There is increasing evidence that several cognitive areas are impaired during the acute phases of bipolar illness and that this impairment persists even in the euthymic periods (Reference Van Gorp, Altshuler and Thebergevan Gorp et al, 1998; Reference Ferrier, Stanton and KellyFerrier et al, 1999; Reference Cavanagh, Van Beck and MuirCavanagh et al, 2002; Reference Clark, Iversen and GoodwinClark et al, 2002; Reference Altshuler, Ventura and van GorpAltshuler et al, 2004; Martinez-Aran et al, Reference Martinez-Aran, Vieta and Colom2004a ,Reference Martinez-Aran, Vieta and Reinares b ; Reference Thompson, Gallagher and HughesThompson et al, 2005). To date investigations on neurocognitive functioning have not distinguished between bipolar subtypes. The bipolar II population has not been assessed in this aspect, mainly because of the small number of patients with type II disorder included in these studies. Furthermore, in recently published studies only patients with bipolar I disorder were investigated (Reference Donaldson, Goldstein and LandauDonaldson et al, 2003; Reference Altshuler, Ventura and van GorpAltshuler et al, 2004; Reference Dixon, Kravariti and FrithDixon et al, 2004; Reference Balanza-Martinez, Tabares-Seisdedos and Selva-VeraBalanza-Martinez et al, 2005; Reference Deckersbach, Savage and DoughertyDeckersbach et al, 2005; Reference Fleck, Shear and StrakowskiFleck et al, 2005; Reference Kravariti, Dixon and FrithKravariti et al, 2005). Factors that have been reported to influence negatively cognitive functioning in bipolar disorder, with a negative impact on the performance of tasks on memory, attention and abstraction (Reference McKay, Tarbuck and ShapleskeMcKay et al, 1995; Reference Zubieta, Huguelet and O'NeilZubieta et al, 2001; Martinez-Aran et al, Reference Martinez-Aran, Vieta and Colom2004a ,Reference Martinez-Aran, Vieta and Reinares b ), are the number of episodes (especially manic episodes), the number of hospitalisations, the occurrence of psychotic symptoms and chronicity defined as duration of the illness. These factors have not, however, been specifically investigated in bipolar II disorder. Cognitive impairment, particularly memory difficulties, may also have negative implications in the functional outcome of patients with bipolar disorder (Martinez-Aran et al, Reference Martinez-Aran, Vieta and Colom2004a ,Reference Martinez-Aran, Vieta and Reinares b ; Reference Martinez-Aran, Vieta and Torrent2006). Between 30% and 50% of patients with bipolar disorder experience significant social disability that may be related to persistent cognitive impairment (Reference Zarate, Tohen and LandZarate et al, 2000; Reference Dickerson, Boronow and StallingsDickerson et al, 2004), but again these studies are not specifically focused on bipolar II disorder. Additionally, sub-syndromal features may have a negative impact in neuropsychological impairment and psychosocial functioning (Reference Cassano, Savino, Akiskal and CassanoCassano & Savino, 1997; Reference FavaFava, 1999; Reference BenazziBenazzi, 2001; Reference Clark, Iversen and GoodwinClark et al, 2002; Reference Martinez-Aran, Vieta and ColomMartinez-Aran et al, 2002).
The main aim of our study was to identify the cognitive performance in patients with bipolar II disorder in comparison with those with bipolar I disorder and a healthy control group. We predicted that the bipolar II group would exhibit an intermediate profile between the bipolar I group and the healthy controls with an emphasis on domains of verbal memory, attention and executive functions, which are the most common cognitive deficits in bipolar illness in general. A further hypothesis was that neuropsychological performance would also influence psychosocial functioning in patients with bipolar II disorder. As far as we know, this is the first study to evaluate specifically cognitive dysfunctions in bipolar II disorder, employing a rigorous definition of euthymia, with a design involving two control groups: one comprising patients with bipolar I disorder and the other healthy participants.
