Depression is a common disorder with a lifetime prevalence of 14–16%. As a result of its major impact on daily functioning and quality of life, depression is currently the third leading contributor to the global burden of disease. 1 Furthermore, patients with depression may have an increased risk of medical diseases such as coronary heart disease, diabetes, dementia, cancer and all-cause mortality. Reference Uzun, Kozumplik, Topic and Jakovljevic2 This has led to the hypothesis that depression (through various pathways leading to cellular damage) is associated with accelerated biological ageing. Reference Wolkowitz, Epel, Reus and Mellon3 Telomeres are DNA–protein complexes composed of tandem TTAGGG repeats at the ends of linear chromosomal DNA, protecting the DNA from damage. During each cell division, telomeres shorten progressively until a critical short length, after which cells become susceptible to senescence or apoptosis. Reference Bojesen4 Telomere length may thus be an indicator of ‘biological age’, and short telomeres have been associated with development of, for example, chronic obstructive pulmonary disease Reference Rode, Bojesen, Weischer, Vestbo and Nordestgaard5 and myocardial infarction. Reference Weischer, Bojesen, Cawthon, Freiberg, Tybjaerg-Hansen and Nordestgaard6
Several cross-sectional studies Reference Schutte and Malouff7 and a few prospective studies have examined the association between telomere length and depression. The first study was published in 2006 and it included 44 patients with mood disorder and 44 age-matched controls. Reference Simon, Smoller, McNamara, Maser, Zalta and Pollack8 It has been followed by a long list of mainly cross-sectional studies with up to 3000 participants Reference Ladwig, Brockhaus, Baumert, Lukaschek, Emeny and Kruse9 with varying adjustment for confounders. In general, the association has been tested in studies including psychiatric in-patients, Reference Simon, Smoller, McNamara, Maser, Zalta and Pollack8,Reference Garcia-Rizo, Fernandez-Egea, Miller, Oliveira, Justicia and Griffith10–Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl and Hultdin13 population-based samples Reference Ladwig, Brockhaus, Baumert, Lukaschek, Emeny and Kruse9,Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel14–Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx21 and in individuals with somatic diseases, Reference Garland, Palmer, Donelson, Gehrman, Johnson and Mao22–Reference Liu, Zhang, Yan, Wang and Li27 with conflicting results. Many of the smaller studies (n<100) have reported a positive association Reference Simon, Smoller, McNamara, Maser, Zalta and Pollack8,Reference Hartmann, Boehner, Groenen and Kalb11–Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl and Hultdin13,Reference Karabatsiakis, Kolassa, Kolassa, Rudolph and Dietrich28 whereas larger studies (n>1000) have shown no association, Reference Ladwig, Brockhaus, Baumert, Lukaschek, Emeny and Kruse9,Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel14,Reference Phillips, Robertson, Carroll, Der, Shiels and McGlynn15,Reference Shaffer, Epel, Kang, Ye, Schwartz and Davidson19 but with important exceptions. Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx21 Most studies have used telomere length measured in blood leukocytes, and only two studies have used telomere length from brain cells, also with conflicting results. Reference Szebeni, Szebeni, DiPeri, Chandley, Crawford and Stockmeier29,Reference Zhang, Cheng, Craig, Redman and Liu30 To date, only a few longitudinal studies have been performed. Hoen et al found that depression did not predict 5-year change in telomere length in 952 individuals with stable coronary heart disease Reference Hoen, de Jonge, Na, Farzaneh-Far, Epel and Lin24 and similarly, telomere length was not associated with depressive symptoms in 75 older men during 7 years of follow-up. Reference Rius-Ottenheim, Houben, Kromhout, Kafatos, van der Mast and Zitman16 Finally, a population-based study of 1037 individuals reported that depression, anxiety and post-traumatic stress disorder were associated with shorter telomere length and accelerated telomere shortening across a 12-year period in men but not in women. Reference Shalev, Moffitt, Braithwaite, Danese, Fleming and Goldman-Mellor20
