Asthma is a T-cell-mediated allergic disease that causes sensitised individuals to develop eosinophilic airway inflammation and mucus hypersecretion in response to inhaled aeroallergens. T-helper cell type 2 (Th2) cells play a central role in the disease progression through their production of the cytokines, particularly IL-4, IL-5 and IL-13, which contribute to IgE production by B cells, growth and differentiation of eosinophils and mast cells and the development of bronchial hyperactivity and goblet cell hyperplasia(Reference Renauld1, Reference Fallon, Jolin and Smith2).
Over the past decades, the prevalence of asthma has dramatically increased and cost of care is more than $11·3 billion per year. According to the records published by the WHO, asthma affects about 300 million people, and 255 000 people died of asthma in 2005. The use of glucocorticosteroids is a major treatment for controlling symptoms of asthma. However, side effects of treatment, such as high blood pressure, osteoporosis, immune system changes, etc., are often inevitable.
Natural health products are generally used to support the treatment of many health problems. They often have fewer side effects and are used as reinforcement for regular medical treatments. Bovine whey protein extract (WPE), which is prepared from milk, is a source of bioactive proteins including lactoferrin, Ig, α-lactoglobulin, β-lactoglobulin and growth factors. It has been scientifically demonstrated to possess many health benefits, including antioxidant, antitumour, hypolipidaemic, anti-viral and anti-bacterial properties(Reference Krissansen3). Many studies have focused on the immune-boosting effect of WPE(Reference Krissansen3–Reference Rusu, Drouin and Pouliot5). However, Penttila et al. (Reference Penttila, Zhang and Bates6) demonstrated that under some circumstances, WPE can also act as an immunosuppressive agent.
The whey protein used in the present study was produced from a bovine whey protein, based on a patented process(Reference Juneau, Drouin and Moroni7), and is rich in the activated form of transforming growth factor-β (TGF-β), at concentrations of 5–50 μg/g powder. A previous study showed that this patented WPE inhibits the production of T-helper 1-related cytokines IFN-γ and IL-2(Reference Drouin, Lamiot and Cantin8). Recently, an open-label clinical trial suggested that WPE has the potential to reduce psoriasis severity(Reference Poulin, Bissonnette and Juneau9, Reference Drouin, Moroni and Cantin10). There were no clinically significant side effects that were associated with the use of 2·5 g of WPE twice daily for 112 d, suggesting that XP-828L may be a safe treatment(Reference Drouin, Lamiot and Cantin8). However, the detailed mechanism regarding this inhibitory effect and whether WPE also has the potential to inhibit Th2-mediated disease remains unclear. In the present study, using an experimental asthma model, we found that the patented WPE was able to alleviate symptoms of asthma, possibly through the induction of regulatory T cell (Treg) population in mice, which in turn inhibited immune responses.
Experimental methods
Animals
BALB/c mice were purchased from the National Animal Center. All of the mice were maintained and bred in an animal facility at the China Medical University. For all of the animal experiments, female mice (aged 8–12 weeks) were used and six to eight mice were assigned to each group. All of the animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the China Medical University.
Reagents
WPE was obtained from EnBio-Life, the Asia exclusive agent of Advitech Solutions. Mouse regulatory T cell staining kit, anti-GATA-3-PE (TWAJ), anti-CD3 (145.2C11) and anti-CD28 (37N.51) were purchased from eBioscience. EasySep CD4+T cell selection kit and EasySep B cell selection kit were purchased from STEMCELL Technologies, Inc. IL-4, IL-5, IL-13 and TGF-β Duo Set ELISA kit were purchased from R&D Systems. Alum (Imject Alum) was purchased from Pierce. Ovalbumin (OVA) and mitomycin C were purchased from Sigma-Aldrich. TGF-β-neutralising antibody (1D11) was purchased from Genzyme Corporation.