METHOD
Participants
Patients participating in this study were enrolled in the Bipolar Disorders Programme of the University Hospital Clinic of Barcelona. All patients met DSM-IV criteria for bipolar disorder type I or II (American Psychiatric Association, 1994) and were euthymic. The clinical state of the patients was determined by a psychiatrist responsible for the follow-up of patients in the Barcelona programme. The remission criteria were prospectively assessed euthymia during monthly visits over a 6-month period, with scores of 8 or less on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960; Reference Ramos-Brieva and Cordero-VillafafilaRamos-Brieva & Cordero-Villafafila, 1988) and 6 or less on the Young Mania Rating Scale (YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978; Reference Colom, Vieta and Martinez-AranColom et al, 2002). A neuropsychological test battery was administered to 33 patients with bipolar II disorder, who were compared with 38 patients with bipolar I disorder and 35 healthy individuals. All patients provided written informed consent. None of the patients had a concomitant medical illness or substance misuse. Ten patients had a history of rapid cycling (n=5 bipolar I, n=5 bipolar II). Patients with learning difficulties were excluded as well as patients who had received electroconvulsive therapy in the past year. The 35 healthy comparison participants with no psychiatric or neurological history were recruited through an advertisement and from a pool of healthy volunteers. All participants were screened for Axis I psychiatric disorders using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; Reference First, Spitzer and GibbonFirst et al, 1997) and it was ensured that none in the control group had a first-degree relative with bipolar disorder. The control group included students, workers, homemakers and hospital staff. Ethical approval for the study was granted by the ethics committee.
Clinical variables were collected as part of the Bipolar Disorders Programme protocol of the University Hospital Clinic of Barcelona. The clinical variables included in this study were number and type of episodes, duration of illness (chronicity); age at onset of illness; number of hospitalisations; suicide attempts; family history of affective disorders; history of psychotic symptoms; and diagnostic type I or II.
Psychosocial functioning was assessed using the Global Assessment of Functioning scale (GAF; American Psychiatric Association, 1994) as a measure of functional outcome. The original GAF instructions call for rating symptoms or functioning. As many other measures of mood symptoms were obtained as part of the evaluation, the rater was instructed to use the GAF to measure psychosocial functioning in the month prior to rating. Occupational adaptation, as an additional measure of functional outcome, was established as ‘good’ when patients were working at a good or acceptable level of functioning or ‘poor’ if they did not work at all or had poor occupational functioning during the 3 years prior to the evaluation. This information was provided by the patient and confirmed by a first-degree relative or a partner. The clinical interview, including psychosocial functioning, was conducted by a trained psychiatrist, and the neuropsychological evaluation was carried out by a trained neuropsychologist, masked to the results of the clinical and psychosocial assessments.
Neuropsychological measures
An extensive review of previous literature on this issue guided our choice of neuropsychological tests. To enhance replication, only tests frequently documented in the neuropsychological literature were used (Reference LezakLezak, 1995). Participants completed a comprehensive battery of tests spanning 4 broad cognitive domains. Tests were administered according to standard instructions and took about 90 min to complete. The tasks were given in the same order to the whole sample. The instruments administered for each domain are described elsewhere (Reference Martinez-Aran, Vieta and ColomMartinez-Aran et al, 2004a ):
-
(a) Estimated premormid IQ: Vocabulary sub-test from the Wechsler Adult Intelligence Scale (WAIS; Reference WechslerWechsler, 1955). Vocabulary has been identified as the single best measure of both verbal and general mental abilities.
-
(b) Frontal executive functions: the Wisconsin Card Sorting Test (WCST; Reference HeatonHeaton, 1981), the Stroop Colour-Word Interference test (SCWT) and the FAS task of the Controlled Oral Word Association Test (Reference Spreen and StraussSpreen & Strauss, 1998), including the animal-naming sub-tests.
-
(c) Attention/concentration and mental tracking: the DigitSpan sub-test from the WAIS and the Trail Making Test (TMT; Reference ReitanReitan, 1958).
-
(d) Verbal learning and memory: the California Verbal Learning Test (CVLT; Reference Delis, Kramer and KaplanDelis et al, 1987).