The nature of the association between depression and biological ageing is thus still not clear. Although most studies have suggested that depression accelerates telomere shortening, one recent study suggested that shorter telomere length in young girls of mothers with depression might predispose them to develop depression as well as other age-related medical illnesses, Reference Gotlib, LeMoult, Colich, Foland-Ross, Hallmayer and Joormann31 suggesting that short telomere length increases risk of depression. Finally, it is also possible that the association between telomere length and depression is explained by confounders such as lifestyle factors or clinical/subclinical chronic disease that influence both depression and telomere length. Here, we tested the hypotheses that depression is associated with short telomere length in the general population, and that short telomere length per se is causally associated with depression, as suggested previously. Reference Gotlib, LeMoult, Colich, Foland-Ross, Hallmayer and Joormann31 First, to mimic previous studies, we tested the hypothesis that participants from the general population who had experienced depression had shorter telomeres than participants who had not experienced an episode of depression. Second, we tested the hypothesis that short telomeres are associated prospectively with increased risk of depression. Finally, we tested whether genetically short telomeres are associated with depression in a Mendelian randomisation study. The Mendelian randomisation approach enables testing of causality between an exposure (such as telomere length) and an outcome (such as depression) by the use of genetic variants as proxies for the exposure as they are randomly allocated during gamete formation and therefore unlikely to be associated with any known or unknown confounders. Reference Lawlor, Harbord, Sterne, Timpson and Davey Smith32 As such, a Mendelian randomisation study is analogous to a randomised controlled trial where participants are randomised to either intervention or placebo, and the randomisation at baseline ensures equal distribution of confounders and excludes the possibility of reverse causation. In this study we used the three single nucleotide polymorphisms with the largest influence on telomere length. Reference Pooley, Bojesen, Weischer, Nielsen, Thompson and Amin Al33 Online Fig. DS1 shows the Mendelian randomisation study design.
Method
The study was approved by Danish ethical committees and by Herlev Hospital. All participants gave written informed consent.
Participants
We used two large independent Danish general population studies, the Copenhagen General Population Study (CGPS) 2003–2013 examination (n = 57 013), Reference Rode, Nordestgaard, Weischer and Bojesen34 and the Copenhagen City Heart Study (CCHS) 1991–1994 and/or 2001–2003 examinations (n = 10 293). Participants from both studies were 20–100 years old and were randomly selected from the national Danish Civil Registration System to represent the Danish general population. All 67 306 participants were White and of Danish descent. Participants filled in a questionnaire, which was reviewed together with an investigator on the day of attendance, had a physical examination performed, and had blood samples drawn for biochemical measurements and for DNA extraction. If a participant took part in both studies, only data from the first examination was included. Because all individuals in Denmark have a unique identification number, we used the national Danish Civil Registration System to register emigration or death for all participants.
Telomere length
DNA was isolated from leukocytes, and telomere length was measured using a modified monochrome multiplex quantitative polymerase chain reaction method, Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber35 as described in detail elsewhere. Reference Weischer, Bojesen, Cawthon, Freiberg, Tybjaerg-Hansen and Nordestgaard6 In brief, we used the reference single-copy gene for albumin to adjust for different amounts of DNA in samples. Telomere length from each participant was measured in quadruplicates and the mean was calculated. Unsuccessful samples were analysed again until valid measurements were available in 99.9% of participants. Of these, a telomere length measurement was obtained after the first analysis in 98.5%, whereas 1.1% of samples were rerun once and 0.5% were rerun twice. The absolute telomere length was derived after calibration with measurement on K562 cell line DNA, included in each plate. The interassay coefficient of variation obtained from the NTERA-2 cell line, included in each plate, was 2% for threshold cycle values (Ct) at a mean of 17.9 cycles for the telomere assay, and 9% for absolute telomere length at a mean of 2534 base pairs. Failed samples were reanalysed resulting in valid measurements in 99.9% of participants. We calculated coefficients of variation (CV) in the whole range of telomere lengths, using the multiple measurements per individual Reference Jones36 and found that CVs for the Ct value remained below 5%, and below 8% for telomere length among >28 000 representative individuals. Samples with measured telomere lengths above 15 000 or below 1000 base pairs were regarded as outliers and were excluded (n = 36).