Experimental model of asthma and treatment of whey protein extract
The mice were sensitised intraperitoneally by injection of 50 μg of OVA that had been emulsified in 4 mg of aluminum hydroxide in a total volume of 200 μl on days 0, 14 and 28, and then challenged with 100 μg OVA in a total volume of 50 μl by intranasal administration for 3 consecutive days and analysed 1 d after they were challenged. Previous studies showed that psoriasis patients who receive orally a minimal dose of WPE of 8–10 mg/kg twice daily for 56 d have improved physician's global assessment score and do not exhibit side effects(Reference Drouin, Lamiot and Cantin8, Reference Poulin, Bissonnette and Juneau9). Based on the body surface area conversion of human to mice(Reference Reagan-Shaw, Nihal and Ahmad11), the dose conversion from human to mice would be 200–1200 mg/kg daily in mice. Therefore, for the treatment, 800 mg/kg (20 mg/mice) WPE in 250 μl of PBS was administered to mice by oral administration daily for 14 d (scheme shown in Fig. 1(a)).

Fig. 1 Airway inflammation was inhibited by whey protein extract (WPE) in a murine asthma model. (a) A brief scheme of animal sensitisation, treatment and challenge that were carried out in the present study. (b) Bronchoalveolar fluid (BALF) total cell number (left) and differential cell counts (right) that were assessed by Pappenheim staining. Values are means of the cell number, with their standard errors represented by vertical bars (n 6). □, Negative control (NC); ■, positive control (PC); ![]() , WPE treated. (c) Representative lung sections stained with haematoxylin and eosin (H&E; 100 × ) or periodic acid-Schiff (PAS; 200 × ) from negative control (left), positive control (middle) or the mice that were treated with WPE before they were challenged (right). The arrows indicate areas of peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). ** Mean values were significantly different compared with the positive control (P< 0·01). Statistical significance was determined using Student's t test. NC, mice that were sensitised and challenged with PBS; PC, mice that were sensitised and challenged with ovalbumin (OVA) without WPE treatment; and WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged. I.P., intraperitoneal; I.N., intranasal.
, WPE treated. (c) Representative lung sections stained with haematoxylin and eosin (H&E; 100 × ) or periodic acid-Schiff (PAS; 200 × ) from negative control (left), positive control (middle) or the mice that were treated with WPE before they were challenged (right). The arrows indicate areas of peribronchiolar cellular infiltrate (H&E) or positive mucus staining (PAS). ** Mean values were significantly different compared with the positive control (P< 0·01). Statistical significance was determined using Student's t test. NC, mice that were sensitised and challenged with PBS; PC, mice that were sensitised and challenged with ovalbumin (OVA) without WPE treatment; and WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged. I.P., intraperitoneal; I.N., intranasal.
Analysis of bronchoalveolar lavage
Mice were euthanised and cells in the bronchoalveolar lavage (BAL) were obtained, as previously described(Reference Cohn, Homer and MacLeod12). Briefly, the BAL fluid was prepared by washing the lungs three times with 1 ml PBS. The cells from individual mice were centrifuged, re-suspended and counted. Cytospin preparations were stained with Pappenheim staining. Differential cell counts were evaluated by counting at least 200 cells to determine the relative percentage of each cell type that was present in the BAL.
Lung histology
Paraffin-embedded lung sections were prepared as previously described(Reference Cohn, Homer and MacLeod12) and stained with haematoxylin and eosin or periodic acid-Schiff. Haematoxylin and eosin images and periodic acid-Schiff images were obtained at 100 × and 200 × total magnification, respectively.
Re-stimulation of mediastinal lymph node cells from challenged mice
After sensitisation and challenge, mediastinal LN cells were isolated. Single-cell suspensions were prepared and stimulated in vitro with 100 μg/ml OVA and syngeneic mitomycin C-treated B cells. The concentrations of cytokines in culture supernatants were measured after 48 h of culturing.
Isolation of CD4 T cells and B cells
CD4+T cells or B cells were isolated from the spleens of mice by negative selection using the EasySep CD4+T cell selection kit or EasySep B cell selection kit, respectively (STEMCELL Technologies, Inc.). All the preparation procedures were carried out following the manufacturer's instructions.