Statistical analyses
The three groups (bipolar I, bipolar II and healthy controls) were compared on clinical and socio-demographic variables using analysis of variance (ANOVA) and chi-squared tests. Multivariate analysis of variance was performed to show overall differences in neuropsychological tests between groups. Since multiple dependent variables were used, a prior protective analysis of covariance was performed with age as covariate and group as a main factor. The differences shown between the scores on the YMRS and HRSD, when controlled for, did not significantly alter the results, so these variables were not finally included as covariates. Since neuropsychological tests are naturally correlated, this procedure was considered better than Bonferroni inequality correction, which would have increased type II error. Group differences between the bipolar I, bipolar II and control samples were tested in one-way ANOVA, followed by Tukey post hoc comparison procedure when significant main effects were present. The effects sizes have been calculated to find the difference between the groups in terms of standard deviation. Pearson correlations were used to analyse which clinical and neurocognitive measures were related to psychosocial functioning, as measured by the GAF, taking into account variables that showed group differences (P0.1). In patients with bipolar II disorder, we used a multiple linear regression model to identify the variables that would be good predictors of psychosocial functioning. The clinical and neuropsychological variables that correlated with the GAF were introduced in the model using a hierarchical stepwise method: clinical variables were introduced in block 1 and neuropsychological variables in block 2. A logistical regression test was also performed to identify predictive variables of occupational adaptation, as defined above. The variables included in the analysis were the same as in the multiple linear regression model. Data analyses were performed using the Statistical Package for the Social Sciences, version 10.0 for Windows.
RESULTS
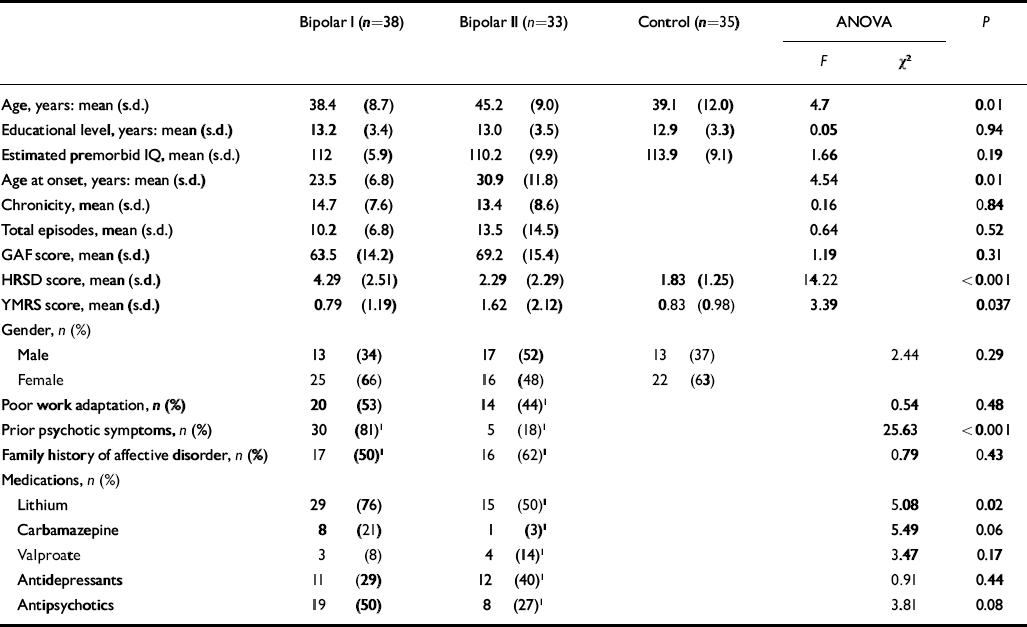
The three groups (bipolar I, bipolar II and healthy controls) did not differ with respect to gender, educational level, functional outcome and total number of episodes (Table 1). They differed on age and age at illness onset, which were lower in the bipolar I group. Patients with type I disorder more commonly had a history of psychotic symptoms and a greater percentage of them were taking lithium (Table 1). Owing to the small sample size there was insufficient statistical power to perform a subanalysis through the groups. For the subgroup of patients who were taking lithium, effect sizes were similar to those of the combined bipolar I and II groups, for example in measures of verbal memory such as recognition (0.45 v. 0.43), cued delayed recall (0.39 v. 0.33) or free short recall (0.32 v. 0.28).