Depression
Depression was defined using two independent sources of information. First, diagnoses of depression for all participants were obtained from the national Danish Patient Registry (94% of diagnoses) with information on all hospital discharge diagnoses of depression from psychiatric and somatic hospitals since 1977 and on diagnoses from emergency rooms and out-patient clinics since 1995. Furthermore, diagnoses were obtained from the national Danish Causes of Death Registry (6% of diagnoses) with information on causes of death for all individuals in Denmark since 1970. Depression was diagnosed using ICD-8 codes 296.0, 296.2, 298.0, and 300.4 until 1994, and ICD-10 codes F32 and F33 from 1994 onwards. 37,38
Second, because antidepressant medication use can only be obtained by prescription in Denmark, we obtained information about every prescription of antidepressant medication use claimed by study participants from 1995 through 2010 from the national Danish Register of Medicinal Product Statistics. We used Anatomical Therapeutic Chemical (ATC) codes for selective serotonin reuptake inhibitors (SSRIs), N06AB, tricyclic antidepressants (TCAs), N06AA, noradrenaline reuptake inhibitors (NARIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), and noradrenergic and specific serotonergic antidepressants (NaSSAs), N06AX. We only assigned this end-point if participants at some point in their life had purchased antidepressant medication use for a period of at least 6 continuous months with an average daily dose of at least 0.75 of a standard World Health Organization (WHO) defined daily dose.
Genetic variants associated with telomere length
We selected the three most significant hits for genetic variants associated with telomere length that all reached genome-wide significance in a recent genome-wide association study. Reference Pooley, Bojesen, Weischer, Nielsen, Thompson and Amin Al33 They are located on different chromosomes: rs1317082 at 3q26.2 in the TERC gene, rs2487999 at 10q24.3 in the OBFC1 gene, and rs7726159 at 5p15.3 in the TERT gene. TERC and TERT encode for telomerase reverse transcriptase and telomerase RNA template, important for maintaining telomere length. Reference Pooley, Bojesen, Weischer, Nielsen, Thompson and Amin Al33 OBFC1 encodes for part of the CST complex (a trimeric complex composed of three components, Cdc13, Stn1 and Ten1), which regulates telomerase activity. Reference Chen, Redon and Lingner39 We used the TaqMan method (Applied Biosystems Foster City, California, USA) to genotype individuals from the CGPS. Individuals from the CCHS were genotyped using an Illumina™ custom genotyping chip (Illumina, San Diego, California, USA). Reference Bojesen, Pooley, Johnatty, Beesley, Michailidou and Tyrer40 Because of reruns, the genotyping call rate was 99.9%. All genotypes were in Hardy–Weinberg equilibrium estimated by χ2 tests (data not shown).
Covariates
Participants reported on date of birth, smoking status (never; former; current), number of alcoholic drinks/week, leisure time physical activity (0–2 h light activity/week; 2–4 h light activity/week; >4 h light activity or 2–4 h vigorous activity/week; >4 h vigorous activity/week), level of education after primary and lower secondary school (no education; shorter education (less than 3 years); basic vocational training (1–3 years); higher education (⩾3 years); university education), level of income (lowest; middle; highest). Body mass index (BMI) was measured as weight in kilograms divided by measured height in metres squared. Plasma levels of C-reactive protein (CRP) were measured with a high-sensitivity assay using latex-enhanced turbidimetry (Dako, Glostrup, Denmark) or nephelometry (Dade Behring, Deerfield, Illinois, USA) at the Department of Clinical Biochemistry, Herlev University Hospital. Chronic disease was ascertained by collecting information on diagnoses from the national Danish Patient Registry, the national Danish Cancer Registry, and the national Danish Causes of Death Registry on ischaemic heart disease, myocardial infarction, stroke, diabetes, hypertension, cancer, pneumonia, chronic obstructive pulmonary disease, asthma, deep venous thrombosis and pulmonary embolism.