Serum antibody and transforming growth factor-β detection
Serum was obtained by cardiac puncturing for the measurement of OVA-specific IgE and TGF-β by ELISA.
Assay of airway hyperresponsiveness
The assays were performed as previously described(Reference Lee, Wang and Lai13). Briefly, airway resistance was assessed as the increase in pulmonary resistance after challenge with aerosolised methacholine in anaesthetised mice. Mice were anaesthetised with 90–120 mg/kg ketamine along with 5–10 mg/kg xylazine. They were then tracheotomised and mechanically ventilated at a rate of 150 breaths per min with a tidal volume of 0·3 ml and a positive end-expiratory pressure of 3–4 cm H2O using a computer-controlled small animal ventilator (flexiVent, SCIPEQ Scientific Respiratory Equipment Inc.). An intravenous catheter tube was inserted into the trachea of each mouse to the level of the thorax and then coupled to a pressure transducer. Air flow was measured by electronic differentiation of the volume signal. Changes in pressure, flow and volume were recorded. Pulmonary resistance was calculated using a software program (flexiVent version 5.2; SCIPEQ Inc.). Methacholine aerosol was generated with an in-line nebuliser and administered directly through the ventilator.
Gene expression analysis by quantitative PCR
RNA was extracted using TRIzol® Reagent (Invitrogen), and reverse transcription were carried out using oligo(dT) (deoxythymidylic acid) as a primer and MMTV Reverse Transcriptase (Invitrogen), according to the manufacturer's protocol (Invitrogen). Quantitative PCR assays were performed using 20 μl of the reaction mixture that contained 8–10 ng of complementary DNA, SYBR Green, deoxyribonucleotide triphosphates, primers and Taq polymerase (ABI). The expression levels were normalised against hypoxanthine-guanine phosphoribosyltransferase.
Flow cytometry analysis
Cells were re-suspended in PBS/5 % fetal calf serum containing 0·01 % sodium azide and incubated with anti-Fc receptor (2·4G2) for 20 min on ice. The cells were then stained with the indicated antibodies for 30 min on ice. Intracellular Foxp3 and GATA-3 staining were conducted according to the manufacturer's protocol in the mouse regulatory T cell staining kit (eBioscience). FACSCalibur analyser was used, and the data were analysed with WinMDI (The Scripps Institute).
Statistical analysis
The results were compared using Student's t test by using the software program GraphPad Prism 5 (GraphPad Software Inc.). The data are presented as means with their standard errors. Probability values (P) of less than 0·05 were considered statistically significant.
Results
Whey protein extract reduces airway inflammation, hyperresponsiveness and T-helper cell type 2 cytokine production in a murine asthma model
In order to test whether WPE has the potential to inhibit Th2-mediated disease such as asthma, a murine asthma model was used. The mice were sensitised and challenged to OVA. For the treatment, mice were orally administered 800 mg/kg (20 mg/mice) of WPE daily for 14 consecutive days before they were challenged. During the period of treatment, the mice showed no signs of illness and weight loss.
To investigate the effect of WPE on airway inflammation, differential cell counts in BAL fluid were analysed. In PBS-sensitised and challenged mice (negative control), no obvious increase in inflammatory cells in BAL fluid was observed; mice sensitised and challenged with OVA (positive control) showed a typical Th2-type, eosinophilic BAL cell differential. After treatment with WPE for 2 weeks before the challenge, less eosinophil infiltration was observed in the lungs (Fig. 1(b)). Histological examinations of the fixed lung tissue that was prepared from these mice revealed that peribronchiolar inflammation (indicated by haematoxylin and eosin staining) and mucus production (indicated by positive periodic acid-Schiff staining) were present in the positive control. In contrast, the extent of cell infiltration and mucus secretion was less in the WPE-treated mice than in the positive control (Fig. 1(c)). Collectively, the results indicate that WPE treatment could inhibit airway inflammation.
We subsequently examined whether WPE treatment could suppress the development of airway hyperresponsiveness. As shown in Fig. 2, WPE-treated mice showed significant decreases in pulmonary resistance compared to the positive control.