Table 1 Demographic and clinical characteristics of the study sample
| Bipolar I (n=38) | Bipolar II (n=33) | Control (n=35) | ANOVA | P | |||
|---|---|---|---|---|---|---|---|
| F | χ2 | ||||||
| Age, years: mean (s.d.) | 38.4 (8.7) | 45.2 (9.0) | 39.1 (12.0) | 4.7 | 0.01 | ||
| Educational level, years: mean (s.d.) | 13.2 (3.4) | 13.0 (3.5) | 12.9 (3.3) | 0.05 | 0.94 | ||
| Estimated premorbid IQ, mean (s.d.) | 112 (5.9) | 110.2 (9.9) | 113.9 (9.1) | 1.66 | 0.19 | ||
| Age at onset, years: mean (s.d.) | 23.5 (6.8) | 30.9 (11.8) | 4.54 | 0.01 | |||
| Chronicity, mean (s.d.) | 14.7 (7.6) | 13.4 (8.6) | 0.16 | 0.84 | |||
| Total episodes, mean (s.d.) | 10.2 (6.8) | 13.5 (14.5) | 0.64 | 0.52 | |||
| GAF score, mean (s.d.) | 63.5 (14.2) | 69.2 (15.4) | 1.19 | 0.31 | |||
| HRSD score, mean (s.d.) | 4.29 (2.51) | 2.29 (2.29) | 1.83 (1.25) | 14.22 | <0.001 | ||
| YMRS score, mean (s.d.) | 0.79 (1.19) | 1.62 (2.12) | 0.83 (0.98) | 3.39 | 0.037 | ||
| Gender, n (%) | |||||||
| Male | 13 (34) | 17 (52) | 13 (37) | 2.44 | 0.29 | ||
| Female | 25 (66) | 16 (48) | 22 (63) | ||||
| Poor work adaptation, n (%) | 20 (53) | 14 (44) 1 | 0.54 | 0.48 | |||
| Prior psychotic symptoms, n (%) | 30 (81) 1 | 5 (18) 1 | 25.63 | <0.001 | |||
| Family history of affective disorder, n (%) | 17 (50) 1 | 16 (62) 1 | 0.79 | 0.43 | |||
| Medications, n (%) | |||||||
| Lithium | 29 (76) | 15 (50) 1 | 5.08 | 0.02 | |||
| Carbamazepine | 8 (21) | 1 (3) 1 | 5.49 | 0.06 | |||
| Valproate | 3 (8) | 4 (14) 1 | 3.47 | 0.17 | |||
| Antidepressants | 11 (29) | 12 (40) 1 | 0.91 | 0.44 | |||
| Antipsychotics | 19 (50) | 8 (27) 1 | 3.81 | 0.08 | |||
ANOVA, analysis of variance; GAF, Global Assessment of Functioning; HRSD, Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale
1. A few patients had missing data for this variable
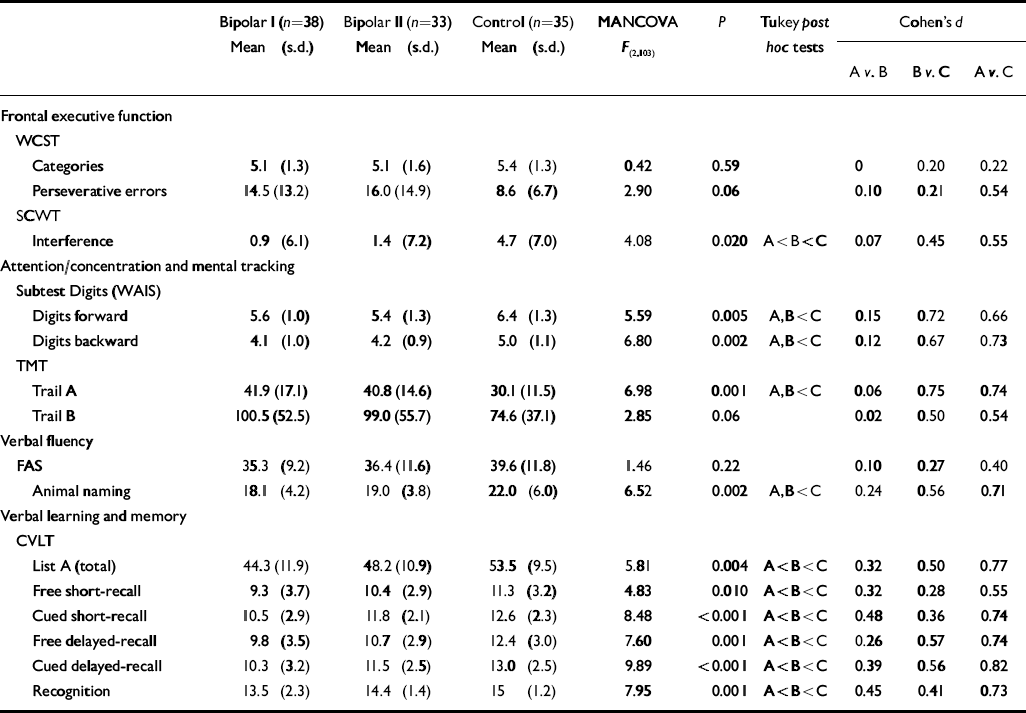
With regard to neuropsychological variables, results are shown in Table 2. Multivariate analysis of covariance yielded Pillai's F=1.952, d.f.=30, 170 (P=0.004) for the main effect, indicating that there were overall differences in neuropsychological performance between groups. For 12 of 15 comparisons the differences reached statistical significance (P<0.05). In general, patients with type II disorder performed poorly on most neuropsychological measures compared with healthy controls, especially on measures related to semantic verbal fluency (animal naming) and verbal learning and memory (CVLT learning