Statistical analyses
Stata version 13.1 was used. To achieve maximal statistical power, data from the CGPS and the CCHS were combined; however, all analyses were adjusted for study and results were similar in the two studies separately. We had 99.9% complete data on covariates. Missing values were imputed based on age and gender.
Cross-sectional analyses
First, we tested whether participants with depression before entry to the study had shorter telomeres compared with participants who had not experienced depression. For these analyses of attendance at hospital for depression, individuals with an episode of depression after entry to the study (n = 1004) and individuals in the control group with any prescription antidepressant medication use before or after study entry (n = 12 816) were excluded. Individuals with an attendance at hospital for depression were then compared with individuals without an attendance at hospital for depression or prescription antidepressant medication use. Similarly, in the analyses of 6-month use of prescription antidepressant medication, individuals with future prescription antidepressant medication use (n = 3010) and individuals in the control group with an attendance at hospital for depression or with any prescription of antidepressant medication before or after study entry (n = 6442) were excluded. To examine the influence of possible confounding on telomere length we examined telomere length in three ways: (a) unadjusted, (b) adjusted for age and gender, and (c) adjusted multifactorially for age, gender, smoking status, alcoholic drinks/week, education, income, physical activity, BMI and date of birth. In a sensitivity analysis, the multifactorially adjusted analysis was further adjusted for plasma CRP and chronic disease. Two-tailed P-values for differences between telomere lengths were determined using multiple linear regression.
Prospective analyses
We also tested whether short telomeres at baseline were associated with increased risk of depression during follow-up. For these analyses, participants with depression before study entry (n = 370 for attendance at hospital for depression, n = 4738 for prescription antidepressant medication use) were excluded from the relevant analyses. We used a Cox proportional hazards regression model with age as the underlying timescale, and left truncation (delayed entry) in 1991–1994, 2001–2003 or 2003–2013 as appropriate, to calculate hazard ratios (HRs) with 95% confidence intervals. Follow-up began at study attendance and participants were censored at first event (attendance at hospital for depression/death with a diagnosis of depression (n = 1004) or prescription of antidepressant medication use (n = 3010), death from other causes (n = 8136), emigration (n = 359) or end of follow-up (April 2013)), whichever came first. We assessed the proportional hazards assumption using Schoenfeld residuals; no important violations were detected. We used two different models adjusted for (a) age and gender, and (b) multifactorially further adjusting for the covariates as in cross-sectional analyses. We also calculated the risk of depression for a 200 base-pair decrease in telomere length stratified on each of the covariates, and tested for interaction between covariates variables and telomere length on risk of depression using a likelihood ratio test. This was performed in order to see whether the association differed between various subgroups. Importantly, in all analyses we adjusted for covariates using the full information on covariates (continuous or categorical), and binary information was only used in the tables and in the stratified analyses.
Mendelian randomisation
For the Mendelian randomisation study design, we first tested whether each genetic variant was associated with telomere length; we then combined all three genetic variants into one allele score based on the number of telomere-shortening alleles. Second, we tested whether the genetic variants and the allele score were directly associated with attendance at hospital for depression/death with depression or prescription antidepressant medication use using unadjusted logistic regression models, as genotypes were not associated with any measured confounders. Third, to test the genetic association between short telomeres and each of the end-points, we performed instrumental variable analysis with a two-stage regression model using genetically lower telomere length to calculate the odds of attendance at hospital for depression/death with depression and prescription antidepressant medication use. The first stage was a linear regression of each of the genetic variants or the allele score as a categorical variable on telomere length. R 2 shows the percentage of telomere length explained by the genetic variant and F-statistics >10 indicated sufficient statistical strength to carry out statistically valid instrumental variable analysis. Reference Lawlor, Harbord, Sterne, Timpson and Davey Smith32 The second stage was a logistic regression of a 200 base-pair lower telomere length determined by the genetic variants/allele score (generated in the first stage) on each of the end-points. Reference Palmer, Sterne, Harbord, Lawlor, Sheehan and Meng41 For comparison, we calculated observational odds ratios (ORs) for the association between a 200 base-pair lower telomere length and each of the end-points using logistic regression.