Fig. 2 Airway hyperresponsiveness was inhibited by whey protein extract (WPE, ![]() ). Airway resistance was measured by invasive body plethysmography. Values are means of the pulmonary resistance (R L), with their standard errors represented by vertical bars (n 4). Mean values were significantly different compared with the control: ** P< 0·01, *** P< 0·001. Statistical significance was determined using Student's t test. Negative control (
). Airway resistance was measured by invasive body plethysmography. Values are means of the pulmonary resistance (R L), with their standard errors represented by vertical bars (n 4). Mean values were significantly different compared with the control: ** P< 0·01, *** P< 0·001. Statistical significance was determined using Student's t test. Negative control (![]() ), mice that were sensitised and challenged with PBS; positive control (
), mice that were sensitised and challenged with PBS; positive control (![]() ), mice that were sensitised and challenged with ovalbumin without WPE treatment; and WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged.
), mice that were sensitised and challenged with ovalbumin without WPE treatment; and WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged.
Elevated levels of IgE in the patients of asthma are also an indicator of allergy. Therefore, we examined whether WPE treatment can reduce the level of OVA-specific IgE in serum. We found that OVA-specific IgE levels were significantly reduced after administration of WPE (Fig. 3(a)).

Fig. 3 Serum ovalbumin (OVA)-specific IgE and cytokine production by T-helper cell type 2 cells was reduced in whey protein extract (WPE)-treated mice. (a) OVA-specific IgE in the serum was measured by ELISA. Values are means of the optical density (OD), with their standard errors represented by vertical bars (n 6). (b) Cytokine production by mediastinal LN cells that were isolated from mice, as indicated after re-stimulation with antigen-presenting cells, and OVA were analysed by ELISA. In each experiment, mediastinal lymph node cells were pooled from six mice per group for culturing. Mean values were significantly different compared with the positive control (PC): * P< 0·05, ** P< 0·01. Statistical significance was determined using Student's t test. NC, negative control; ND, non-detectable.
To further establish whether Th2 functions were inhibited by WPE treatment, we evaluated the ability of mediastinal lymph node cells to produce Th2-type cytokines after they were challenged. Upon re-stimulation with OVA-pulsed B cells in vitro, mediastinal lymph node cells that were isolated from mice that had been sensitised and challenged with OVA produced Th2 cytokines such as IL-4, IL-5 and IL-13. However, the production of IL-4, IL-5 and IL-13 by the re-stimulated mediastinal lymph node cells from the WPE-treated mice was decreased (Fig. 3(b)).
Whey protein extract can induce the generation of regulatory T cells and reduce the CD4+GATA-3+ cell population in vivo
The WPE that we used was prepared by acid precipitation according to a patented process(Reference Juneau, Drouin and Moroni7), and is rich in TGF-β (5–50 μg/g powder). TGF-β is a potent regulatory cytokine and has the ability to inhibit the development and progression of various immunopathological diseases(Reference Li, Wan and Sanjabi14). We therefore examined whether TGF-β levels could be elevated after oral administration of WPE. Indeed, elevated TGF-β levels in the serum of the WPE-fed mice were observed (Fig. 4). TGF-β was found to be able to induce Foxp3 expression and the generation of Treg(Reference Li, Sanjabi and Flavell15, Reference Zhou, Lopes and Chong16), which in turn block immune cell function. To explore the mechanisms by which WPE elicits its inhibitory effects, we first examined whether WPE can induce the generation of Treg. As shown in Fig. 5(a), an increased proportion of Treg cells was observed in the blood and lungs from the mice that had been fed with WPE for 14 d before they were challenged. Treg were found to be able to inhibit T-cell proliferation and the expression of GATA-3, which is an important transcription factor that regulates the expression of the Th2 cytokines IL-4 and IL-5(Reference Lee, Takemoto and Kurata17–Reference Jager and Kuchroo19). Therefore, we next examined whether the Th2 cell (identified as being CD4+GATA-3+) population and/or GATA-3 expression were inhibited in the WPE-fed mice. We found that the Th2 cell population was increased in OVA-sensitised and challenged mice; however, it was reduced when the mice were fed with WPE before they were challenged (Fig. 5(b)). Interestingly, the expression levels of GATA-3 in the Th2 cells were similar among the groups (Fig. 5(b)), indicating that the decrease in Th2-related cytokine production observed in the WPE-fed mice was due to the decrease in Th2 cell numbers and not due to the decreased GATA-3 expression levels in the Th2 cells.