task, cued short-delay and long-delay-recall and recognition hits). Both bipolar disorder groups performed worse than the control group on attention (TMT part A and Digit-Span Forwards) and working memory measures (DigitSpan Backwards). In another measure of working memory (TMT part B) only a trend towards a poorer performance was detected in patients compared with controls. Patients with type II disorder, as well as the bipolar I group, showed a trend towards a higher number of WCST perseverative errors compared with healthy controls (F=2.90. P=0.06). Tukey post hoc analysis showed that the bipolar I group performed worse on most measures than the bipolar II group, who in turn performed worse than the control group, so patients with bipolar II disorder showed an intermediate cognitive profile between patients with type I disorder and healthy participants.
Table 2 Performance on neuropsychological tests
| Bipolar I (n=38) Mean (s.d.) | Bipolar II (n=33) Mean (s.d.) | Control (n=35) Mean (s.d.) | MANCOVA F (2,103) | P | Tukey post hoc tests | Cohen's d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A v. B | B v. C | A v. C | |||||||||
| Frontal executive function | |||||||||||
| WCST | |||||||||||
| Categories | 5.1 (1.3) | 5.1 (1.6) | 5.4 (1.3) | 0.42 | 0.59 | 0 | 0.20 | 0.22 | |||
| Perseverative errors | 14.5 (13.2) | 16.0 (14.9) | 8.6 (6.7) | 2.90 | 0.06 | 0.10 | 0.21 | 0.54 | |||
| SCWT | |||||||||||
| Interference | 0.9 (6.1) | 1.4 (7.2) | 4.7 (7.0) | 4.08 | 0.020 | A<B<C | 0.07 | 0.45 | 0.55 | ||
| Attention/concentration and mental tracking | |||||||||||
| Subtest Digits (WAIS) | |||||||||||
| Digits forward | 5.6 (1.0) | 5.4 (1.3) | 6.4 (1.3) | 5.59 | 0.005 | A,B<C | 0.15 | 0.72 | 0.66 | ||
| Digits backward | 4.1 (1.0) | 4.2 (0.9) | 5.0 (1.1) | 6.80 | 0.002 | A,B<C | 0.12 | 0.67 | 0.73 | ||
| TMT | |||||||||||
| Trail A | 41.9 (17.1) | 40.8 (14.6) | 30.1 (11.5) | 6.98 | 0.001 | A,B<C | 0.06 | 0.75 | 0.74 | ||
| Trail B | 100.5 (52.5) | 99.0 (55.7) | 74.6 (37.1) | 2.85 | 0.06 | 0.02 | 0.50 | 0.54 | |||
| Verbal fluency | |||||||||||
| FAS | 35.3 (9.2) | 36.4 (11.6) | 39.6 (11.8) | 1.46 | 0.22 | 0.10 | 0.27 | 0.40 | |||
| Animal naming | 18.1 (4.2) | 19.0 (3.8) | 22.0 (6.0) | 6.52 | 0.002 | A,B<C | 0.24 | 0.56 | 0.71 | ||
| Verbal learning and memory | |||||||||||
| CVLT | |||||||||||
| List A (total) | 44.3 (11.9) | 48.2 (10.9) | 53.5 (9.5) | 5.81 | 0.004 | A<B<C | 0.32 | 0.50 | 0.77 | ||
