prepared with no sample as a negative control to quantify the SCFA produced by substrate originating from the faecal sample or fermentation media. Glucose was run as a positive control to confirm viability of the inoculum. Samples (0·5 g) were hydrated for 12 h in 40 ml sterile trypticase peptone media fortified with minerals at 4°C(Reference McBurney and Thompson8). At 2 h before inoculation with the faecal solution, sample bottles were warmed to 37°C.
Faecal samples from three healthy human subjects consuming a non-specified Western diet who had not taken antibiotics for the previous 6 months were pooled and diluted with PBS at a ratio of 1:6(Reference McBurney and Thompson8, Reference Velazquez, Davies and Marett9). The solution was homogenised in a blender. Reducing solution was added to the faecal inoculum to obtain a ratio of fifteen parts faecal inoculum to two parts reducing solution(Reference Goering, Van Soest and US Department of Agriculture10).
Faecal inoculum (10 ml) was added into each serum bottle along with 0·8 ml Oxyrase® oxygen-reducing enzyme (Oxyrase Inc., Mansfield, OH, USA). The bottles were immediately flushed with CO2 to generate anaerobic conditions. The bottles were gently shaken in a 37°C water-bath. One sample bottle for each fibre was removed at 0, 4, 8, 12 and 24 h. Immediately upon removal, 1 ml copper sulfate (200 g/l) was added to each bottle to cease fermentation. Two 2 ml samples were removed for SCFA analysis.
Samples were centrifuged and supernatant fractions were mixed with 25 % meta-phosphoric acid to precipitate protein(Reference Pylkas, Juneja and Slavin11). The samples were centrifuged and the pH of the supernatant fraction was adjusted to 6·5 with 4 m-KOH. Oxalic acid (0·1 ml; 0·3 m) was added to obtain a final concentration of 0·03 %. Samples were frozen at − 20°C until GC analysis. Acetate, propionate, butyrate and total SCFA were determined by GC-flame ionisation detection (Hewlett Packard model 6890; Hewlett Packard, Palo Alto, CA, USA)(Reference Raeth-Knight, Linn and Jung12). Total SCFA concentration was the sum of acetate, propionate, butyrate, isobutyrate, 2-methylbutyrate, isovalerate, lactate and valerate.
The experiment was a randomised complete block. One block consisting of pooled faecal samples from three humans was used. The treatments were arranged in a 5 × 5 factorial pattern with five fibres measured at five time points. Each fibre × time point combination was measured in duplicate. SCFA produced by each fibre were corrected for the SCFA concentration produced in the control (no added fibre) fermentation bottle. Molar percentages of SCFA were calculated by dividing the concentration of the respective SCFA by the sum of acetate, propionate and butyrate. Statistical analyses were completed with the SAS statistical software package (version 8.0; SAS Institute, Cary, NC, USA). ANOVA with Tukey pair-wise comparison was conducted to compare the mean SCFA concentrations. Statistical significance was achieved at P values less than 0·05.
Results
One sample for fine by-product at 12 h contained excessive particulate matter and was unsuitable for SCFA analysis. Statistical comparisons of SCFA at 12 h do not include fine-by product. Glucose (positive control) produced the highest SCFA concentrations at all time points (data not shown).
Total SCFA concentrations increased over 24 h for all samples (Fig. 1 (A)). From 0 to 12 h, total SCFA concentrations were similar among samples. Fine by-product produced the highest total SCFA concentrations at 24 h (P = 0·0019). Aleurone produced higher total SCFA concentrations than large-particle bran, but lower than fine by-product.