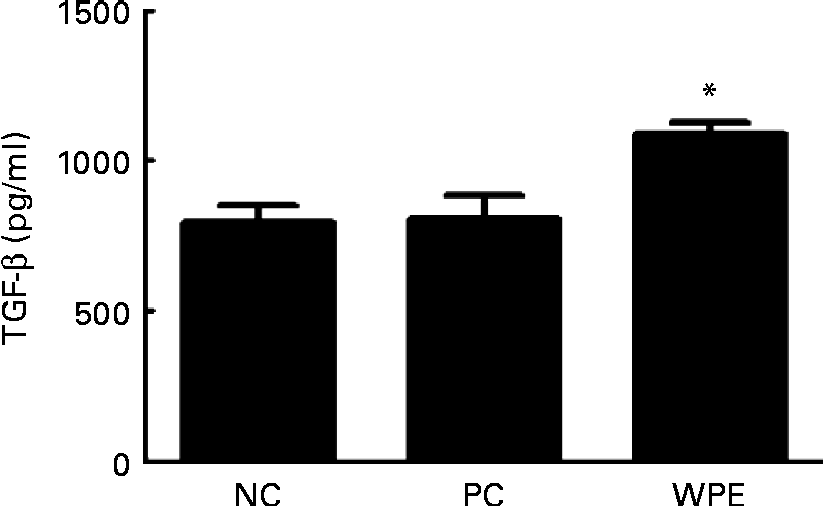
Fig. 4 Concentrations of transforming growth factor-β (TGF-β) in the blood. The concentrations of TGF-β in the serum samples were measured by ELISA. Values are means of the concentrations, with their standard errors represented by vertical bars (n 6). * Mean value was significantly different compared with the positive control (P< 0·05). Statistical significance was determined using Student's t test. Negative control (NC), mice that were sensitised and challenged with PBS; positive control (PC), mice that were sensitised and challenged with ovalbumin without whey protein extract (WPE) treatment; WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged.

Fig. 5 Generation of regulatory T cells (Treg) and expression levels of GATA-3 in vivo. (a) Treg populations in the blood (left) and lungs (right) were identified as CD4+Foxp3+ cells using flow cytometry analysis. Values are means of the Treg proportion in the blood, with their standard errors represented by vertical bars (n 5). (b) CD4+GATA-3+ cell population (left) and the mean fluorescence intensity (MFI) of GATA-3 expression in the CD4+GATA-3+ cells in the lungs were analysed using flow cytometry analysis. Values are means of the CD4+GATA-3+ cell proportion (left) or MFI (right), with their standard errors represented by vertical bars (n 4). Mean values were significantly different compared with the positive control (PC): * P< 0·05, ** P< 0·01. Statistical significance was determined using Student's t test. Negative control (NC), mice that were sensitised and challenged with PBS; positive control (PC), mice that were sensitised and challenged with ovalbumin without whey protein extract (WPE) treatment; WPE, the mice that were administered with WPE orally for 2 weeks before they were challenged.
Transforming growth factor-β is the active component in whey protein extract that induces the generation of regulatory T cells
Finally, we aimed to investigate whether TGF-β is a major component in WPE that can induce the generation of Treg. We observed that WPE induced the generation of Treg when CD4 T cells were cultured in the presence of WPE (Fig. 6). However, this induction could be completely abolished when a TGF-β-blocking antibody (1D11) was used to neutralise the activity of TGF-β (Fig. 6). This finding indicates that without TGF-β, WPE lost its ability to induce the generation of Treg.