| Free short-recall | 9.3 (3.7) | 10.4 (2.9) | 11.3 (3.2) | 4.83 | 0.010 | A<B<C | 0.32 | 0.28 | 0.55 | ||
| Cued short-recall | 10.5 (2.9) | 11.8 (2.1) | 12.6 (2.3) | 8.48 | <0.001 | A<B<C | 0.48 | 0.36 | 0.74 | ||
| Free delayed-recall | 9.8 (3.5) | 10.7 (2.9) | 12.4 (3.0) | 7.60 | 0.001 | A<B<C | 0.26 | 0.57 | 0.74 | ||
| Cued delayed-recall | 10.3 (3.2) | 11.5 (2.5) | 13.0 (2.5) | 9.89 | <0.001 | A<B<C | 0.39 | 0.56 | 0.82 | ||
| Recognition | 13.5 (2.3) | 14.4 (1.4) | 15 (1.2) | 7.95 | 0.001 | A<B<C | 0.45 | 0.41 | 0.73 | ||
CVLT, California Verbal Learning Test; MANCOVA, multivariate analysis of variance; SCWT, Stroop Colour–Word Interference Test; TMT, Trail Making Test; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test
The bipolar II group showed an intermediate level of performance, between the bipolar I and control groups, on the Stroop interference task and on all measures of verbal memory (CVLT). In this regard medium effect sizes were observed, as shown in Table 2 (Cohen's d values; Reference CohenCohen, 1988). Analysis of the effect sizes pointed to small differences between the patient groups, suggesting that cognitive deficits are present in both groups but these dysfunctions are quantitatively more marked in bipolar I disorder. Cognitive dysfunction was present in the bipolar II group relative to the control group but differences were medium in terms of effect size. Pearson correlations were also used in order to establish which clinical variables correlated with the neuropsychological measures in the patient groups. In the bipolar II group we found a correlation between psychosocial functioning as measured by the GAF and the age at illness onset (R=-0.42, P=0.026), the HRSD (R=-0.48, P=0.004) and the Trail Making Test part B (R=-0.45, P=0.009). Patients with longer illness duration showed more slowness or diminished attention (TMT part A), more working memory dysfunctions (DigitSpan Backwards sub-test) and more deficits in executive functions (animal naming, and higher perseverative errors from the WCST).
In the bipolar I group psychosocial functioning was related to some frontal executive functions such as the FAS (R=0.41, P=0.009), the DigitSpan Backwards sub-test (R=0.39, P=0.013) and the TMT part B (R=-0.36, P=0.025), as well as the learning (R=0.37, P=0.019), short-delay recall (R=0.35, P=0.027), free and cued long-delay recall (R=0.39, P=0.013); (R=0.37, P=0.021) and recognition (R=0.32; P=0.045) measures from the CVLT.