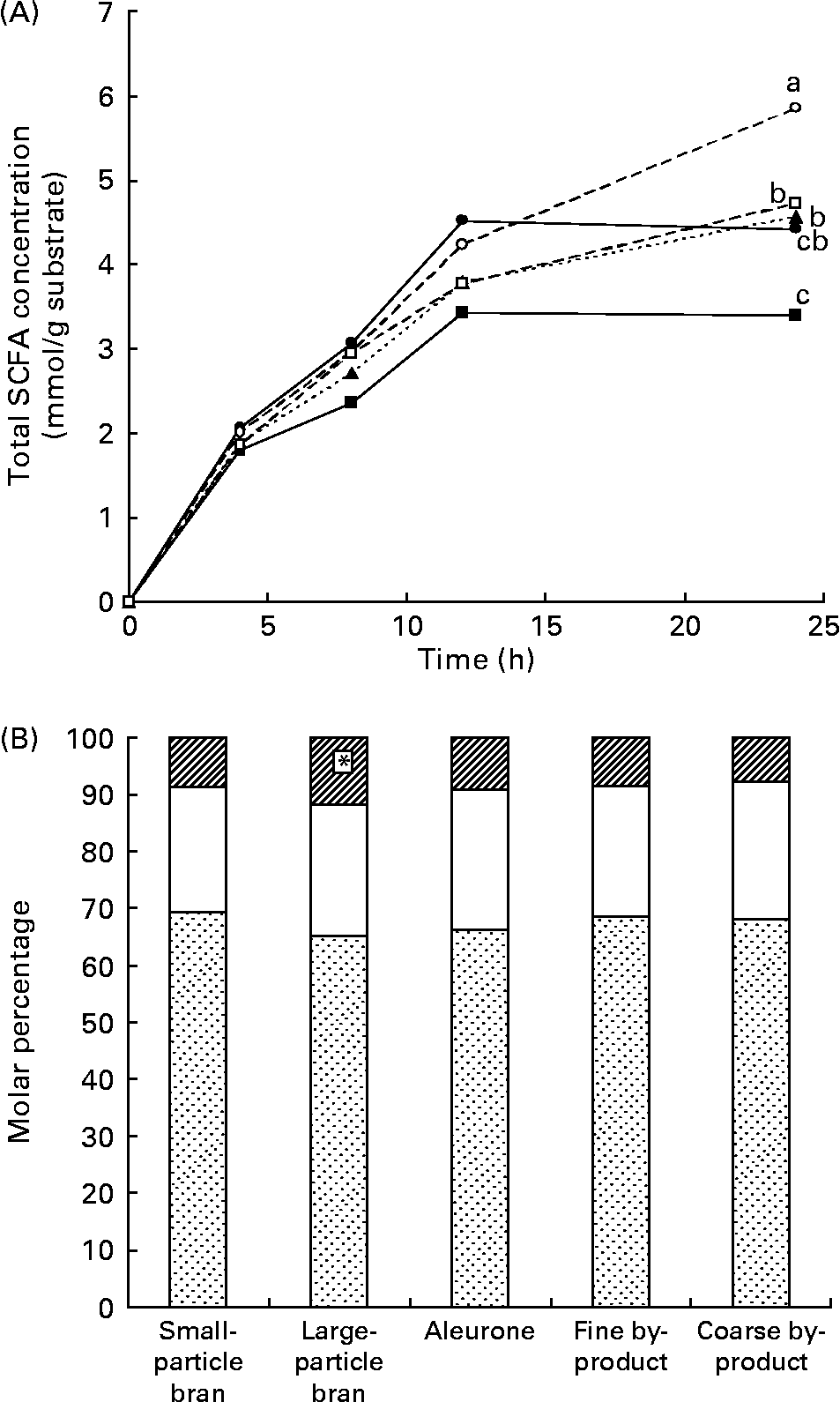
Fig. 1 (A) Total SCFA production during 24 h in vitro fermentation of small-particle bran (![]() ), large-particle bran (
), large-particle bran (![]() ), aleurone (
), aleurone (![]() ), fine aleurone by-product (
), fine aleurone by-product (![]() ) and coarse aleurone by-product (
) and coarse aleurone by-product (![]() ). Values are means. a,b,c Mean values with unlike letters were significantly different (P = 0·002). (B) Molar percentages of SCFA (acetate (
). Values are means. a,b,c Mean values with unlike letters were significantly different (P = 0·002). (B) Molar percentages of SCFA (acetate (![]() ), propionate (□) and butyrate (
), propionate (□) and butyrate (![]() )) at 24 h. * Butyrate molar proportion for large-particle bran was significantly greater than for the other samples (P = 0·003).
)) at 24 h. * Butyrate molar proportion for large-particle bran was significantly greater than for the other samples (P = 0·003).
Bran samples (both large-particle and small-particle) produced lower SCFA concentrations at 24 h than the by-product samples, but coarse by-product was not different from small-particle bran. Aleurone produced SCFA concentrations similar to small-particle bran at 24 h for all SCFA. Particle size affected SCFA concentration for the by-products but not the bran.
Molar percentages of acetate and propionate produced at 24 h did not differ among fibres. However, butyrate percentage was significantly greater for large-particle bran than the other fibres (P = 0·0026) (Fig. 1 (B)).