Fig. 6 Removal of transforming growth factor-β (TGF-β) could abolish the effect of whey protein extract (WPE) on regulatory T cell (Treg) differentiation. (a) Naive CD4+T cells were stimulated with anti-CD3 and anti-CD28 in the presence of indicated concentrations of WPE for 3 d. Treg were identified as CD4+Foxp3+ cells using flow cytometry analysis. Values are means of the fold increase in Treg population compared with the cells without WPE treatment from four independent experiments, with their standard errors represented by vertical bars. (b) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence or absence of 250 μg/ml WPE with the indicated concentrations of anti-TGF-β antibodies for 3 d. Treg were identified as CD4+Foxp3+ cells using flow cytometry analysis. Values are means of the Treg population in the culture, with their standard errors represented by vertical bars (n 6). Mean values were significantly different compared with the one with WPE treatment, but without the presence of anti-TGF-β antibodies: * P< 0·05, ** P< 0·01, *** P< 0·001. Statistical significance was determined using Student's t test.
Discussion
Whether consumption of farm milk can prevent allergic diseases has always been controversial(Reference Braun-Fahrlander and von Mutius20). Recently, the results of a large epidemiological study (GABRIELA study) revealed that the consumption of unheated farm milk is inversely associated with childhood asthma and allergies(Reference Loss, Apprich and Waser21). Elevated levels of TGF-β were found in unpasteurised cow milk(Reference Peroni, Piacentini and Bodini22). Further, milk TGF-β has been shown to help reduce the development of allergies in early childhood(Reference Oddy and Rosales23). All of the available evidence implicates the important role played by TGF-β in the prevention of allergic diseases in early life. Therefore, during manufacturing, enriching bioactive TGF-β may be useful for allergy prevention. In the present study, we showed that the patented WPE, which is enriched in TGF-β, has the potential to alleviate the symptoms of asthma, including airway inflammation, serum IgE level, production of Th2-related cytokines (IL-4, IL-5 and IL-13) and airway hyperresponsiveness, which might be due to the induction of Treg in mice after ingestion of WPE.
Treg has been shown to maintain immune homoeostasis by suppressing T-cell proliferation and the functional maturation of dendritic cells(Reference Serra, Amrani and Yamanouchi24). It plays a central role in the prevention of extensive immune-mediated damage and autoimmune disease. Several Treg subsets have been described: one is the cells that originate from the thymus and called naturally occurring Treg(Reference Sakaguchi, Sakaguchi and Asano25). Another is the subset that is induced in the periphery and thus called inducible Treg, which can be further divided into IL-10-secreting Treg and TGF-β-secreting Treg. The subset that is induced by WPE could be TGF-β-secreting Treg. The suppressive effect mediated by Treg is known to be not antigen specific(Reference Thornton and Shevach26) and involves cell–cell interaction or the inhibitory effects of TGF-β that is secreted by Treg(Reference Corthay18).
Treg from atopic individuals are defective in suppressing Th2 cell function compared with those from non-atopic individuals. Numerous experiments, carried out by either depleting Treg or adoptive transfer of antigen-specific regulatory cells, revealed that Treg can suppress allergic inflammation and hyperresponsiveness(Reference Umetsu, Akbari and Dekruyff27–Reference Lloyd and Hawrylowicz29). Interestingly, the present data show that WPE can induce the generation of Treg. WPE contains many bioactive proteins, including lactoferrin, Ig, α-lactoglobulin, β-lactoglobulin and growth factors that have been demonstrated to regulate immune functions(Reference Krissansen3). However, none of them has been shown to be able to induce the generation of Treg, except TGF-β that is enriched in this patented WPE(Reference Zhou, Lopes and Chong16, Reference Bettelli, Carrier and Gao30). Research has demonstrated that orally administered TGF-β can still retain and exert its biological activity in the intestinal mucosa and can affect immune responses locally or systemically(Reference Ando, Hatsushika and Wako31). Furthermore, the latent from of TGF-β, such as the TGF-β in whey-derived natural products, can be activated by gastric acid(Reference Nakamura, Miyata and Ando32). Indeed, increased concentrations of TGF-β in the serum from the WPE-fed mice were observed in the present study. We therefore hypothesise that the inhibitory effect that was elicited by WPE might have been due to the generation of Treg that were induced by the TGF-β enriched in the WPE.