In sensitivity analyses we repeated analyses using a weighted allele score (i.e. where each genotype is included based on its effect on telomere length). Furthermore, to exclude confounding from covariates we adjusted all genetic analyses for the covariates used in the observational analyses. Finally, to further exclude confounding we did a two-stage analysis in which the first stage was a regression analysis of all the covariates except for telomere length on each of the end-points. We obtained residuals between the observed and expected outcome and in the second stage we did a regression analysis of the allele score on the residuals.
Results
Baseline characteristics of the 67 306 participants by telomere length quartiles are listed in online Table DS1, by end-points in online Table DS2 and by allele score in online Table DS3. Telomere length was associated with all potential confounders, most strongly with age (P<1 × 10−300). Furthermore, almost all of the potential confounders were associated with each of the end-points, but importantly not with the allele score. The latter illustrates that genotype can be used as a largely unconfounded proxy for telomere length free of reverse causation to study the relationship between telomere length and depression.
Telomere length in participants with and without depression
Participants with attendance at hospital for depression before study entry (n = 370) had a shorter mean telomere length compared with participants without attendance at hospital for depression or prescription antidepressant medication use (3175 bp (95% CI 3089–3261) v. 3404 bp (95% CI 3396–3412), P = 3 × 10−6) (Fig. 1). This was still significant after adjustment for age and gender (coefficient −104.19, P = 0.01) and after multifactorial adjustment (coefficient −82.38, P = 0.04). All coefficients from the multifactorially adjusted model are shown in online Table DS4. When chronic disease and CRP were included in the multifactorially adjusted model, results were no longer significant (coefficient −76.01, P = 0.06) (online Table DS5). For prescription antidepressant medication use, participants with prescription antidepressant medication (n = 4738) had shorter telomere length compared with participants without attendance at hospital for depression or prescription antidepressant medication use (3253 bp (95% CI 3228–3278) v. 3404 bp (95% CI 3396–3412), P = 3 × 10−5) (Fig. 1). This was still significant after adjustment for age and gender (coefficient −35.60, P = 2 × 10−3) but not after multifactorial adjustment (coefficient −12.40, P = 0.29). Coefficients from the multifactorially adjusted model are shown in online Table DS6. When chronic disease and CRP were included in the multifactorially adjusted model, results were similar (coefficient −9.83, P = 0.40) (online Table DS7).
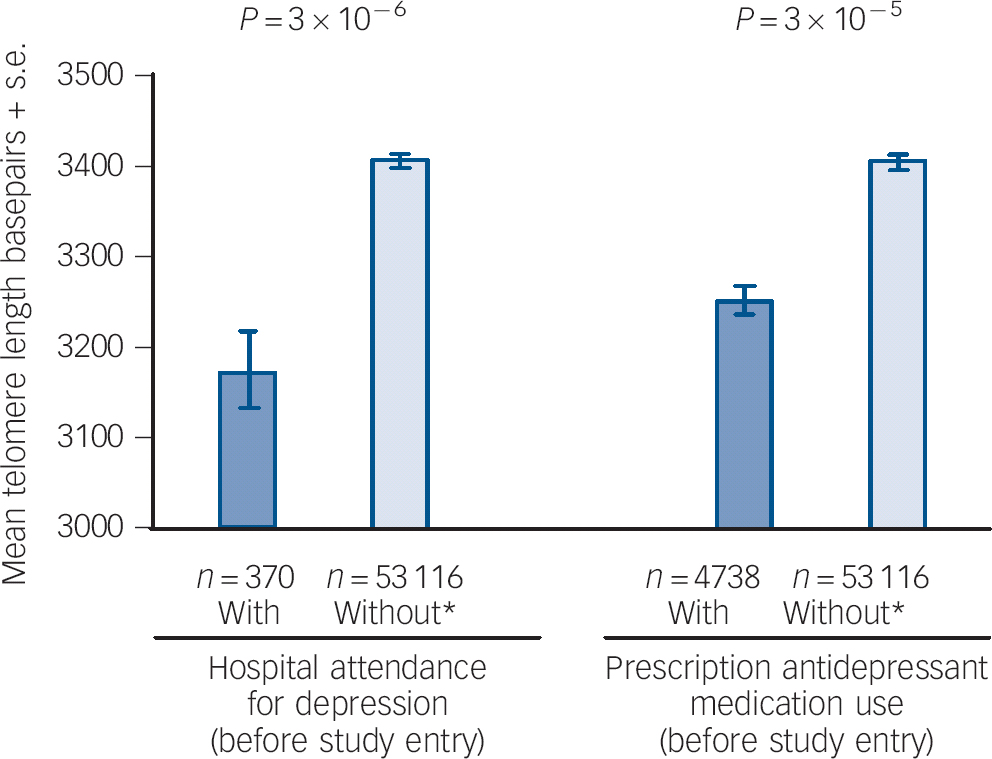
Fig. 1 Mean telomere length and depression.