Discussion
The small-particle bran and fine by-product produced greater SCFA concentrations than their large-particle or coarse counterparts. These differences are probably due to the increased accessible surface area as particle size decreases. Bacterial enzymes have a larger contact area to access fermentable carbohydrates. The small-particle bran had a similar fermentability to aleurone for all measures at all time points. Particle sizes of the small-particle bran encompassed a wider range of sizes than aleurone and the mean particle size of small-bran particle was greater than that of aleurone. Based on the role of particle size in SCFA production, it was expected that aleurone would produce greater SCFA concentrations than small-particle bran. However, this was not true. Our data show that the bran fraction influences SCFA production as well as particle size.
Other in vitro studies reported slightly lower acetate (52–65 %), slightly lower propionate (14–21 %) and higher butyrate percentages when wheat bran is fermented (16–23 %) compared with the present study(Reference Pedersen, Knudsen and Eggum5, Reference McBurney and Thompson8, Reference McBurney and Thompson13–Reference Wood, Arrigoni and Miller17). Differences are probably due to varying microflora, as only certain bacteria are efficient butyrate producers. However, as shown in the present study, particle size of the brans may also influence the molar percentages of SCFA.
Breath H2 is a marker of fermentation in the proximal colon. In line with our findings, early fermentation of coarse (50 % >150 μm) and fine (50 % < 42 μm) whole-wheat flours did not differ as assessed by breath H2 in human subjects(Reference Hallfrisch and Behall18). Butyrate concentrations in human faecal samples increased significantly in the fine wheat bran treatment compared with medium wheat bran, which we confirmed with the by-products but not the bran samples (data not shown)(Reference Jenkins, Kendall and Vuksan19). Nutrient composition data were not published in Jenkins et al. (Reference Jenkins, Kendall and Vuksan19), so it is unclear what role composition played in fermentation. In the present study, the coarse by-product was 47 % total dietary fibre while the fine by-product was 41 % total dietary fibre. Differences in fibre content do not support the differences in butyrate production. Fine by-product contained a higher percentage of starch and protein than coarse by-product, which may have affected butyrate concentrations, particularly if the starch fraction contained resistant starch.
Aleurone showed average fermentability when compared with the other samples. However, the SCFA profile for aleurone in the present study differed from other published results. Amrein et al. reported molar ratios of 52, 21 and 21 for acetate, propionate and butyrate respectively(Reference Pedersen, Knudsen and Eggum5). Differences in molar ratios may be the result of in vitro aleurone digestion before fermentation. Aleurone contains digestible proteins and carbohydrates that may not normally reach the colon(Reference Pedersen, Knudsen and Eggum5). If undigested, these components may contribute to SCFA production. Additionally, bacteria in the fermentation inoculum probably differed among studies, which may influence the concentrations and proportions of SCFA produced.
Total dietary fibre and protein content of the samples were similar among the bran preparations, as shown in Table 1. However, starch content ranged from 13 to 21 %, which may have contributed to differences in fermentation. Fine by-product had the highest percentage of starch and also produced the greatest SCFA concentration at 24 h. Aleurone, which had the lower percentage of starch, did not exhibit the lowest fermentability of the fibres, indicating that starch was not a main contributor to fermentation.
Particle size influences the physiological effects of wheat bran. SCFA production and faecal moisture are increased at the expense of other physiological effects such as delayed gastric emptying, increased mean transit time and increased stool weight when particle size decreases(Reference Heller, Hackler and Rivers20–Reference Brodribb and Groves22).
Limitations of the present study include the small range of particle sizes and the limited number of bran and by-product samples. Further research should be conducted with whole bran, aleurone and aleurone by-product of identical particle sizes to more specifically determine the differences between bran fractions. Additionally, a greater range of particle sizes within each fraction should be examined to identify optimal particle sizes for specific physiological effects. In vitro digestion before fermentation may provide fermentation data that are more relevant to humans. The present study is the first to examine the fermentability of the fraction of bran remaining after the aleurone layer is removed (by-product). Aleurone is perceived to be the best bran fraction due to its high vitamin and mineral content. Although the by-product lacks some of the vitamins and minerals contained in the aleurone layer, it is fermentable and may still confer beneficial health effects. Particle size and fraction of wheat bran along with composition should be considered with choosing products for food manufacturing.
Acknowledgements
We would like to thank Mary Raeth-Knight (University of Minnesota) for her assistance with SCFA analysis and Keith Petrofsky (University of Minnesota) for his assistance with particle-size measurement.
Funding was provided by the University of Minnesota.
M. L. S. conducted the experiments, conducted data analysis and prepared the manuscript. J. L. S. designed the experiments and prepared the manuscript.
The authors declare no conflicts of interest.