In the bipolar II group, after selecting all the variables that were correlated with the GAF, stepwise multiple linear regression analysis showed that the variables that best predicted psychosocial functioning, as measured through the GAF, were higher HRSD score, TMT part B score and the age at illness onset. This model accounted for nearly half (49.7%) of the variance (F=9.55, P<0.001). The TMT part B accounted for nearly 18% of the variance after controlling for the effect of the clinical variables (β=-0.41, t=-2.93, P=0.007). On the other hand, 14 of 33 patients showed poor occupational adaptation. Consistently with these results, logistical regression analysis also showed that higher TMT part B scores appear to be nearly significant as an indicator of poor occupational adaptation (Exp(B)=1.021, P=0.058).
DISCUSSION
To the best of our knowledge, none of the previous cognitive studies in bipolar disorder focused on neuropsychological dysfunction in type II disorder. Our study suggests that cognitive dysfunctions in bipolar disorder are not limited to the traditional bipolar I subtype. Our findings indicate that euthymic patients with type II disorder also show (although to a lesser degree) the persistent cognitive deficits seen in patients with a type I diagnosis. This was already anticipated as a clinical observation (Reference Vieta, Colom, Martinez-Aran, Maj, Akiskal and Lopez-IborVieta et al, 2002) and was confirmed with this study.
Cognitive performance in bipolar II disorder
Patients with bipolar II disorder had many verbal memory deficits compared with healthy controls. When compared with bipolar I patients, the bipolar I group showed quantitatively more dysfunctions than the bipolar II. This is consistent with a growing body of evidence that people with bipolar disorder experience impairment in verbal learning and memory which persists during the euthymic state (Reference Cavanagh, Van Beck and MuirCavanagh et al, 2002; Reference Glahn, Bearden and NiendamGlahn et al, 2004; Martinez-Aran et al, Reference Martinez-Aran, Vieta and Colom2004a ,Reference Martinez-Aran, Vieta and Reinares b ; Reference Balanza-Martinez, Tabares-Seisdedos and Selva-VeraBalanza-Martinez et al, 2005; Reference Kieseppa, Tuulio-Henriksson and HaukkaKieseppa et al, 2005). A longitudinal study would better address the differences in cognitive performance in hypomania and mania, but all studies so far have been cross-sectional.
Regarding executive functions, patients with type II disorder seem to make more perseverative errors in the Wisconsin Card Sorting Test. Perseverative errors may also be related to greater impulsivity, so this could be related to a higher comorbidity related to the impulsivity spectrum in type II disorder (Reference Goldberg, Harrow, Goldberg and HarrowGoldberg & Harrow, 1999; Reference Vieta, Colom and Martinez-AránVieta et al, 2000).
After controlling for age, the bipolar I and II groups had a worse performance than the control group on working memory measures (DigitSpan Backwards and TMT part B) and attention (TMT part A). Patients in the bipolar II group showed an intermediate level of performance between the bipolar I and control groups in verbal memory and executive functions (Stroop interference task). This suggests that working memory may be correlated with illness severity. However, bipolar II disorder has been reported to be not just a milder form of bipolar illness, but a particularly malignant subtype with regard to frequency of episodes (Reference Vieta, Gasto and OteroVieta et al, 1997). In fact, participants with bipolar II disorder in this study had on average three more episodes than those with bipolar I disorder, but differences did not reach statistical significance owing to the higher standard deviation of the bipolar II sample.