Left: unadjusted telomere length for participants with hospital attendance for depression before study entry v. participants without attendance at hospital with depression or antidepressant medication use before study entry. Participants with admission to hospital/death with depression after study attendance were excluded. Right: unadjusted telomere length for participants with 6-month prescription antidepressant medication use before study entry v. participants without prescription antidepressant medication use or admission to hospital with depression before study entry. Participants with 6-month prescription antidepressant medication use after study entry were excluded. Based on 67 306 participants from the Copenhagen General Population Study and Copenhagen City Heart Study combined. Bp, base pairs. * Without attendance at hospital with depression and prescription antidepressant medication use.
Prospective analyses
Mean follow-up was 7.6 years (range 0.0–21.5). In prospective analyses, short telomeres at baseline were not associated with high risk of future attendance at hospital for depression/death with depression in the analyses adjusted for age and gender (P for trend 0.21 across quartiles and P for trend 0.27 across octiles) or adjusted multifactorially (P for trend 0.21 across quartiles and P for trend 0.26 across octiles), with a corresponding multifactorially adjusted hazard ratio of 1.09 (95% CI 0.90–1.31) for participants with short telomere length (in the lowest quartile) compared with those with the longest telomere lengths (in the highest quartile), and a hazard ratio of 1.21 (0.94–1.55) for participants with short telomere length (in the lowest octile) compared with those with the longest telomere length (the highest octile) (Fig. 2). Further adjustment for chronic disease and CRP slightly attenuated results (data not shown). For prescription antidepressant medication use, short telomere length was associated with prescription antidepressant medication use when adjusting for age and gender (P for trend 6 × 10−3 across quartiles and P for trend 4 × 10−3 across octiles) but not when adjusting multifactorially (P for trend 0.14 across quartiles and P for trend 0.11 across octiles), with corresponding multifactorially adjusted hazard ratios of 1.07 (0.96–1.19) and 1.04 (0.90–1.21). Further adjustment for chronic disease and CRP slightly attenuated results (data not shown).

Fig. 2 Prospective associations between telomere length and attendance at hospital for depression/death ((a) quartiles and (b) octiles) or prescription antidepressant medication use ((c) quartiles and (d) octiles) in the general population.
Overall based on 67 306 participants from the Copenhagen General Population Study and Copenhagen City Heart Study combined; however, as individuals either with attendance at hospital for depression/death or prescription antidepressant medication use at baseline were excluded, the number of individuals in the different analyses is smaller than 67 306. Multifactorially adjusted was for age, gender, smoking status, alcoholic drinks/week, education, income, physical activity, body mass index, plasma C-reactive protein and chronic disease. bp, base pair.