TGF-β, produced by Treg, has diverse effects on various types of immune cells other that T cells, including dendritic cells, mast cells and granulocytes(Reference Li, Wan and Sanjabi14). Dendritic cells play a key role in linking innate and adaptive immune responses. Impaired dendritic cell functional maturation hampers the differentiation of Th2 cells. Evidence has shown that TGF-β negatively regulates dendritic cell maturation and its ability to present antigen to T cells(Reference Geissmann, Revy and Regnault33, Reference Dong, Tang and Letterio34). The mediators that are released by activated mast cells and eosinophils aggravate allergic inflammation. Cell activation depends on cross-linking of IgE to FcɛRI. Previous data have shown that TGF-β can inhibit the expression of FcɛRI(Reference Gomez, Ramirez and Rivera35). Taking the evidence together, the inhibitory effect of WPE during the disease progression of asthma might not be simply due to the inhibition of T cell function. The influence of WPE on other cells would be interesting to study.
Although we speculate that TGF-β serves as a key component that renders this patented WPE immunosuppressive, we cannot exclude the possibility that components other than TGF-β might also contribute to the alleviation of symptoms of asthma. For instance, whey protein is a source of cysteine-rich protein and may facilitate the synthesis of glutathione (GSH), which is a potent antioxidant found in the airways. It has been shown that GSH levels decrease in the lungs during the early stage of asthmatic reaction(Reference Kloek, Mortaz and van Ark36). GSH has also been shown to decrease allergen-induced contractions by relaxing airway smooth muscle and preventing histamine- and allergen-induced airway contractions(Reference Kloek, Mortaz and van Ark36, Reference Kloek, van Ark and Bloksma37). A whey-based GSH-enhancing diet has also been shown to decrease allergen-induced airway contractions in a guinea pig model of asthma(Reference Kloek, Mortaz and Van Ark38). Therefore, the reduced airway hyperresponsiveness that we observed in the WPE-fed mice might be due to the increased GSH levels that reduced the oxidative stress in the airway. Lactoferrin, which is an Fe-binding protein and has the ability to inhibit the potential causative agent of asthma, tryptase, was demonstrated to be able to abolish bronchoconstriction and airway hyperresponsiveness in an allergic sheep model(Reference Elrod, Moore and Abraham39). Furthermore, lactoferrin was shown to inhibit the migration of eosinophils(Reference Bournazou, Mackenzie and Duffin40). Currently, a phase II clinical trial using orally administered recombinant human lactoferrin against asthma is underway. However, considering that the proportion of lactoferrin in this patent WPE is only 0–2 %, lactoferrin might not be the major component that alleviated symptoms of asthma in the present study.
Much research has focused on identifying nutrients with value more than nutrition itself, but also with an ability to modulate the immune system. Undoubtedly, milk is one of the important interests. The present data further demonstrate that WPE can act as an immune modulator and that it might be potentially beneficial for patients with not only T-helper 1-mediated, but also Th2-mediated immunopathological diseases.
Acknowledgements
The present project was supported by the China Medical University (CMU97-227 and CMU99-N1-20), the Teh-Tzer Study Group for Human Medical Research Foundation (B991043) and EnBio-Life, the Asia exclusive agent of Advitech Solutions, Canada. J.-H. C. and P.-H. H. contributed equally to the present paper. H.-C. C. and J.-H. C. designed the research protocol; P.-H. H. conducted the research; H.-C. C., J.-H. C. and P.-H. H. analysed the data; P.-H. H. prepared the figures and tables; H.-C. C. wrote the paper; C.-C. L. provided technical support and reagents that were used for airway hyperresponsiveness experiments. P.-Y. C. was involved in revising the paper with respect to important intellectual content. All of the authors read and approved the final manuscript and declare no conflict of interest.