Role of clinical and social factors
A severe illness course probably has a negative impact on social and occupational functioning as well as on cognition. The correlations found between psychosocial outcome and verbal memory in the bipolar I group are consistent with other findings by our research group (Martinez-Aran et al, Reference Martinez-Aran, Vieta and Colom2004a ,Reference Martinez-Aran, Vieta and Reinares b ; Reference Martinez-Aran, Vieta and Torrent2006). Patients with type II disorder initially showing a better clinical profile than those with type I disorder may have a worse illness course because of the greater number of episodes, with significantly more major and minor depressive episodes and shorter inter-episode intervals (Reference Vieta, Gasto and OteroVieta et al, 1997; Reference Judd, Akiskal and SchettlerJudd et al, 2003). In bipolar II disorder, patients experience more severe and longer depressions than in bipolar I disorder (Reference Ayuso-Gutierrez and Ramos-BrievaAyuso-Gutierrez & Ramos-Brieva, 1982) and have more persistent residual depressive symptoms (Reference Cassano, Savino, Akiskal and CassanoCassano & Savino, 1997; Reference BenazziBenazzi, 2001). Partial remission as well as cognitive dysfunctions may lead to impaired psychosocial functioning in bipolar disorder. These subtle depressive symptoms might explain why patients with bipolar II disorder have more cognitive complaints and cognitive dysfunctions than healthy individuals even when the effect of subtle affective symptoms is controlled for. Rapid-cycling might carry higher risk of cognitive impairment, but as these patients were equally split between the two groups, there is a little chance that this factor could explain the differences between type I and II disorder in our study. Other possible factors involved when comparing executive function between the two types of bipolar disorder are prior psychotic symptoms and lithium treatment, which were both more frequent in participants with bipolar I disorder. However, looking at the effect sizes we cannot conclude that taking or not taking lithium would explain the differences in cognitive performance between the two groups (P=0.023). In one study (Reference Stip, Dufresne and LussierStip et al, 2000) it was observed that medium-term lithium administration did not impair explicit memory and attention in healthy participants.
Regarding psychotic symptoms, the important reduction of the effect size (approximately 50%) may mean that the higher prevalence of psychotic symptoms in bipolar I disorder would partially explain the differences in performance v. type II disorder. The presence of psychotic symptoms is a baseline diagnostic difference between the two diagnostic categories (Reference Vieta, Gasto and OteroVieta et al, 1997) and the specific effect of psychotic features on cognitive function in bipolar disorder has not been adequately examined. A recent study did not reveal any correlation between prior history of psychotic symptoms and cognitive impairment (Reference Selva, Salazar and Balanza-MartinezSelva et al, 2006). Frontal executive dysfunctions, specifically related to working memory impairment, may be related to a poorer psychosocial functioning in bipolar II disorder. Working memory dysfunctions have been found to be present in euthymic patients with bipolar disorder, even when residual depressive symptoms were covaried for (Reference Ferrier, Stanton and KellyFerrier et al, 1999). Therefore, executive dysfunctions are likely to constitute good predictors of social and occupational difficulties in patients with type II disorder, whereas problems in retaining and recovering information may be more relevant in type I disorder. These results suggest that perhaps different neurocognitive processes are involved in the psychosocial difficulties of the two bipolar subtypes. However, further research would be required to clarify our findings.
Limitations of the study
Our study was cross-sectional, whereas a longitudinal follow-up might provide more information about the progression of cognitive dysfunctions. It remains unclear whether cognitive dysfunction is a premorbid issue or actually progressive in the course of the illness. A larger sample size would have allowed more sophisticated analyses and might have shown clearer differences between the groups, for instance with respect to the executive functions. Another relevant issue is the baseline difference between patients and controls in terms of medication and history of psychotic symptoms. In the bipolar I group there was a significantly higher percentage of patients with a previous history of psychotic symptoms compared with the bipolar II group, so the potential impact of this variable on cognition deserves specific attention in further research.
Clinical implications
Persistent cognitive dysfunctions, including deficits in attention, executive function and verbal memory, exist in bipolar II disorder as in type I disorder, so cognitive functioning should be routinely examined in patients with either subtype. In patients with bipolar II disorder, working memory dysfunction seems to be a good predictor of functional impairment, after controlling for the effect of sub-syndromal symptoms. Rehabilitation interventions should take into account potential cognitive differences between the two subtypes, especially regarding their impact on functioning. An early diagnosis of type II disorder is important to prevent or remediate as much as possible the cognitive problems of these patients.
Acknowledgements
The study was supported by grants from the Fundacio Marató de TV3 (2510/01), the Instituto Carlos III FIS051542 and Stanley Medical Research Institute, Bethesda, Maryland, USA. The authors thank C. Corchero from the University Politècnica of Barcelona for statistical support.





eLetters
No eLetters have been published for this article.