A 200 base-pair decrease in telomere length was not associated prospectively with attendance at hospital for depression/death with depression or prescription antidepressant medication use after multifactorial adjustment (online Fig. DS2). There was no convincing interaction between confounding variables and telomere length on risk of depression.
Mendelian randomisation study
All genetic variants and the allele score were associated with telomere length (P for trend 3 × 10−23 to 2 × 10−75). Compared with individuals with 0–2 telomere decreasing alleles, individuals with 5–6 telomere decreasing alleles had telomeres that were on average 229 base pairs shorter (Fig. 3). However, participants with genetically shorter telomeres did not have increased odds of attendance at hospital for depression/death with depression or prescription antidepressant medication use (P for trend >0.05). When we adjusted the analyses for all mentioned covariates, results were similar (data not shown). Furthermore, when we did a two-stage model as described in the Method section, the residuals from the first stage were not associated with the allele score, which further suggests that the genotypes were not associated with either of the end-points after adjustment for covariates.

Fig. 3 Associations between each genetic variant and telomere length (first column), attendance at hospital for depression/death (second column), and prescription antidepressant medication use (last column).
Based on 65 107 participants with the rs1317082 genotype, 65 488 with the rs2487999 genotype, 65 719 with the rs7726159 genotype and 65 096 with all three genotypes from the Copenhagen General Population Study and Copenhagen City Heart Study combined. P-values for trend were calculated using Cuzick's extension of the Wilcoxon rank sum test. Odds ratios were unadjusted, as genotypes did not associate with measured potential confounders (see online Table DS3). bp, base pair; He, heterozygote; Ho, homozygote; Wt, wildtype. a. Events of attendance at hospital for depression/death/events of antidepressant medication use.
For attendance at hospital for depression/death with depression, instrumental variable analysis yielded a genetic odds ratio of 0.97 (95% CI 0.83–1.14) for a 200 base-pair shorter telomere length estimated from the allele score, and the corresponding multifactorially adjusted observational odds ratio was 1.01 (95% CI 0.99–1.02) (Fig. 4). For prescription antidepressant medication use, corresponding odds ratios were 1.01 (95% CI 0.94–1.09) genetically and 1.00 (95% CI 1.00–1.01) observationally. When we adjusted the genetic analyses for covariates or when using a weighted allele score, results were similar (data not shown).

Fig. 4 Observational and genetic risk estimates for (a) attendance at hospital for depression/death and (b) prescription antidepressant medication use for a 200 base-pairs shorter telomere length.
Based on 65 107 participants with the rs1317082 genotype, 65 488 with the rs2487999 genotype, 65 719 with the rs7726159 genotype and 65 096 with all three genotypes from the Copenhagen General Population Study and Copenhagen City Heart Study combined. F and partial R 2 are from the linear regression of telomere length on each of the genetic variants and the allele score.
Discussion
Main findings
The principal finding of this study of 67 306 individuals from the Danish general population was that individuals who attended hospital for depression had shorter telomere length compared with individuals who did not attend hospital for depression or use prescription antidepressant medication. However, our results also showed, that a large part of this association was explained by confounders such as age, gender and lifestyle factors. Furthermore, for prescription antidepressant medication use results were not significant after multifactorial adjustment. Finally, short telomeres were not prospectively or genetically associated with increased risk of depression. These last findings are novel.
Results from studies examining the association between telomere length and depression have been conflicting. Many of the smaller studies (n<100) have reported a positive association, Reference Simon, Smoller, McNamara, Maser, Zalta and Pollack8,Reference Hartmann, Boehner, Groenen and Kalb11–Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl and Hultdin13,Reference Karabatsiakis, Kolassa, Kolassa, Rudolph and Dietrich28 whereas larger studies (n>1000) have shown no association. Reference Ladwig, Brockhaus, Baumert, Lukaschek, Emeny and Kruse9,Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel14,Reference Phillips, Robertson, Carroll, Der, Shiels and McGlynn15,Reference Shaffer, Epel, Kang, Ye, Schwartz and Davidson19 This could be explained by publication bias, or because smaller studies may be less well-adjusted compared with larger studies. One exception is the study by Verhoeven et al including 1095 patients with current depression, 802 patients with remitted depression and 510 controls. Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx21 However, similar to the cross-sectional part of our study, they did find that the association between depression (both current and remitted) and short telomeres was attenuated after multifactorial adjustment. Importantly, as opposed to our study, Verhoeven et al did not adjust for plasma CRP levels. When we included plasma CRP and chronic disease, our results were no longer significant. This could be because CRP and chronic disease are on the causal pathway between depression and telomere length. Our results from the prospective and Mendelian randomisation study further shows that short telomere length in itself is not causally associated with depression. This suggests that short telomere length per se does not increase risk of depression. Rather, our results suggest that the association may be explained by depression leading to shorter telomere length or by residual confounding.
Strengths and limitations
An important strength of our study is the large sample size of 67 306 individuals from the general population. Furthermore, we included prospective analyses and a Mendelian randomisation study using three genetic variants associated with telomere length. Other strengths include the completeness of the Danish registers, which enabled us to perform prospective analyses of attendance at hospital for depression/death with depression and prescription antidepressant medication use with no losses to follow-up, and to adjust results for register-based chronic diseases such as cardiovascular disease, lung diseases and cancer, which otherwise may confound the association.
A potential limitation of our study is that depression was not ascertained using a diagnostic test. Instead we used information from two independent registers on attendance at hospital for depression/death with depression and prescriptions of a minimum of 6 months of antidepressant medication use. Because most people with depression in Denmark are treated in general practice or at private psychiatrists, using hospital discharge diagnoses might have underestimated the number of participants with depression. However, we found similar results when the hypothesis was tested using the independent end-point of prescription antidepressant medication use. Conversely, this might have overestimated the number of participants with depression since depression is not the only indication for these pharmaceuticals. However, as may be the case in other cohort studies we cannot exclude some selection bias as individuals with depression may not participate as often as individuals without depression, which may be why the prevalence of depression is lower than the general population prevalence. Selection bias in our study can also be demonstrated as only 370 individuals had a previous attendance at hospital for depression before entrance to the study whereas 1004 had an attendance at hospital for depression/death with depression during the mean 7.6-year follow-up. Another potential limitation to our study is that we only measured telomere length in leukocytes and not in brain cells. However, leukocyte telomere length correlates with telomere length in other tissues including brain cells, Reference Friedrich, Griese, Schwab, Fritz, Thon and Klotz42,Reference Lukens, Van, Clark, Xie and Johnson43 and leukocytes are easily accessible whereas measurement of brain leukocytes is difficult in a large population-based study. Another potential limitation to our study is that all participants were White, and therefore our results may not necessarily apply to other populations; however, as genotypes may have different allele frequencies in different populations, this meant that our Mendelian randomisation was less prone to population stratification bias. Furthermore, we are not aware of data to suggest that our results for the association between depression and telomeres should not be applicable to other populations. Finally, a fundamental assumption in a Mendelian randomisation analysis is that the genotypes used should influence the outcome only through the exposure of interest (i.e. telomere length). Although we cannot exclude the possibility that the genetic variants may have pleiotropic effects, by including genetic variants in three different genes on three different chromosomes, any possible pleotropic effects are likely to be different for the three genetic variants and therefore individually have smaller effect.
In conclusion, we could not detect a difference in telomere length between individuals with and without depression after adjustment for lifestyle factors, levels of inflammation and chronic disease in our study. Furthermore, short telomeres were not associated prospectively with risk of depression. Finally, our Mendelian randomisation study indicated that short telomeres were not causally associated with depression.
Funding
This study was supported by Herlev Hospital, Copenhagen University Hospital and The Danish Council for Independent Research, Medical Sciences (FSS).
Acknowledgements
We thank the participants and staff of the Copenhagen General Population Study and the Copenhagen City Heart Study for their important contributions, and lab technician Anja Jochumsen for assistance with the large-scale telomere length measurements.







eLetters
No eLetters have been published for this article.